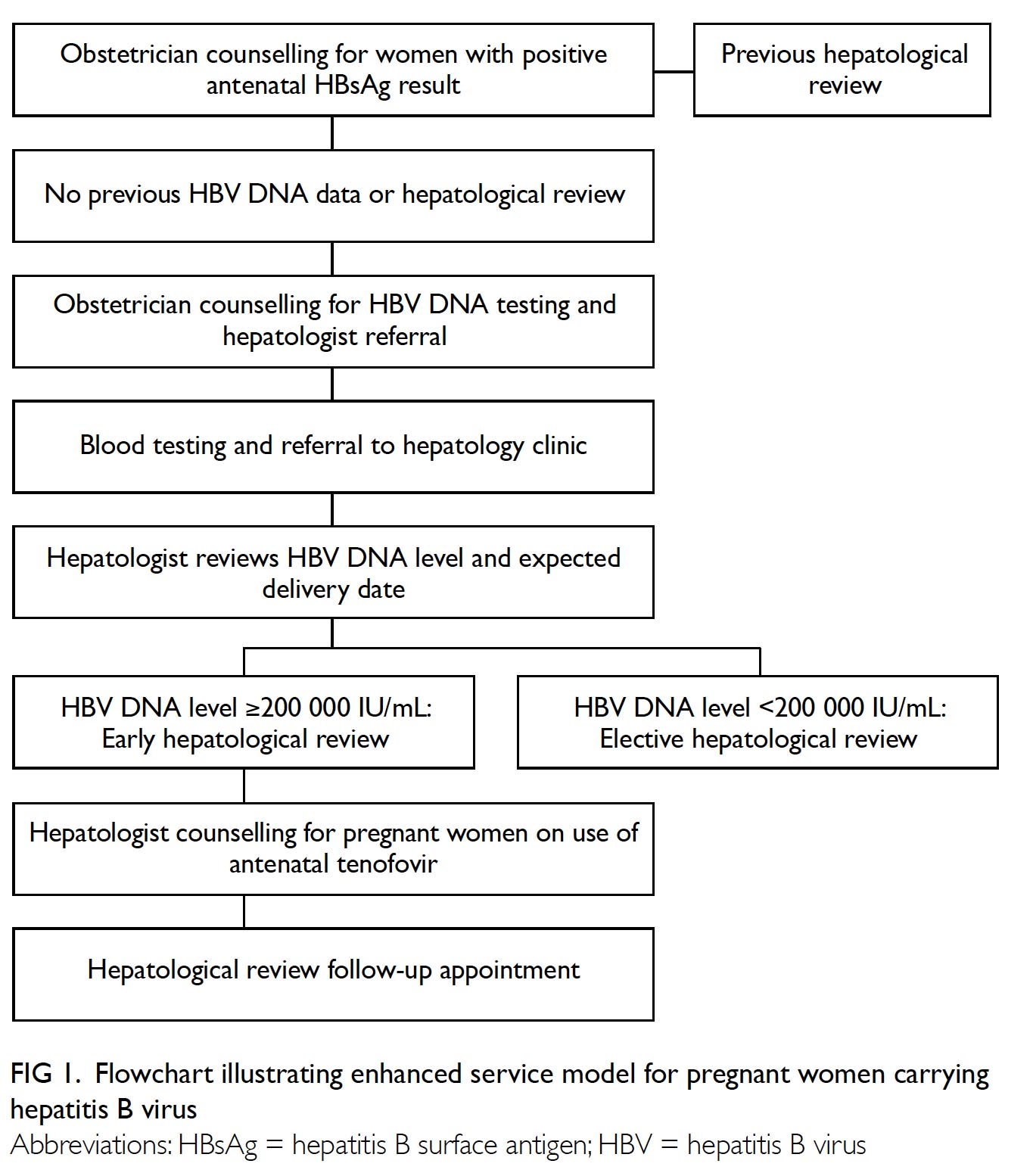
