Parapharyngeal abscess presenting as masticatory otorrhoea-persistent foramen tympanicum as a route of drainage: a case report
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Parapharyngeal abscess presenting as masticatory
otorrhoea-persistent foramen tympanicum as a route of drainage: a case
report
KH Lee, FRCR, FHKCR; YL Li, MB, BS, FRCR; ML Yu,
MB, ChB, FRCR
Department of Radiology, Queen Mary Hospital,
Pokfulam, Hong Kong
Corresponding author: Dr KH Lee (viclkh88@gmail.com)
Case report
A 26-year-old woman presented to the emergency
department of Queen Mary Hospital in August 2014 with fever and right
otorrhea following extraction of her right lower third molar 5 days
previously. Physical examination revealed right buccal swelling, limited
mouth opening, and pus-like discharge in the right external auditory canal
(EAC) exacerbated by mastication. There was no hearing loss or facial
nerve palsy. Otoscopy revealed granulation tissue and pus arising from the
anterior wall of the inner right EAC.
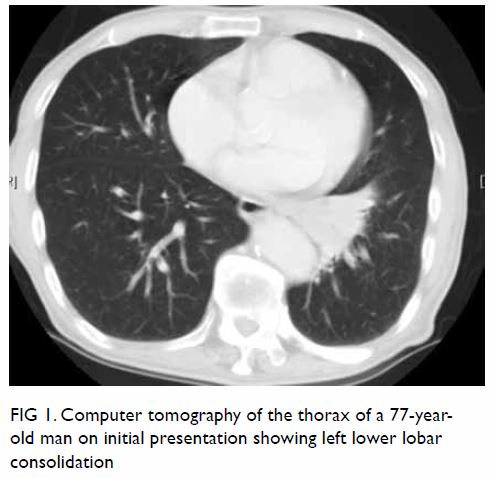
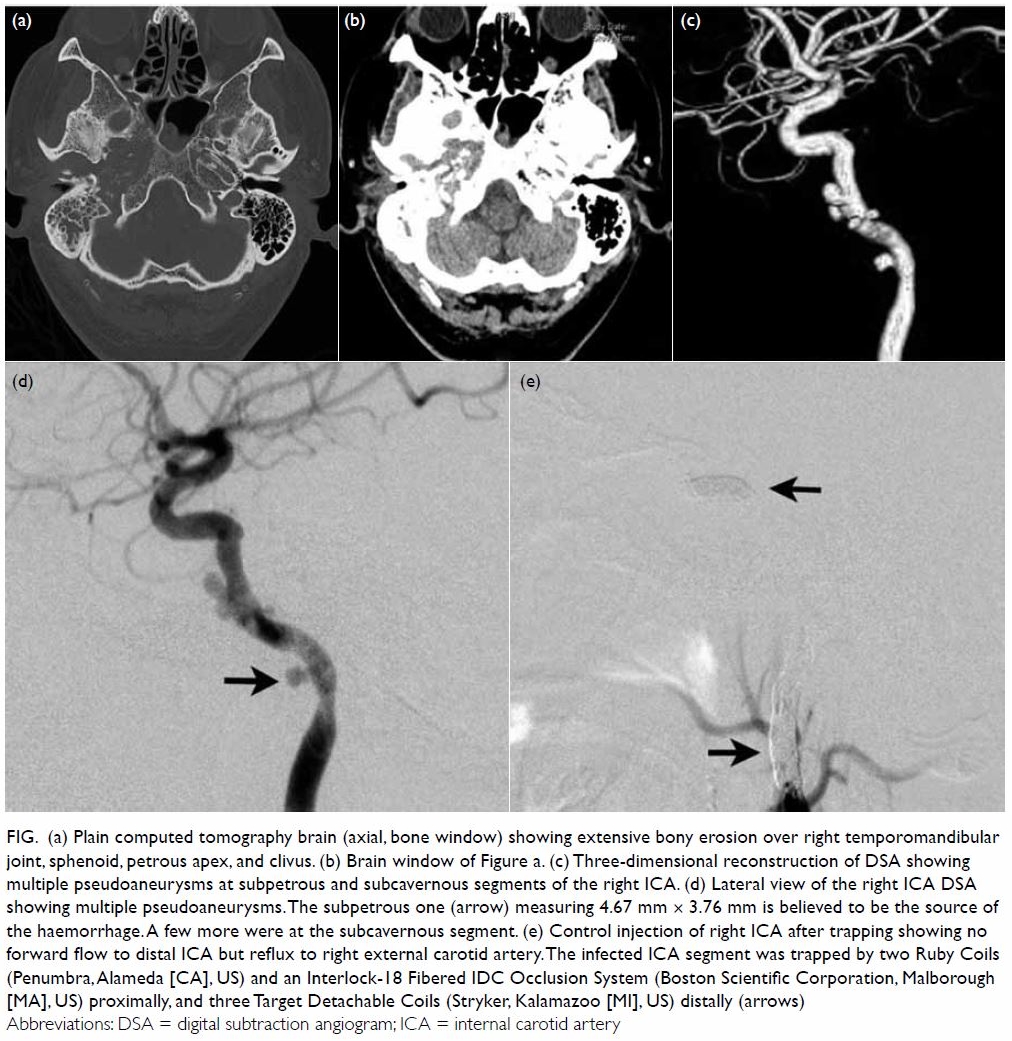
Contrast-enhanced computed tomography scan showed a
right parapharyngeal rim enhancing collection (Fig 1) tracking into the right
temporomandibular fossa. Enhancing soft tissue was seen at the
inner one-third of the right EAC. A bony defect was present at its
anterior wall, compatible with a persistent foramen tympanicum (also known
as foramen of Huschke), allowing communication between the EAC and the
temporomandibular fossa (Fig 2). Overall findings were compatible with right
parapharyngeal abscess discharging via a persistent foramen tympanicum
into the right EAC.
