Bilateral breast multiple myeloma: a case report
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Bilateral breast multiple myeloma: a case report
Cyrus KM Mo, MB, ChB, FRCR1; Alta YT Lai, FRCR, FHKAM (Radiology)1; Sherwin SW Lo, FRCR, FHKAM (Radiology)2; TS Wong, MB, BS3; Wendy WC Wong1, FRCR, FHKAM (Radiology)1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Radiology, Gleneagles Hospital, Hong Kong
3 Department of Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Corresponding author: Dr Cyrus KM Mo (mkm463@ha.org.hk)
Introduction
Multiple myeloma (MM) is a malignant disorder
characterised by excessive proliferation of single
clonal plasma cells derived from B cells in the bone
marrow with increased formation of monoclonal
immunoglobulins.1 Multiple myeloma may
involve extramedullary organs or soft tissues
(extramedullary plasmacytoma), commonly in
the upper aerodigestive tract with a predilection
for the head and neck.1 Breast plasmacytoma is
very rare. We present a case of MM with extensive
extramedullary involvement including bilateral
breasts, and mainly focus on the imaging features of
breast plasmacytoma across multi-modalities.
Case report
A 59-year-old female presented for scheduled
ultrasound scan of abdomen for follow-up of
a complicated renal cyst in December 2019.
