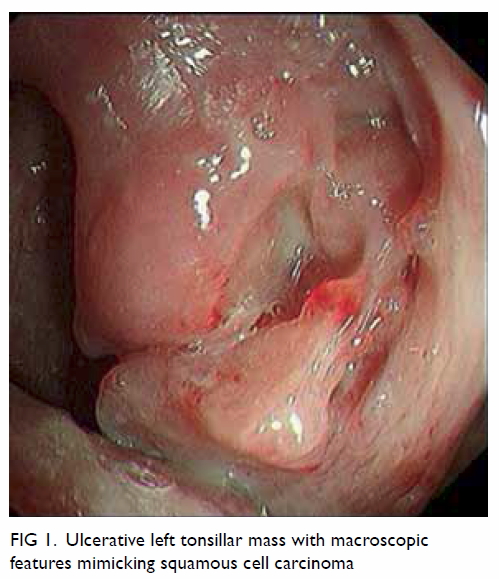
Feasibility of short double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with surgically altered gastrointestinal anatomy: experience in a regional centre
DOI: 10.12809/hkmj164987
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Feasibility of short double-balloon
enteroscopy-assisted endoscopic retrograde
cholangiopancreatography in patients with
surgically altered gastrointestinal anatomy:
experience in a regional centre
SW Cheung, MRCP, FHKCP1; KS Cheng, MRCP, FHKCP1; WM Yip, MRCP, FHKCP2; KK Li, MBBS, FRCP1
1 Department of Medicine and Geriatrics, Tuen Mun Hospital, Tuen Mun, Hong Kong
2 Department of Medicine and Geriatrics, Pok Oi Hospital, Yuen Long, Hong Kong
Corresponding author: Dr SW Cheung (saiwahc@hotmail.com)
 A video clip showing double-balloon enteroscopy–assisted endoscopic
retrograde cholangiopancreatography is available at www.hkmj.org
A video clip showing double-balloon enteroscopy–assisted endoscopic
retrograde cholangiopancreatography is available at www.hkmj.orgCase Reports
Endoscopic retrograde cholangiopancreatography
(ERCP) is a standard endoscopic technique for
treating biliary obstruction and cholangitis. The
presence of surgically altered gastrointestinal
anatomy, however, poses a major technical difficulty
to the procedure due to the long and tortuous access
to the small bowel. We report a three-case series
with successful attempts at short double-balloon
enteroscopy (DBE)–assisted ERCP in patients
with postoperative gastrointestinal anatomy. The
enteroscope employed was EC-450BI5, Fujifilm
endoscopy (Fig 1) and the sedation agents used
in all procedures were dexmedetomidine and
fentanyl continuous infusion combined with bolus
midazolam.
Case 1
A 76-year-old man was admitted to our hospital in
December 2015 with acute cholangitis that presented
as fever and deranged liver function tests (LFT)
with predominant elevation of alkaline phosphatase
(ALP). He had a history of stroke, hyperlipidaemia,
and a Billroth II gastrectomy performed 30
years ago. Blood cultures grew Escherichia coli.
Ultrasonography of the hepatobiliary system
revealed a 5-mm stone in the common bile duct
(CBD).
The first ERCP using a standard side-view
duodenoscope and end-view gastroscope failed
to identify the papilla. He continued to receive
antibiotic treatment, with the fever reduced but
liver biochemistry remained elevated. The DBE-assisted
ERCP was performed 2 weeks later with an
enteroscope of 152-cm working length. The papilla
was reached over the afferent limb of the small bowel
by manipulation of the overtube only (no balloon
inflation), followed by successful cannulation of
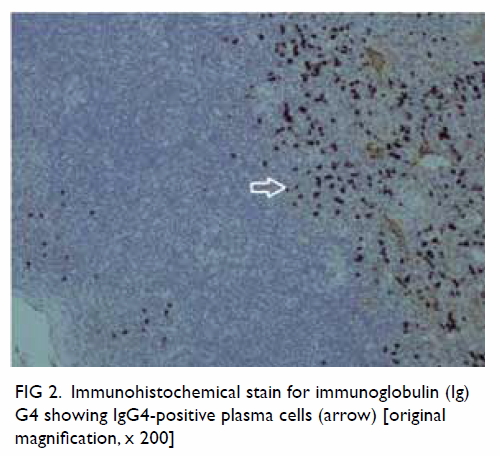
the CBD. Cholangiogram revealed a 1.2-cm dilated
CBD with two intraductal filling defects (Fig 2a).
