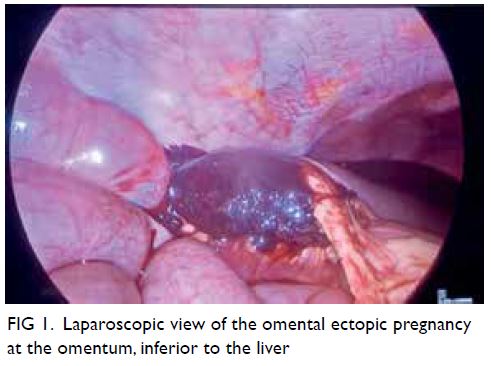
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Primary omental pregnancy after intrauterine
insemination: a case report
Tony PL Yuen, MB, BS1; Winnie Hui, MRCOG, FHKAM (Obstetrics and Gynaecology)1; MK Ho, MB, BS1; Richard WC Wong, FRCPA, FHKAM (Pathology)2
1 Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Corresponding author: Dr Tony PL Yuen (ypl634@ha.org.hk)
Introduction
Ectopic pregnancy (EP), a condition in which a fertilised ovum does not implant in the endometrial
cavity, occurs in 1% to 2% of all pregnancies.1 Up
to 97% of EPs occur within the fallopian tube, but
implantation can also occur at locations such as
the cervix, ovary, uterine cornua and abdomen.
Abdominal EPs are extremely rare, making up
less than 1% of EPs.1 Their presentation can be
non-specific and they are classified as primary or
secondary abdominal pregnancies. We present a
case of primary omental pregnancy with laparoscopy
and omentectomy performed.
Case summary
In January 2020, a 33-year-old gravida 1 para 0 woman
was admitted to our gynaecology unit with right-sided
abdominal pain. The patient’s past health was good
and she had no history of gynaecological surgery,
sexually transmitted disease or pelvic inflammatory
disease. She had been treated in the private sector
3 weeks before to admission with ovulation induction
and subsequent intrauterine insemination (IUI)
for coital problems. Serum beta-human chorionic
gonadotropin (HCG) was 48 mIU/L on day 18 and
768 mIU/L on day 22 after IUI. Ultrasound of the
pelvis at 5 weeks of gestation showed no intrauterine
sac. She also complained of mild per-vaginal
bleeding on admission. Abdominal examination
revealed tenderness over the right abdomen, next
to the umbilicus. Transvaginal ultrasound of the
pelvis on admission showed a linear endometrial
lining, with no adnexal masses or pelvic free fluid
identified. Blood tests showed a haemoglobin level
of 12 g/dL and beta-HCG level of 1366 mIU/mL.
Diagnostic laparoscopy was offered to the patient
in view of her abdominal pain but she opted for
beta-HCG monitoring as she was worried about a
negative laparoscopy. She subsequently complained
of severe right abdominal pain about 6 hours after
admission. Repeat transvaginal ultrasound revealed
no adnexal masses but a moderate amount of free
fluid in the pouch of Douglas. Due to the increased abdominal pain and suspicion of a ruptured EP, the
patient agreed to undergo laparoscopy.
Laparoscopy showed haemoperitoneum of
200 mL and a normal uterus, bilateral fallopian tubes
and ovaries. Survey of the peritoneal cavity revealed
a 5 × 5 cm haematoma attached to the omentum at
the right hepatic flexure, with mild oozing from the
site of attachment (Fig 1). The rest of the abdomen
was unremarkable. General surgeons were consulted
and omentectomy (including the site of bleeding)
was performed.
The patient made an uneventful postoperative
recovery and haemoglobin was stable. She was
discharged on day 4 after surgery. Pathological
examination revealed products of gestation mixed
with inflammatory and reactive mesothelial cells
(Fig 2). Beta-HCG monitoring after surgery
showed a satisfactory drop to a non-pregnant level:
366 mIU/mL, 144 mIU/mL, and 1.7 mIU/mL on
days 2, 4, and 18 after surgery.
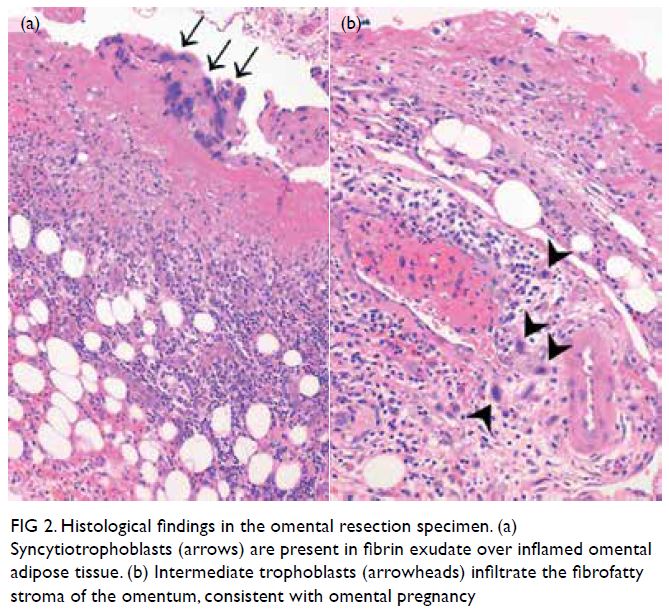
Figure 2. Histological findings in the omental resection specimen. (a) Syncytiotrophoblasts (arrows) are present in fibrin exudate over inflamed omental adipose tissue. (b) Intermediate trophoblasts (arrowheads) infiltrate the fibrofatty stroma of the omentum, consistent with omental pregnancy
Discussion
Among EPs, abdominal pregnancy is most rare. They
have been classified as either primary or secondary.