She had a previous history of diabetes mellitus,
hypertension, hyperlipidaemia and hepatitis B
infection. She was diagnosed with MM 10 years
previously and had previously failed hematopoietic
stem cell transplantation. Ultrasound scan revealed
an enlarged hypoechoic nodule in the liver that
corresponded to the liver lesion detected on earlier
computed tomography (CT) urogram. She also
complained of a palpable nodule in the right lower
quadrant of the abdomen, present for a few months.
Magnetic resonance imaging (MRI) with contrast
of liver was arranged to assess the liver lesion and
abdominal wall nodule.
The MRI 1 month later showed significant
enlargement of the liver lesion with heterogeneous
contrast enhancement and restricted diffusion.
No definite contrast washout was demonstrated
in the portovenous phase. The nodule in the right
lower quadrant of the abdominal wall demonstrated
contrast enhancement and restricted diffusion as
well. Features of both lesions were suggestive of
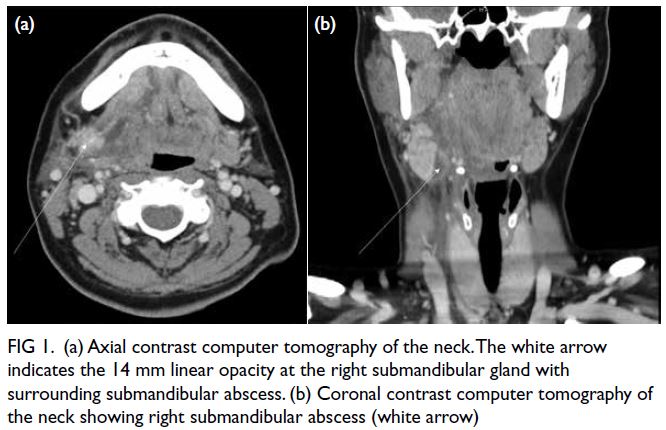
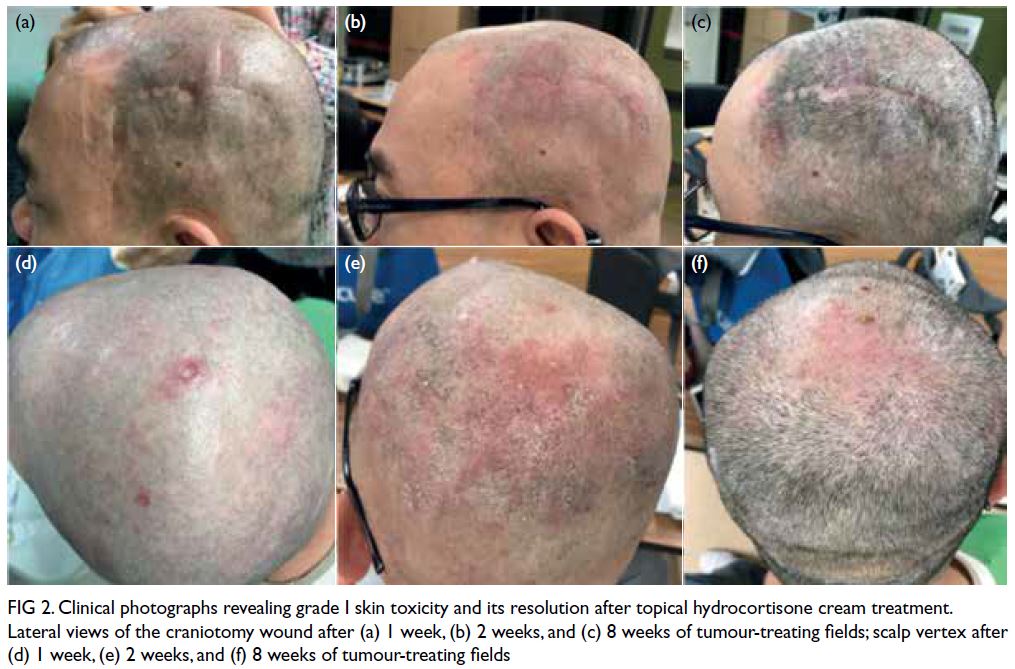
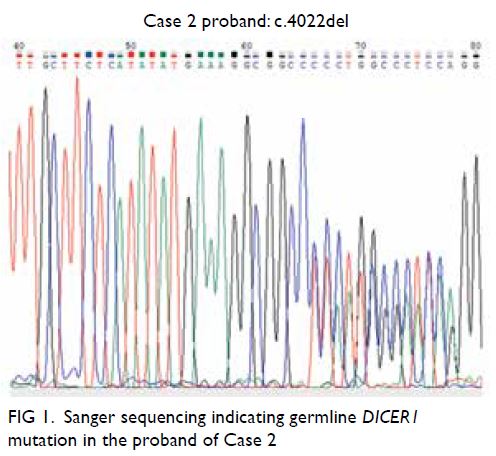
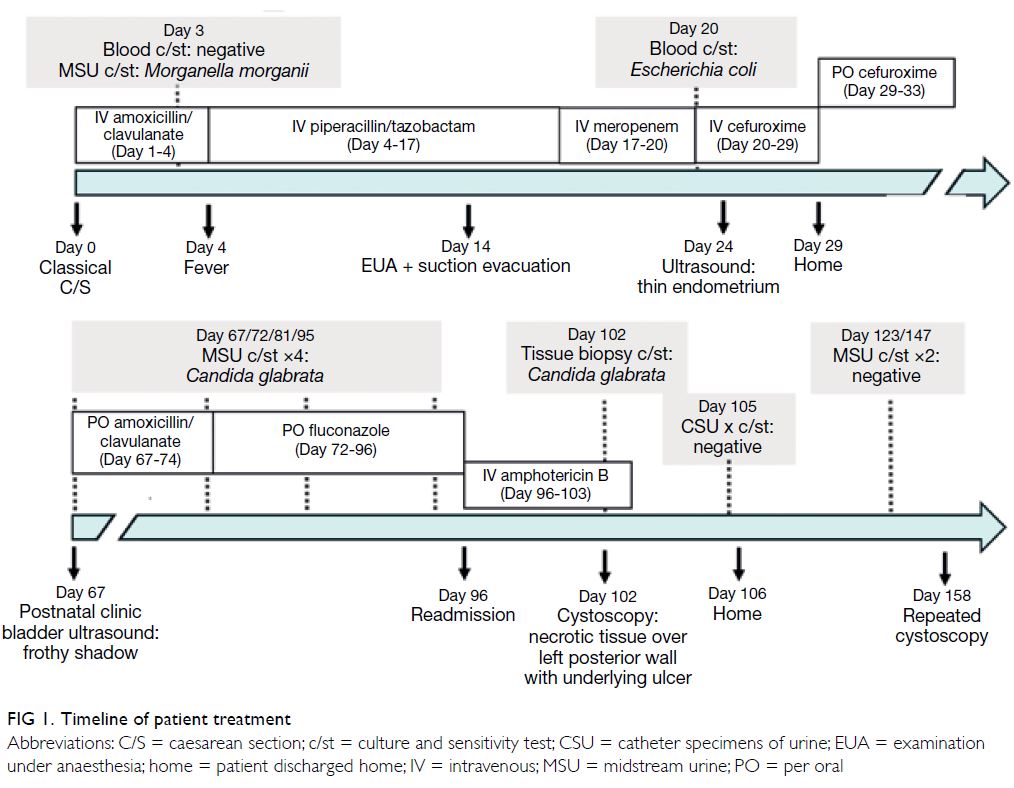
malignancy. The MRI also revealed a 1.3 cm T1
isointense T2 hyperintense contrast enhancing
nodule with restricted diffusion in the outer lower
quadrant of the left breast (Fig 1).