Pre-cut papillotomy on-stent was performed with
pre-insertion of a 7-French 7-cm plastic biliary stent.
The plastic stent was removed after papillotomy
and the papilla was dilated using a controlled radial
expansion (CRE) balloon dilator of 12-mm size and
subsequently stones were removed by a basket. The
ERCP procedure was completed in 115 minutes and
there were no complications. The patient’s LFT had
normalised at a follow-up 3 weeks later.
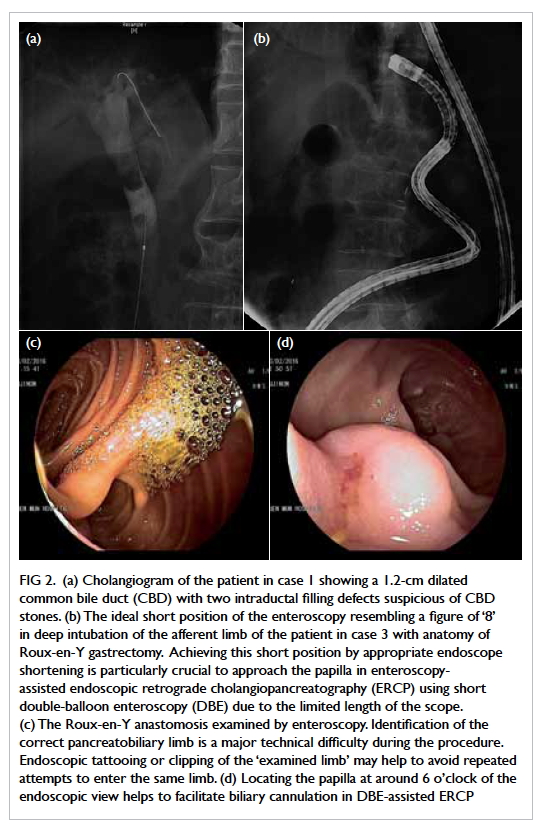
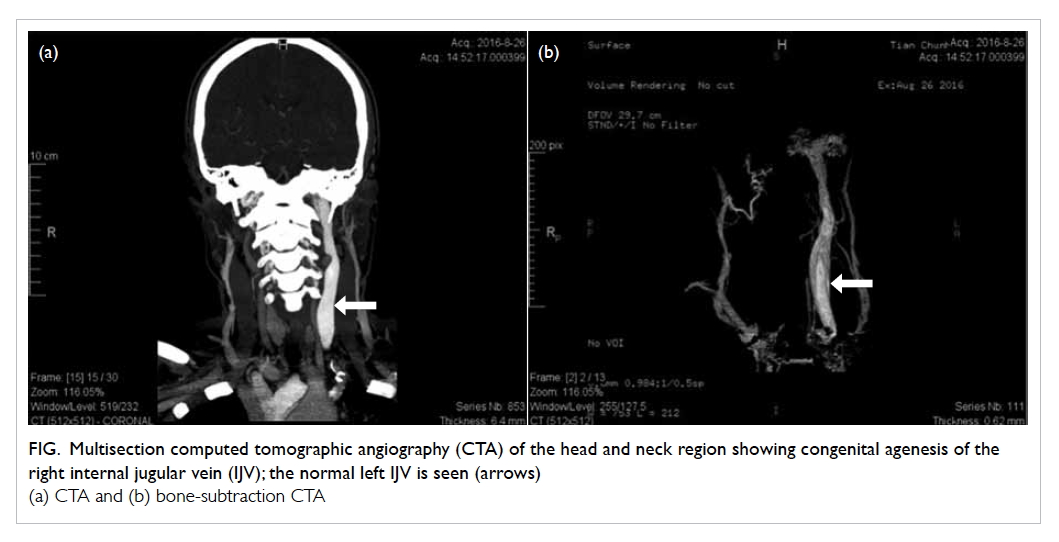
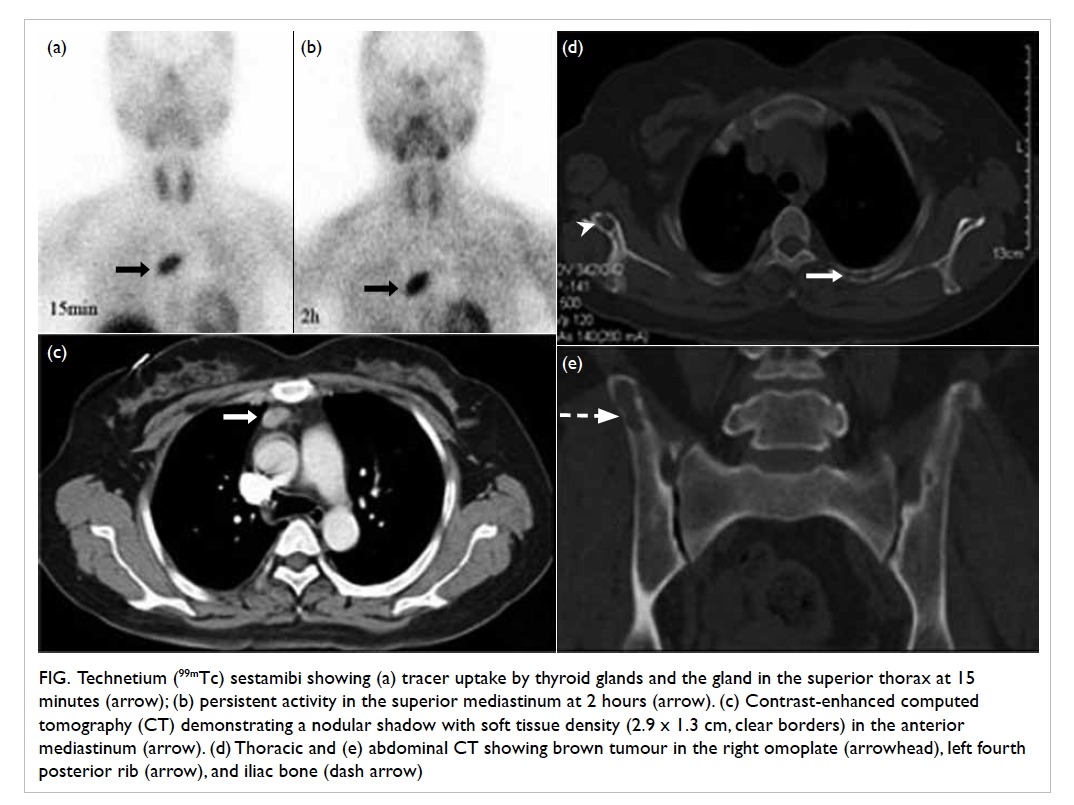
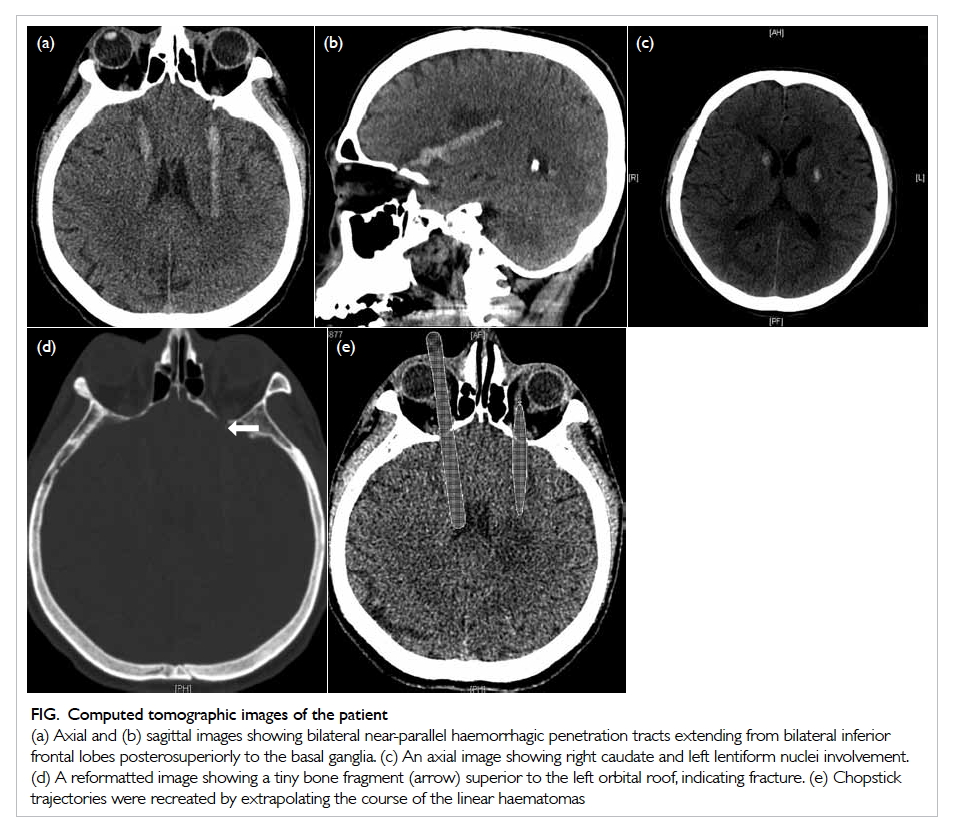
Figure 2. (a) Cholangiogram of the patient in case 1 showing a 1.2-cm dilated common bile duct (CBD) with two intraductal filling defects suspicious of CBD stones. (b) The ideal short position