Our case meets the criteria established by Studdiford2
for a primary abdominal pregnancy: normal, bilateral
fallopian tubes and ovaries with no recent or remote
injury; absence of any uteroperitoneal fistula; and
presence of a pregnancy related exclusively to the
peritoneal surface and diagnosed early enough to
exclude the possibility of secondary implantation
after primary nidation elsewhere.
Early preoperative diagnosis of an abdominal
EP is very difficult in many cases. A systematic
review by Poole et al3 showed that among patients
with a final diagnosis of omental EP, none had a
preoperative diagnosis of abdominal pregnancy. As
a result of the diagnostic difficulty, there is usually
a delay from presentation to definitive treatment
with some cases requiring diagnosis by serial HCG
monitoring supplemented with magnetic resonance
imaging. A high level of vigilance is therefore vital
when monitoring the symptoms and vital signs of a suspected case, and early surgical intervention should
be considered if there is clinical deterioration. In our
case, we elected to perform emergent laparoscopy in
view of increased abdominal pain and free fluid in
the pouch of Douglas.
Laparotomy with excision of the embryo
has been the classic management for abdominal
pregnancy.4 However, with its widespread availability,
laparoscopy should be the modality of choice,
especially when the patient is haemodynamically
stable, as in our case, and the required expertise is
available. Laparoscopic management is associated
with fewer morbidities, reduced intraoperative blood
loss and a shorter hospital stay. The importance of
a general peritoneal survey is paramount; in cases
of normal fallopian tubes and ovaries, extra care
must be taken not to miss an EP elsewhere in the
peritoneum and prematurely commit to a negative
laparoscopy. If a difficult resection is encountered,
the expertise of a general surgeon will be of benefit.
Alternatives to surgical treatment have also been
reported,3 such as intralesional methotrexate,
intramuscular methotrexate, intracardiac potassium
chloride injection and artery embolisation. However,
the prerequisites for non-surgical treatment
include reliable imaging and for the patient to be
haemodynamically stable.
Assisted reproductive techniques are known
to be associated with an increased risk of EP.
Some reports state an incidence of up to 4.5% with
assisted reproductive technology compared with
a spontaneous pregnancy.5 With regard to IUI, the
incidence of EP is reported to be 2.05% compared
with 3.33% for in vitro fertilisation. A higher risk
of EP is also associated with stimulated cycles
(compared with natural cycles: 2.62% vs 0.99%) and
use of husband sperm (compared with donor sperm:
3.54% vs 1.08%). Many postulations have been made
regarding the mechanism of an abdominal EP.3 As
ovarian induction was performed in this case, the risk
of EP was increased. In the setting of IUI, it is possible
that the fertilised embryo develops as a primary tubal
pregnancy that subsequently passes through the
fimbrial end and implants into the omentum.
Although omental EPs are extremely rare, and
in our case, the first of such a condition found after
IUI, clinical suspicion must be high in a patient who
presents with symptoms suggestive of EP but with
normal uterus and adnexa during intraoperative
exploration. Clinicians should always be vigilant with
regard to the patient’s clinical condition, and there
should be a low threshold for surgical intervention
if clinical deterioration is noted. In addition, with
the rising application of assisted reproductive
technology, the risk of EPs, and by extension the
risk of abdominal EPs, is also increased, making
the diagnosis and treatment of this potentially life-threatening
condition evermore challenging.
Author contributions
All authors contributed to the design of the report, acquisition
of data, drafting of the manuscript and critical revision for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Informed consent was obtained for all treatment involved as well as for publication of this article and
accompanying images.
References
1. Fylstra DL. Ectopic pregnancy not within the (distal)
fallopian tube: etiology, diagnosis, and treatment. Am J
Obstet Gynecol 2012;206:289-99. Crossref
2. Studdiford WE. Primary peritoneal pregnancy. Am J Obstet Gynecol 1942;44:487-91. Crossref
3. Poole A, Haas D, Magann EF. Early abdominal ectopic
pregnancies: a systematic review of the literature. Gynecol
Obstet Invest 2012;74:249-60. Crossref
4. Yip SL, Tan WK, Tan LK. Primary omental pregnancy. BMJ
Case Rep 2016;2016: bcr2016217327. Crossref
5. Bu Z, Xiong Y, Wang K, Sun Y. Risk factors for ectopic
pregnancy in assisted reproductive technology: a 6-year,
single-center study. Fertil Steril 2016;106:90-4. Crossref