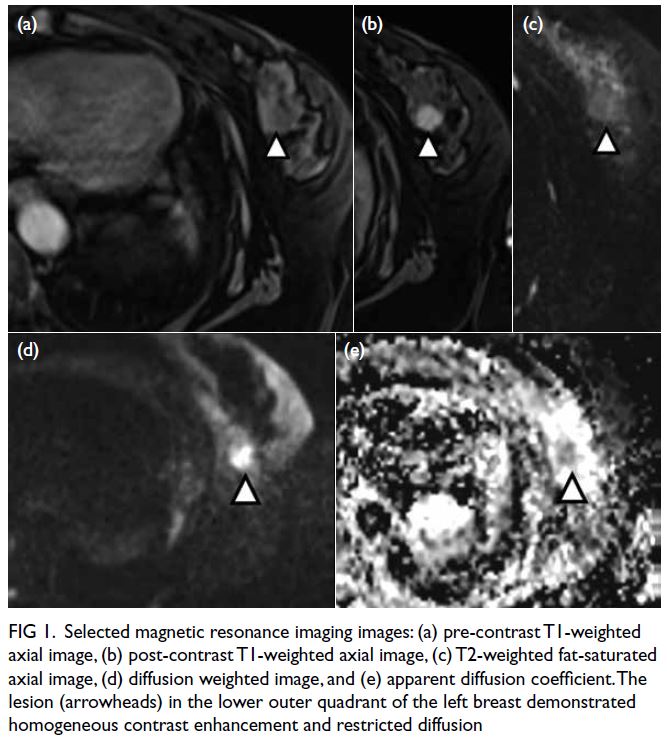
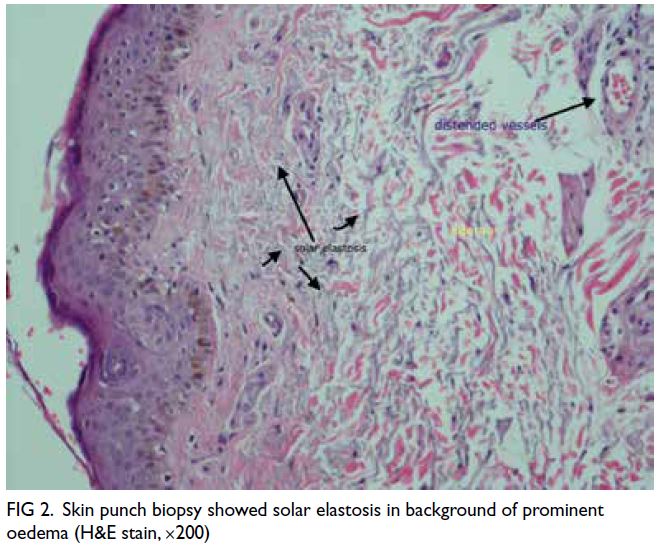
Figure 1. Selected magnetic resonance imaging images: (a) pre-contrast T1-weighted axial image, (b) post-contrast T1-weighted axial image, (c) T2-weighted fat-saturated axial image, (d) diffusion weighted image, and (e) apparent diffusion coefficient. The lesion (arrowheads) in the lower outer quadrant of the left breast demonstrated homogeneous contrast enhancement and restricted diffusion
Ultrasound-guided fine needle aspiration of
the right lower quadrant abdominal wall nodule
was performed in February 2020. The pathological
diagnosis was plasmacytoma.
Dual-tracer positron emission tomography
(PET)/CT was performed in early March 2020 at
a private institution to assess disease involvement.