of the enteroscopy resembling a figure of ‘8’ in deep intubation of the afferent limb of the patient in case 3 with anatomy of Roux-en-Y gastrectomy. Achieving this short position by appropriate endoscope shortening is particularly crucial to approach the papilla in enteroscopy-assisted endoscopic retrograde cholangiopancreatography (ERCP) using short double-balloon enteroscopy (DBE) due to the limited length of the scope. (c) The Roux-en-Y anastomosis examined by enteroscopy. Identification of the correct pancreatobiliary limb is a major technical difficulty during the procedure. Endoscopic tattooing or clipping of the ‘examined limb’ may help to avoid repeated attempts to enter the same limb. (d) Locating the papilla at around 6 o’clock of the endoscopic view helps to facilitate biliary cannulation in DBE-assisted ERCP
Case 2
An 80-year-old man presented in January 2016
with a history of hypertension, pulmonary fibrosis,
and cerebral infarct. A Billroth II gastrectomy
had been performed 10 years previously for
gastrointestinal bleeding. He had an episode of acute
cholangitis 4 months ago. Endoscopic retrograde
cholangiopancreatography using a standard end-view
gastroscope failed to reach the papilla and the
infection resolved after a course of antibiotic. Follow-up
magnetic resonance cholangiopancreatography 3
weeks earlier had revealed a 1.2-cm distal CBD stone
with the CBD dilated to 1.7 cm. He then attended the
hospital again for fever, E coli septicaemia, and an
obstructive pattern of liver derangement; bilirubin
level was 93 µmol/L and ALP level was 1224 U/L.