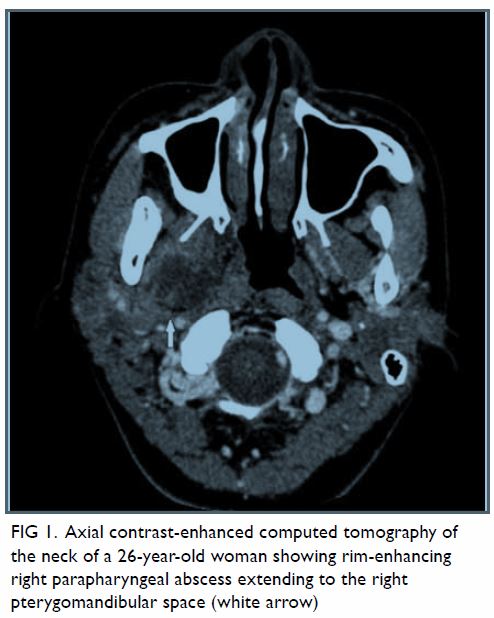
Figure 1. Axial contrast-enhanced computed tomography of the neck of a 26-year-old woman showing rim-enhancing right parapharyngeal abscess extending to the right pterygomandibular space (white arrow)
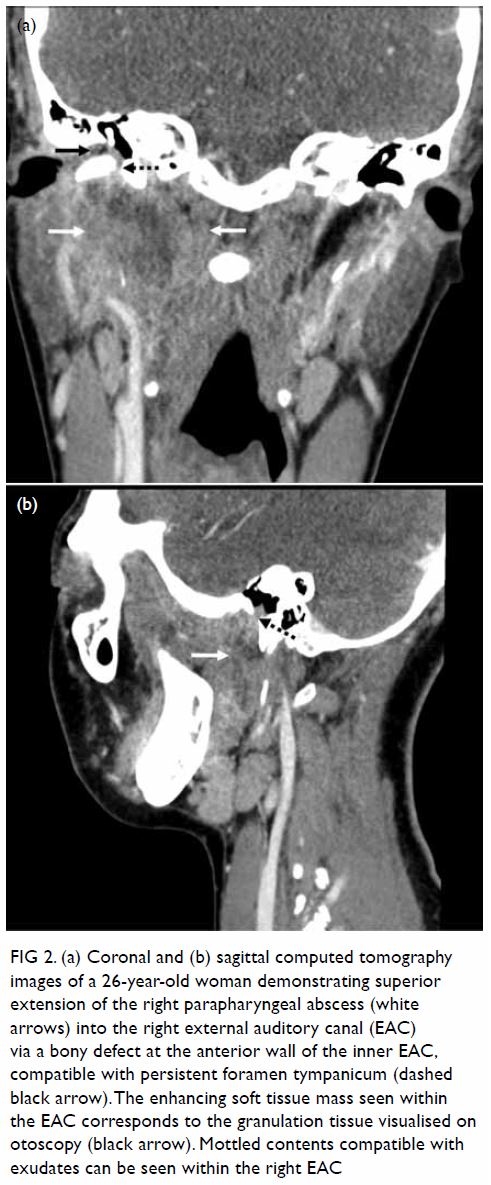
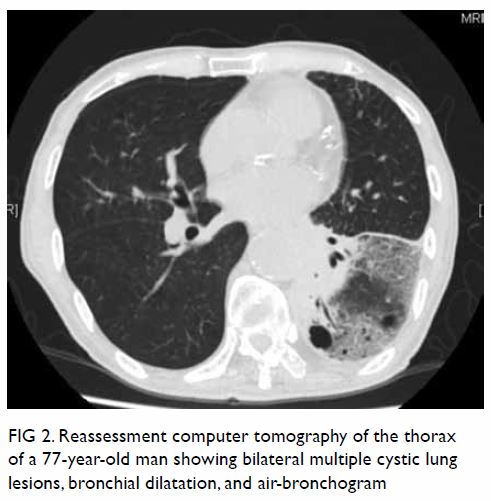
Figure 2. (a) Coronal and (b) sagittal computed tomography images of a 26-year-old woman demonstrating superior extension of the right parapharyngeal abscess (white arrows) into the right external auditory canal (EAC) via a bony defect at the anterior wall of the inner EAC, compatible with persistent foramen tympanicum (dashed black arrow). The enhancing soft tissue mass seen within the EAC corresponds to the granulation tissue visualised on otoscopy (black arrow). Mottled contents compatible with exudates can be seen within the right EAC
The patient was treated with a course of
antibiotics in view of her stable condition and lack of airway compromise.