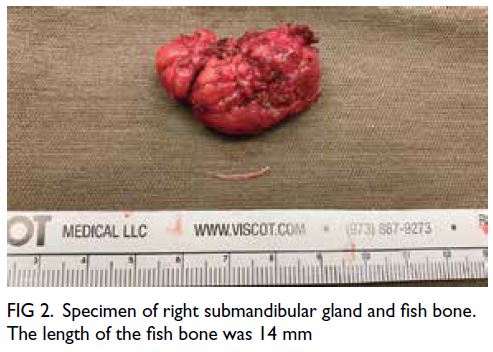
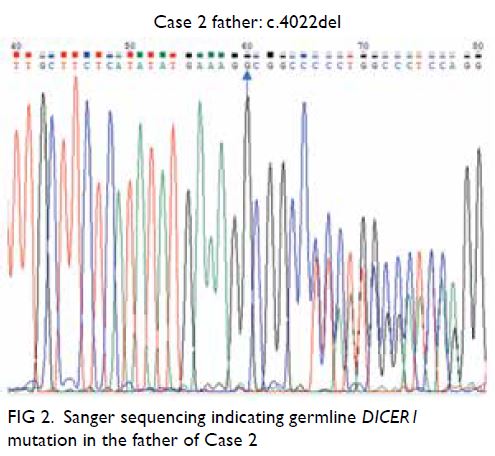
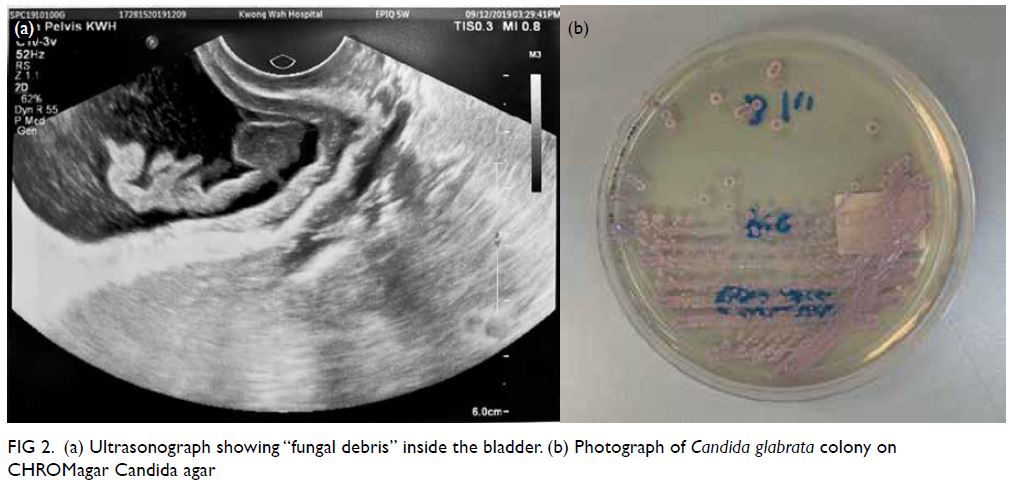
There were multiple acetate (Ac) and fludeoxyglucose
(FDG)-avid soft tissue nodules in bilateral breasts,
measuring up to 1.5 × 1.4 cm with FDG maximum
standard unit value (SUVmax) 8.4 and Ac SUVmax
5.0 in left breast (Fig 2a) and 1.4 × 1.0 cm with FDG
SUVmax 7.0 and Ac SUVmax 6.6 in right breast.
There were a few left axillary level I lymph nodes
measuring up to 0.9 × 0.8 cm with FDG SUVmax 3.3
and Ac SUVmax 3.9.