Percutaneous transhepatic biliary drainage was
established and antibiotics were continued until
DBE-assisted ERCP was performed 2 weeks later.
With the push-pull manoeuvre, the blind end of
the afferent loop was reached. Initially, a nearby
minor papilla was mistaken as the major papilla and
repeated attempts failed to cannulate it.
The true major papilla was then identified
5 cm proximal to the minor papilla and pre-cut
papillotomy was performed due to difficult
cannulation. The impacted CBD stone was removed
following papillotomy and balloon sphincteroplasty
was performed with a 12-mm CRE balloon.
Subsequent cholangiogram was clear with a 1.5-cm
dilated CBD. The operating time was 163 minutes.
The patient remained asymptomatic and LFT had
normalised at a follow-up 1 month later.
Case 3
A female patient aged 81 years with a history
of mild Parkinson’s disease had undergone
gastrectomy 30 years ago for peptic ulcer disease.
She presented to our hospital in February 2016 for
biliary pancreatitis with sudden onset of jaundice
associated with epigastric pain. Amylase level was
elevated to 698 U/L, bilirubin level to 35 µmol/L,
and an ALP level of 1001 U/L. Ultrasonography of
the hepatobiliary system detected grossly dilated
intrahepatic ducts (IHD) and the CBD measured
up to 2.4 cm in diameter with one obstructing
CBD stone measuring 1.5 cm. The first gastroscopy
confirmed a previous total gastrectomy with Roux-en-Y reconstruction. Percutaneous transhepatic
biliary drainage was attempted but failed due to the
resolution of the IHD dilatation. The patient was
then managed conservatively with antibiotics and
a DBE-assisted enteroscopy was scheduled 3 weeks
later. The 152-cm DBE identified the blind end of
the afferent limb while the endoscope advanced to
140 cm from the incisors after push-pull manoeuvre
(Fig 2b to d). Successful guidewire cannulation was
followed with a cholangiogram that showed a dilated
CBD with no filling defect. In view of the recent
history of biliary obstruction and the possibility of
a passed stone, the papilla was dilated to 13.5 mm
with the CRE balloon and good bile drainage was
observed. The operating time was 130 minutes. The
major difficulty encountered was that the tip of the
enteroscope was very unstable making it difficult
to maintain the distal blind end and it was easily
slipped out proximally. As such, repeated endoscope
manipulation was required to achieve optimal
positioning. Postoperatively, the patient was well,
bilirubin normalised, and ALP level had reduced to
266 U/L at 1-month follow-up.
Discussion
Balloon enteroscopy–assisted ERCP using either
single-balloon enteroscopy or DBE has been
reported to be an effective modality for ERCP in these
patients.1 2 3 Traditional DBE with 200-cm length and
narrow (2.2-mm) accessory working channel limits
the utilisation of conventional ERCP accessories.
Recent attempts with a short 152-cm DBE have
been highly successful.1 2 4 To date, there have been
approximately 200 reports of patients who have
undergone ERCP using this short DBE procedure.
The success rate for reaching the blind end of the
afferent limb was 86% to 100%; it is not inferior to
that achieved using a long-type balloon enteroscope.4
The short-type enteroscope accommodates the use
of most available accessories to perform ERCP-related
procedures such as sphincterotomy, balloon
dilatation, stone extraction, and deployment of
plastic or metallic stents.
In our small series, all patients ran an
uneventful course during the perioperative and
postoperative period and none had any apparent
complications. The overall procedural complication
rate has been reported to be 8% to 10%, with the rate
of major complications of perforation or emphysema
being around 3.5%.4 The risk of complications,
however, may vary according to different surgical
approaches with consequent significant differences
in the endoscopic techniques anticipated.3 4 5 Since
it is a relatively new and evolving endoscopic
technique, these rates are still experience-dependent
and patients should be closely observed after the
procedure.
