Hong Kong Med J 2022 Feb;28(1):73–5 | Epub 6 Dec 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Thoracoscopic repair of congenital oesophageal
atresia in a newborn: a case report
Michelle ON Yu, FCSHK, FHKAM (Surgery)1; Patrick HY Chung, FCSHK, FHKAM (Surgery)1; Mabel Wong, FHKAM (Paediatrics)2; Anne Kwan, FHKAM (Anaesthesiology)3; Yee-Eot Chee, FHKAM (Anaesthesiology)3; Kenneth KY Wong, FCSHK, FHKAM (Surgery)1
1 Department of Surgery, Queen Mary Hospital, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital, Hong Kong
3 Department of Anaesthesiology, Queen Mary Hospital, Hong Kong
Corresponding author: Dr Patrick HY Chung (chungphy@hku.hk)
Case report
In July 2021, a 1.5-kg baby girl presented with
polyhydramnios on antenatal ultrasound scan at
27 weeks that had resolved by 35 weeks. Physical
examination showed no dysmorphic features. She
was born by caesarean section at 35 weeks due to
discordant growth in a dichorionic diamniotic twin
pregnancy. She was intubated at birth but resistance
was noted during orogastric tube insertion. Chest
X-ray revealed coiling of the gastric tube at the
upper pouch of the oesophagus with the presence
of intestinal gas, which is a classic feature of type C
oesophageal atresia (Fig 1). Echocardiogram revealed
several cardiac anomalies including a ventricular
septal defect, a moderate patent ductus arteriosus,
and a large atrial septal defect.
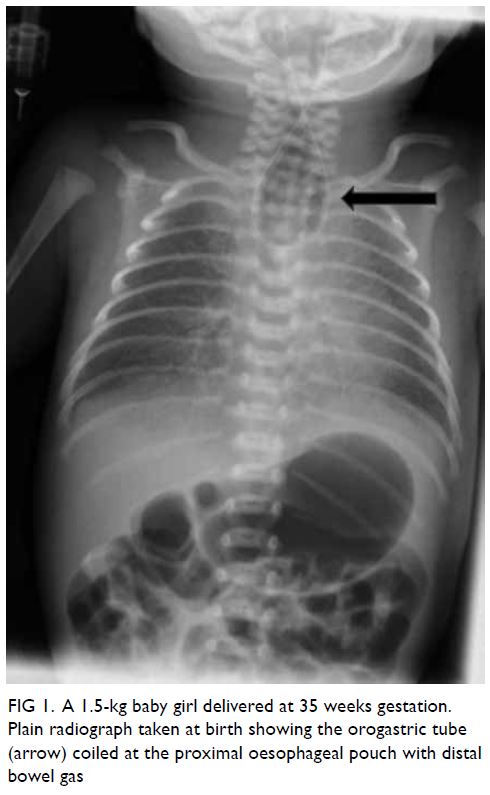
Figure 1. A 1.5-kg baby girl delivered at 35 weeks gestation. Plain radiograph taken at birth showing the orogastric tube (arrow) coiled at the proximal oesophageal pouch with distal bowel gas
Thoracoscopic repair of oesophageal
atresia was scheduled for the next day following
stabilisation. The patient was positioned in a semi-prone
position with the right chest slightly elevated.
She was put on conventional ventilation without
single lung ventilation, as per our practice. A camera
port was inserted at the 5th intercostal space and two
further 5-mm working ports were inserted under
direct vision. Pneumothorax of 3 to 4 mm Hg was
created using CO2. The azygous vein was cauterised
and divided with monopolar diathermy and the
tracheoesophageal fistula readily identified. This was
closed by Weck Hem-o-lok (Teleflex; Wayne [PA],
United States) and divided (Fig 2). The proximal
oesophageal stump was identified and opened with
scissors. End-to-end esophago-oesophagostomy
was performed using single-layer interrupted 5/0
polydioxanone sutures (Ethicon; Bridgewater [NJ],
United States). A 12-Fr chest drain was inserted
at the end of the procedure. The operation was
uneventful and completed in 145 minutes.
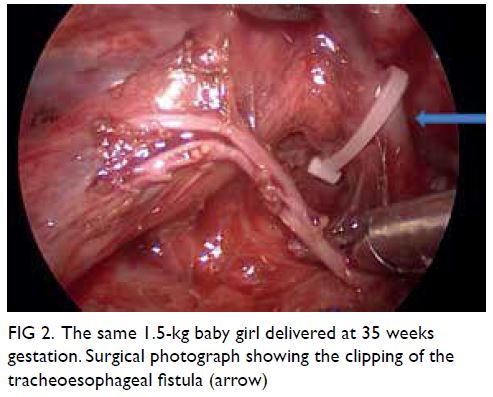
Figure 2. The same 1.5-kg baby girl delivered at 35 weeks gestation. Surgical photograph showing the clipping of the tracheoesophageal fistula (arrow)
After surgery, the patient was managed in the
neonatal intensive care unit according to our usual
protocol. On day 1 after surgery she developed
sudden profound desaturation with CO2 retention
when the endotracheal tube was secured at 8 cm from the upper lip. Bedside bronchoscopy showed
a tracheal pouch (fistula remnant) close to the
tip of the endotracheal tube. The tip completely
entered the pouch with minimal advancement of
the endotracheal tube. The endotracheal tube was
then withdrawn to 7.5 cm from the upper lip and no
further desaturation was noted.