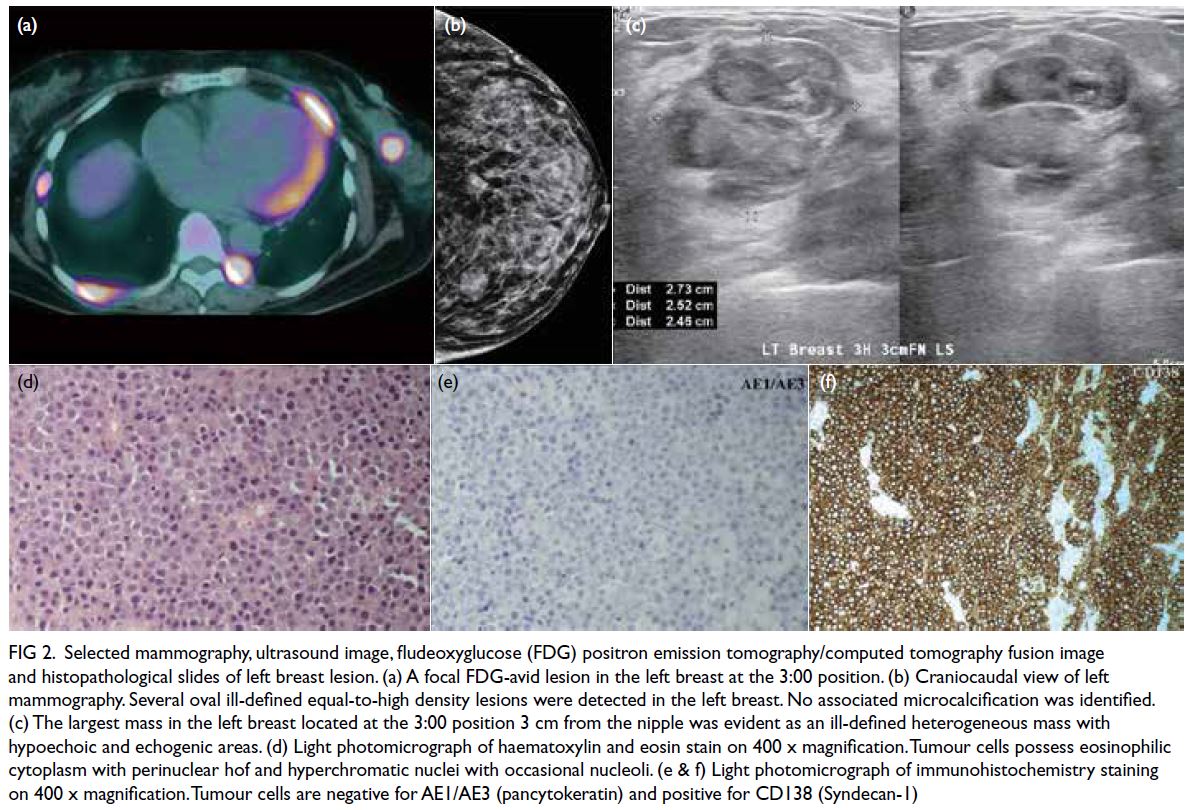
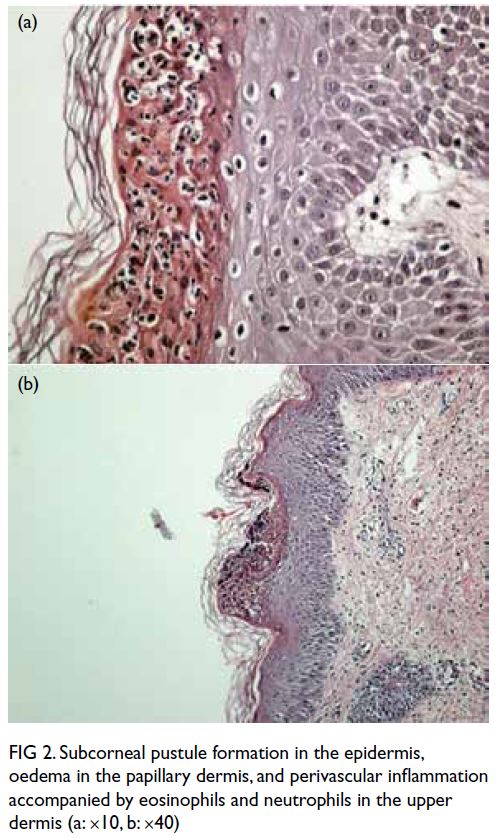
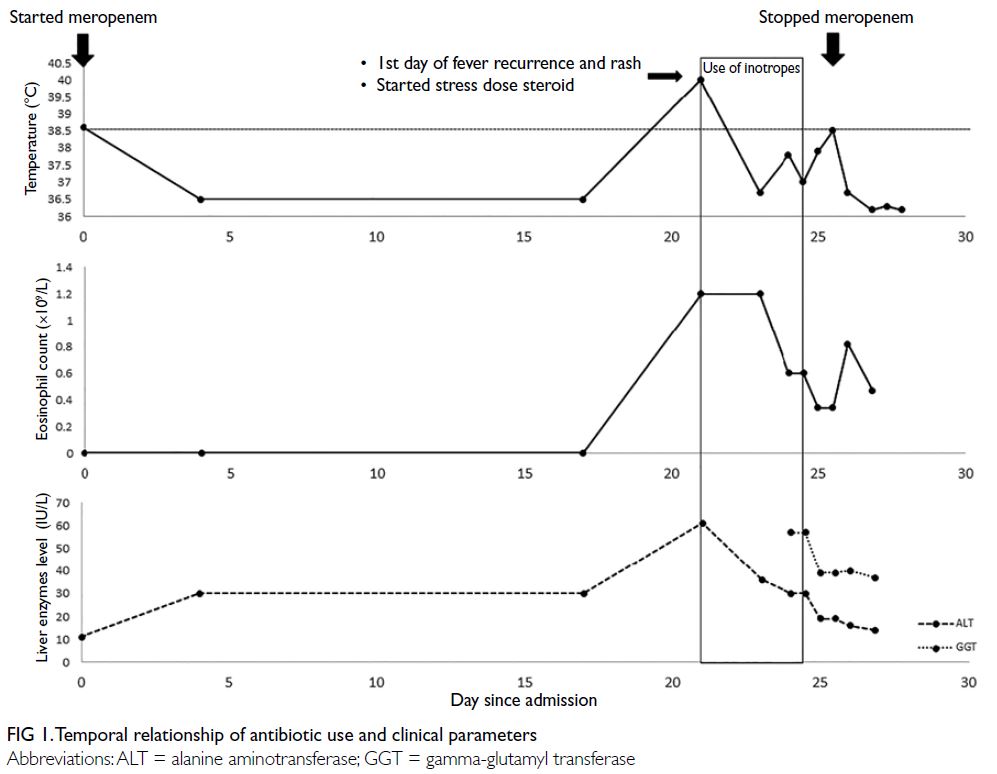
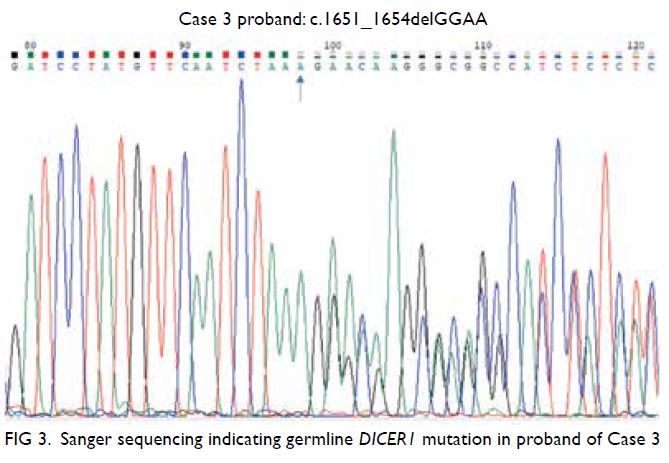
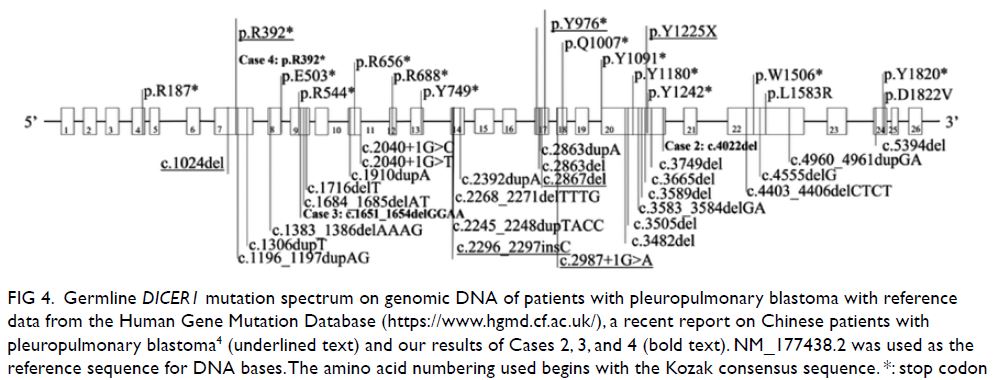
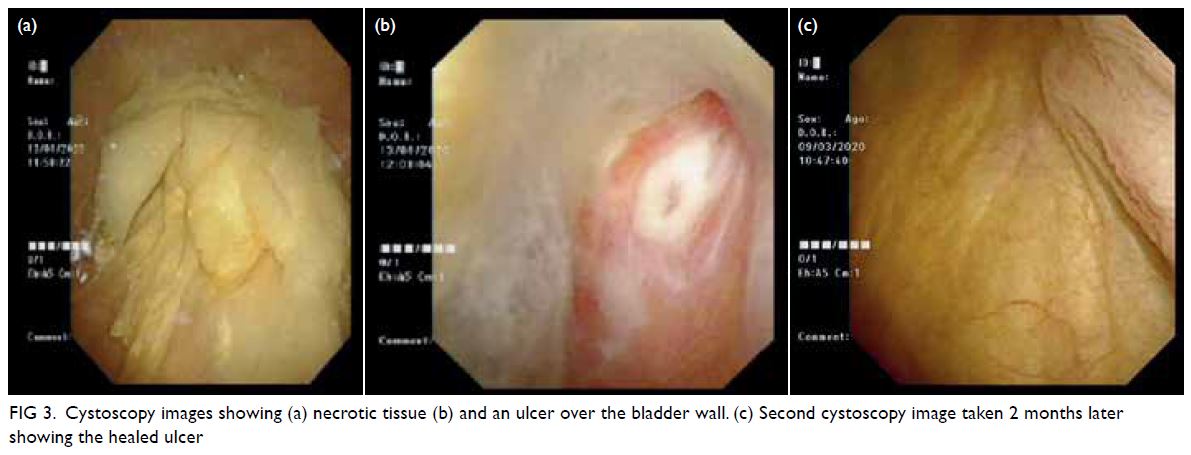
Figure 2. Selected mammography, ultrasound image, fludeoxyglucose (FDG) positron emission tomography/computed tomography fusion image and histopathological slides of left breast lesion. (a) A focal FDG-avid lesion in the left breast at the 3:00 position. (b) Craniocaudal view of left mammography. Several oval ill-defined equal-to-high density lesions were detected in the left breast. No associated microcalcification was identified. (c) The largest mass in the left breast located at the 3:00 position 3 cm from the nipple was evident as an ill-defined heterogeneous mass with hypoechoic and echogenic areas. (d) Light photomicrograph of haematoxylin and eosin stain on 400 x magnification. Tumour cells possess eosinophilic cytoplasm with perinuclear hof and hyperchromatic nuclei with