The ERCP in the patient with Roux-en-Y reconstruction posed particular technical
difficulties. The endoscopist should review previous
surgical reports in detail to map and anticipate
the endoscopic view at the anastomotic site. On
reaching the Roux-en-Y anastomosis, identification
of the pancreatobiliary limb is often difficult. After
choosing either limb, the endoscopist could perform
endoscopic marking by a clip or Indian ink tattoo at
the entrance of the chosen limb (Fig 2c). If the chosen
limb is confirmed wrong under fluoroscopy, the
endoscopist can return the endoscope to the Roux-en-Y anastomosis and then insert the endoscope into
the other limb. A marking at the entrance of a limb
has been shown to be useful as it avoids repeated
misidentification of the limb to be entered.6
Barotrauma is the major cause of intestinal
perforations and may be a result of excessive air
insufflation forming a closed loop between the
blind end and the inflated overtube or enteroscope
balloon.7 Use of carbon dioxide insufflation instead
of air insufflation may reduce the chance of this
closed-loop phenomenon. Additionally, use of a
transparent hood at the tip of the enteroscope with
instillation of water into the intestinal lumen has
also been suggested to maintain the endoscopic
view without gas insufflation and avoid consequent
barotrauma.8
With regard to the cannulation techniques,
the catheter exits from a 7 o’clock direction of
the enteroscope during DBE-assisted ERCP.
Endoscopists should attempt to locate the papilla
at 6 o’clock of the endoscopic view to facilitate
biliary cannulation (Fig 2b). In case of an unstable
endoscopic manoeuvre, an inflated enteroscope
balloon may help to grip the intestine and stabilise
the manipulation. In cases where the papilla is
located at 11 to 12 o’clock in the endoscopic view,
the overtube balloon should remain inflated and the
enteroscope rotated to solve difficult cannulation.6
In conclusion, short DBE-assisted ERCP is
a safe and effective endoscopic method to treat
patients with surgically altered anatomy and
biliary conditions. Nonetheless local experience in
enteroscopy-assisted ERCP, particularly using the
short DBE, is scant because of the unavailability
of the endoscopic devices. Further discussion and
training opportunities are encouraged to consolidate
experience and minimise procedural complications
in future practice.
References
1. Moreels TG. Altered anatomy: enteroscopy and ERCP
procedure. Best Pract Res Clin Gastroenterol 2012;26:347-57. Crossref
2. Cheng CL, Liu NJ, Tang JH, et al. Double-balloon
enteroscopy for ERCP in patients with Billroth II anatomy:
results of a large series of papillary large-balloon dilation
for biliary stone removal. Endosc Int Open 2015;3:E216-22. Crossref
3. Mönkemüller K, Bellutti M, Neumann H, Malfertheiner P.
Therapeutic ERCP with the double-balloon enteroscope in
patients with Roux-en-Y anastomosis. Gastrointest Endosc
2008;67:992-6. Crossref
4. Kato H, Tsutsumi K, Harada R, Okada H, Yamamoto K.
Short double-balloon enteroscopy is feasible and effective
for endoscopic retrograde cholangiopancreatography in
patients with surgically altered gastrointestinal anatomy.
Dig Endosc 2014;26 Suppl 2:130-5. Crossref
5. Katanuma A, Yane K, Osanai M, Maguchi H. Endoscopic
retrograde cholangiopancreatography in patients
with surgically altered anatomy using balloon-assisted
enteroscope. Clin J Gastroenterol 2014;7:283-9. Crossref
6. Hatanaka H, Yano T, Tamada K. Tips and tricks of double-balloon
endoscopic retrograde cholangiopancreatography
(with video). J Hepatobiliary Pancreat Sci 2015;22:E28-34. Crossref
7. De Koning M, Moreels TG. Comparison of double-balloon
and single-balloon enteroscope for therapeutic endoscopic
retrograde cholangiography after Roux-en-Y small bowel
surgery. BMC Gastroenterol 2016;16:98. Crossref
8. Yamamoto H. Be aware of the fatal risk of air embolism.
Dig Endosc 2014;26:23. Crossref