The patient responded clinically and repeat computed tomography scan 1
week after initial presentation showed complete resolution of the right
parapharyngeal abscess and enhancing soft tissue within the right EAC.
Subsequent bacterial culture of the right ear discharge yielded Streptococcus
anginosus, a common cause of oral infection.
Discussion
Parapharyngeal abscesses are deep cervical
infections with potential serious complications such as shock,
mediastinitis, jugular vein thrombosis, upper airway obstruction, and
death. Tonsillitis and odontogenic infection are the most common
aetiologies. The clinical presentation typically involves fever, neck
pain, odynophagia, neck oedema, and upper airway obstruction.1
Otorrhea is typically caused by external or middle
ear pathologies such as otitis. Otorrhoea as a presenting symptom for a
neck abscess is highly unusual. In our literature review, only two cases
were found. Biron et al2 described
a patient with a submandibular abscess that tracked into the ipsilateral
external auditory meatus via the parapharyngeal and masticator spaces.
Pepato et al3 reported a case of
lower third molar infection presenting as purulent ear discharge, with
persistent foramen tympanicum found in a follow-up cone-beam computed
tomography study. The route of spread was postulated to be either via the
Santorini fissures (the tiny defects in the anterior wall of the
cartilaginous EAC) or via a persistent foramen tympanicum.
Persistent foramen tympanicum, first described by
Emil Huschke in 1844, represents a failure of ossification of the tympanic
part of the temporal bone and normally occurs from birth with completion
by age 5 years. The foramen is located at the anteroinferior aspect of the
EAC, just posterior to the temporomandibular joint. Incidence is quoted
from 4.6% to 22.7% based on radiological and cadaveric studies.4
The majority of individuals with the foramen are
asymptomatic although various complications have been reported. Most are
benign, such as salivation from the ear during mastication and spontaneous
herniation of the temporomandibular joint into the EAC leading to otalgia
and tinnitus. Iatrogenic middle ear injury is possible when the foramen is
inadvertently traversed during temporomandibular joint arthroscopy.5 It is possible that the lack of bony integrity reduces
mechanical resistance to pathological processes, such as the spreading of
parapharyngeal abscess in our case. Parotid pleomorphic adenomas have also
been reported to herniate through the foramen to present as an EAC mass.
To the best of our knowledge, this is the first
case to provide direct radiological evidence of persistent foramen
tympanicum as a route for drainage leading to masticatory otorrhoea. It is
important for doctors to be aware of the clinical presentation to permit
diagnosis and subsequent treatment.
Author contributions
All authors contributed to the concept, image
acquisition, image and data interpretation, manuscript drafting, and
critical revision for important intellectual content. All authors had full
access to the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Patient consent was obtained for the purpose of
this case study.
References
1. Brito TP, Hazboun IM, Fernandes FL, et
al. Deep neck abscesses: study of 101 cases. Braz J Otorhinolaryngol
2017;83:341-8. Crossref
2. Biron A, Halperin D, Sichel JY, Eliashar
R. Deep neck abscess of dental origin draining through the external ear
canal. Otolaryngol Head Neck Surg 2005;133:166-7. Crossref
3. Pepato AO, Yamaji MA, Sverzut CE,
Trivellato AE. Lower third molar infection with purulent discharge through
the external auditory meatus. Case report and review of literature. Int J
Oral Maxillofac Surg 2012;41:380-3. Crossref
4. Akbulut N, Kursun S, Aksoy S, Kurt H,
Orhan K. Evaluation of foramen tympanicum using cone-beam computed
tomography in orthodontic malocclusions. J Craniofac Surg 2014;25:e105-9.
Crossref
5. Nakasato T, Nakayama T, Kikuchi K et al.
Spontaneous temporomandibular joint herniation into the external auditory
canal through a persistent foramen tympanicum (Huschke): radiographic
features. J Comput Assist Tomogr 2013;37:111-3. Crossref