A contrast swallow study on day 18 after
surgery showed an intact anastomosis. The patient was extubated successfully on day 19 after surgery.
Bolus feeding via a feeding tube was established after
extubation, and oromotor training was commenced.
She was transferred back to the referring hospital for
management of her cardiac disorders. At 6 weeks
after surgery, she had good weight gain with full oral
feeding and no clinical gastroesophageal reflux.
Discussion
The introduction of minimally invasive surgery has
undoubtedly revolutionised the treatment of many
surgical disorders. The role of minimally invasive
surgery in common neonatal procedures such as
inguinal hernia repair and pyloromyotomy is well
established. However, this operative approach in
more complex procedures is still limited by various
factors including equipment size, surgical expertise,
and perioperative support.
Among all the neonatal operations, repair of
oesophageal atresia is one of the most challenging.
In addition to the need for meticulous surgical skill,
adequate support from a dedicated neonatal intensive
care unit and expert paediatric anaesthetists are
equally important. The first successful thoracoscopic
repair of oesophageal atresia was reported in 1999.1
A subsequent international multicentre study
published 15 years ago further confirmed that
thoracoscopic repair of oesophageal atresia was at
least as good as traditional thoracotomy.2 However,
this operative approach is still not widely practised.
In our unit, we started to perform thoracoscopic
surgery in 2007. In our early experience reported in
2012,3 we selected patients with a reasonably large
body size for minimally invasive surgery. With the
accumulation of neonatal operative experience, we
became confident performing these operations on
smaller-sized babies. Prior to our case, Son et al4
published their experience in thoracoscopic repair of oesophageal atresia in babies <2 kg, and Rothenberg1
reported a successful experience in a 1.2-kg baby.
We believe that thoracoscopic repair of oesophageal
atresia in neonates can be performed safely in
experienced centres with proper case selection.
Traditionally, the Spitz classification has been
considered the prognostic indicator. Neonates
with oesophageal atresia with birth weight <1.5 kg
and major cardiac anomalies (as in our patient)
are predicted to have only a 50% survival rate with
a traditional open surgical approach. However,
advances in surgical skills, neonatal care, and
anaesthesia combined with the ability to optimise
the surgical approach have resulted in improved
surgical outcomes and consequent survival rates.1 4
The challenges faced in this operation
included the patient’s small size and presence
of cardiopulmonary complications before and
during surgery. The pneumothorax created during
thoracoscopy may compress the lung causing
difficulty in ventilation and compromise cardiac
function, further increasing the complexity of
anaesthesia. We overcame this by limiting the
pressure to 3 to 4 mm Hg, resulting in less operative
space but better patient tolerance. In our opinion,
the reduced working space is not a major hurdle for a
surgeon competent in minimally invasive surgery. In
a baby with congenital heart disease, an anaesthetist
who has experience in neonatal thoracic surgery is
essential for optimum intra-operative management.
Holcomb et al2 demonstrated in a multi-institutional
study that there were no significant
differences in reported postoperative complication
rates between minimally invasive surgery and open
repair. A minimally invasive approach has the
additional benefits of smaller wounds and less pain.
More specifically, thoracoscopic repair allows for
clearer magnification. Although CO2 pneumothorax
will compress the right lung during surgery, its effect
is significantly less than that of manual compression
during open surgery. Long-term studies of
musculoskeletal problems also reveal a superior
outcome for thoracoscopic surgery.1
In conclusion, we report a successful
thoracoscopic repair of oesophageal atresia in a
high-risk neonate with very low birth weight. To
the best of our knowledge, this is the smallest baby
with oesophageal atresia in Hong Kong to have this
operation. While proper case selection to ensure
patient safety remains the top priority, small body
size should not preclude a thoracoscopic surgical
approach. The combined efforts and advances in
surgery, anaesthesia and neonatal care are key to
success.
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
As the editor of the journal, KKY Wong was not involved in the peer review process for this article. Other authors have no
conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient’s parents provided informed consent for all treatments and procedures and provided consent for
publication.
References
1. Rothenberg SS. Thoracoscopic repair of esophageal atresia
and tracheo-esophageal fistula in neonates: evolution of a
technique. J Laparoendosc Adv Surg Tech A 2012;22:195-9. Crossref
2. Holcomb GW 3rd, Rothenberg SS, Bax KM, et al.
Thoracoscopic repair of esophageal atresia and
tracheoesophageal fistula: a multi-institutional analysis.
Ann Surg 2005;242:422-8. Crossref
3. Huang J, Tao J, Chen K, et al. Thoracoscopic repair of oesophageal atresia: experience of 33 patients from two tertiary referral centres. J Pediatr Surg 2012;47:2224-7. Crossref
4. Son J, Jang Y, Kim W, et al. Thoracoscopic repair of esophageal atresia with distal tracheoesophageal fistula: is
it a safe procedure in infants weighing less than 2000 g?
Surg Endosc 2021;35:1597-601. Crossref