occasional nucleoli. (e & f) Light photomicrograph of immunohistochemistry staining on 400 x magnification. Tumour cells are negative for AE1/AE3 (pancytokeratin) and positive for CD138 (Syndecan-1)
Bilateral mammography and breast ultrasound
were arranged in early April 2020 to assess bilateral
breast lesions. There were multiple oval and ill-defined
equal-to-high density lesions in both
breasts. The largest lesion located at the upper
outer quadrant of the right breast measured 3.6 × 2.8 cm and was palpable. No associated suspicious
microcalcifications were detected. There was no
architectural distortion, skin thickening or enlarged
lymph nodes on mammography (Fig 2b).
Subsequent breast ultrasound on the same
section revealed scattered oedematous areas in both
breasts. A heterogeneous mass with hypoechoic and
echogenic areas was detected in the left breast at a
3:00 position, 3 cm from the nipple (Fig 2c). Posterior
enhancement was evident. This mass corresponded
to the lesion detected on previous MRI. The size was
2.7 × 2.5 × 2.5 cm. There was interval enlargement
compared with previous MRI (which was 1.3 cm).
Multiple hypoechoic, echogenic, and heterogeneous
masses and nodules were identified in other areas
of both breasts. The largest one in the right breast
located at a 9:00 position 5 cm from the nipple
measured 4.5 × 2.6 × 4.1 cm. There was an irregular hypoechoic enlarged left axillary lymph node with
loss of fatty hilum. Ultrasound-guided fine needle
aspiration of this node and core biopsies of the
dominant masses in each breast were performed
in the same section. The pathological diagnoses of
the breast masses and left axillary lymph node were
consistent with plasmacytoma (Fig 2d-f). Two weeks
later (mid-April 2020), the patient was admitted
with multilobar pneumonia and severe metabolic
acidosis and disseminated intravascular coagulation.
Unfortunately, she passed away 4 days later.
Discussion
Breast plasmacytoma is extremely rare.
Approximately 50 cases have been reported in the
literature since 1925.2 3 4 5 The prevalence is unknown.
Surov et al6 reported a prevalence of 1.5% for breast
plasmacytoma among patients with plasmacytoma
in their institution. Involvement of the breast was a
secondary event of MM in 85% and more than half of
the lesions were unilateral.6
There are some differences in the
ultrasonographic features of breast MM between
this case and those reported in the literature. For the cases described by Ali et al2 and Park5, breast MM
was revealed as a well-defined oval hypoechoic mass
on ultrasound. In our patient, it was an indistinct
oval heterogeneous mass with hypoechoic and
echogenic areas.
Compared with typical primary breast cancer
(invasive ductal carcinoma) that is usually revealed
as an irregular spiculated high-density mass on
mammogram and an anti-parallel hypoechoic
irregular spiculated mass on ultrasound, there are
no characteristic imaging features of breast MM. On
mammography, it can present as a single or multiple
high-density round or oval lesion(s) that is/are
circumscribed or ill-defined.2 It can show as diffuse
infiltration. Association with microcalcifications
is rarely reported. On ultrasound, the features are
well-defined echo-poor, hypoechoic, or hyperechoic
solid masses with hypervascularity.2 Mixed hypo- to
hyper-echoic masses with indistinct margins are also
possible. Posterior acoustic features are variable.
Posterior acoustic enhancement can be evident but
absence of acoustic transmission or even posterior
acoustic shadowing has been reported in some
cases.
There are limited case reports of MRI and PET/
CT features of breast MM. On MRI, it shows as a T1-weighted intermediate to hypointense T2-weighted
hyperintense lesion with homogeneous or rim
enhancement. Restricted diffusion and early rapid
contrast enhancement with washout kinetics are the
reported features.5 It appears as a homogeneous soft
tissue lesion with high FDG uptake on PET/CT.
No case report has discussed the features of
breast MM on dual-tracer PET/CT. It was revealed
as an Ac-avid lesion in our patient, indicative of high
metabolism of malignant plasma cells. There are
provisos in this case report. The MRI protocols did
not relate specifically to breast imaging and contrast
kinetics was not performed.
Conclusion
There are no specific radiological features of breast MM. In bilateral multiple breast masses, the
differential diagnoses are lymphoma, metastasis,
synchronous primary breast cancer, secondary
involvement of haematological disorder or benign conditions such as fibroadenoma. Biopsy for
histopathological diagnosis is advised.
Author contributions
Concept or design: CKM Mo, AYT Lai.
Acquisition of data: SSW Lo, TS Wong.
Analysis or interpretation of data: SSW Lo, TS Wong.
Drafting of the manuscript: CKM Mo, AYT Lai, WWC Wong.
Critical revision of the manuscript for important intellectual content: CKM Mo, AYT Lai, WWC Wong.
Acquisition of data: SSW Lo, TS Wong.
Analysis or interpretation of data: SSW Lo, TS Wong.
Drafting of the manuscript: CKM Mo, AYT Lai, WWC Wong.
Critical revision of the manuscript for important intellectual content: CKM Mo, AYT Lai, WWC Wong.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Informed consent for publication was unobtainable
from the deceased patient's next-of-kin despite all reasonable
efforts.
References
1. Angtuaco EJ, Fassas AB, Walker R, Sethi R, Barlogie B. Multiple myeloma: clinical review and diagnostic imaging.
Radiology 2004;231:11-23. Crossref
2. Ali HO, Nasir Z, Marzouk AM. Multiple myeloma breast involvement: a case report. Case Rep Radiol
2019;2019:2079439. Crossref
3. Kaviani A, Djamali-Zavareie M, Noparast M, Keyhani-Rofagha S. Recurrence of primary extramedullary
plasmacytoma in breast both simulating primary breast
carcinoma. World J Surg Oncol 2004;2:29. Crossref
4. Lee HS, Kim JY, Kang CS, Kim SH, Kang JH. Imaging
features of bilateral breast plasmacytoma as unusual initial
presentation of multiple myeloma: case report and literature
review. Acta Radiol Short Rep 2014;3:2047981614557666. Crossref
5. Park YM. Imaging findings of plasmacytoma of both breasts as a preceding manifestation of multiple myeloma.
Case Rep Med 2016;2016:6595610. Crossref
6. Surov A, Holzhausen HJ, Ruschke K, Arnold D, Spielmann RP. Breast plasmacytoma. Acta Radiol
2010;51:498-504. Crossref