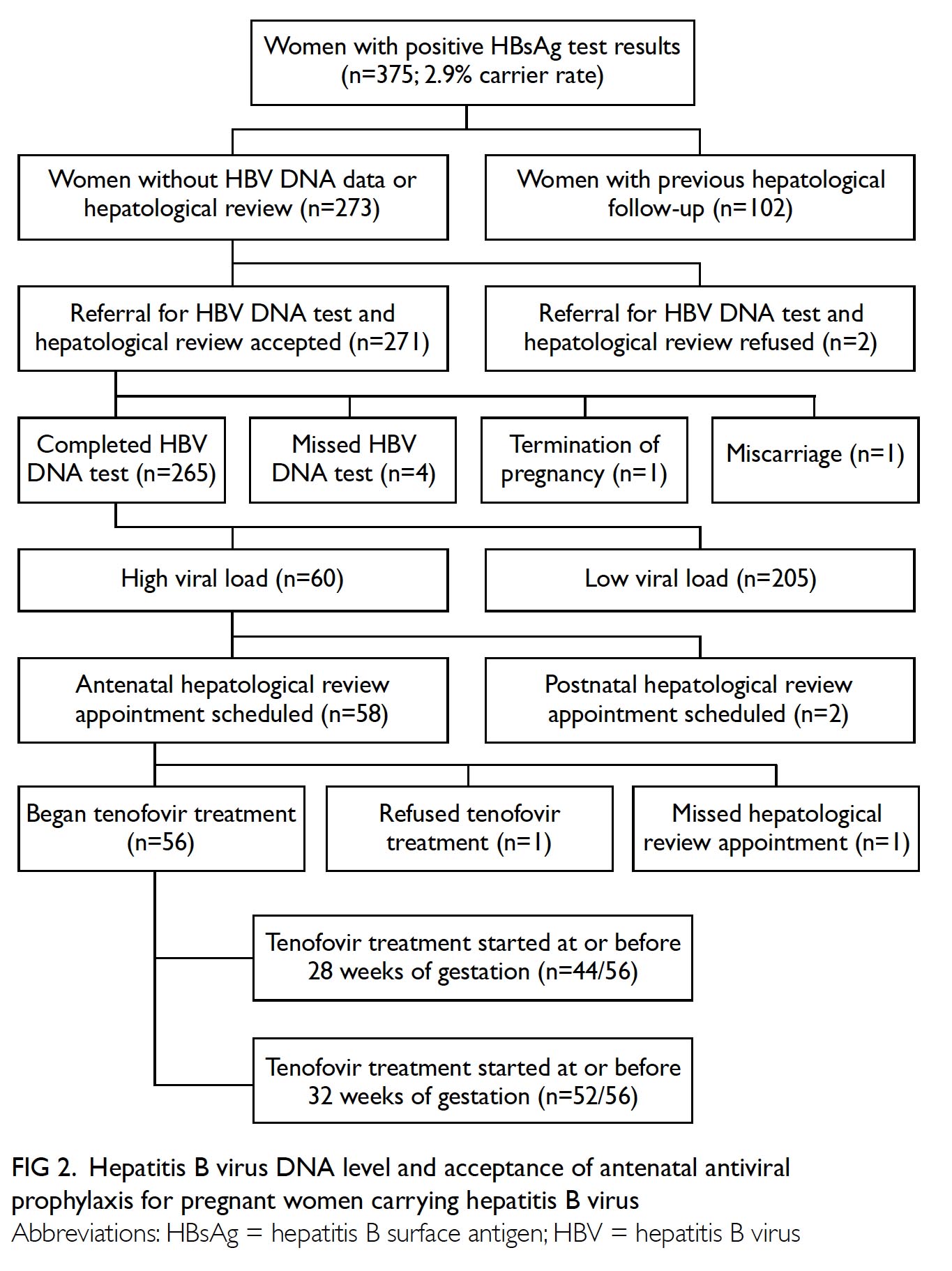
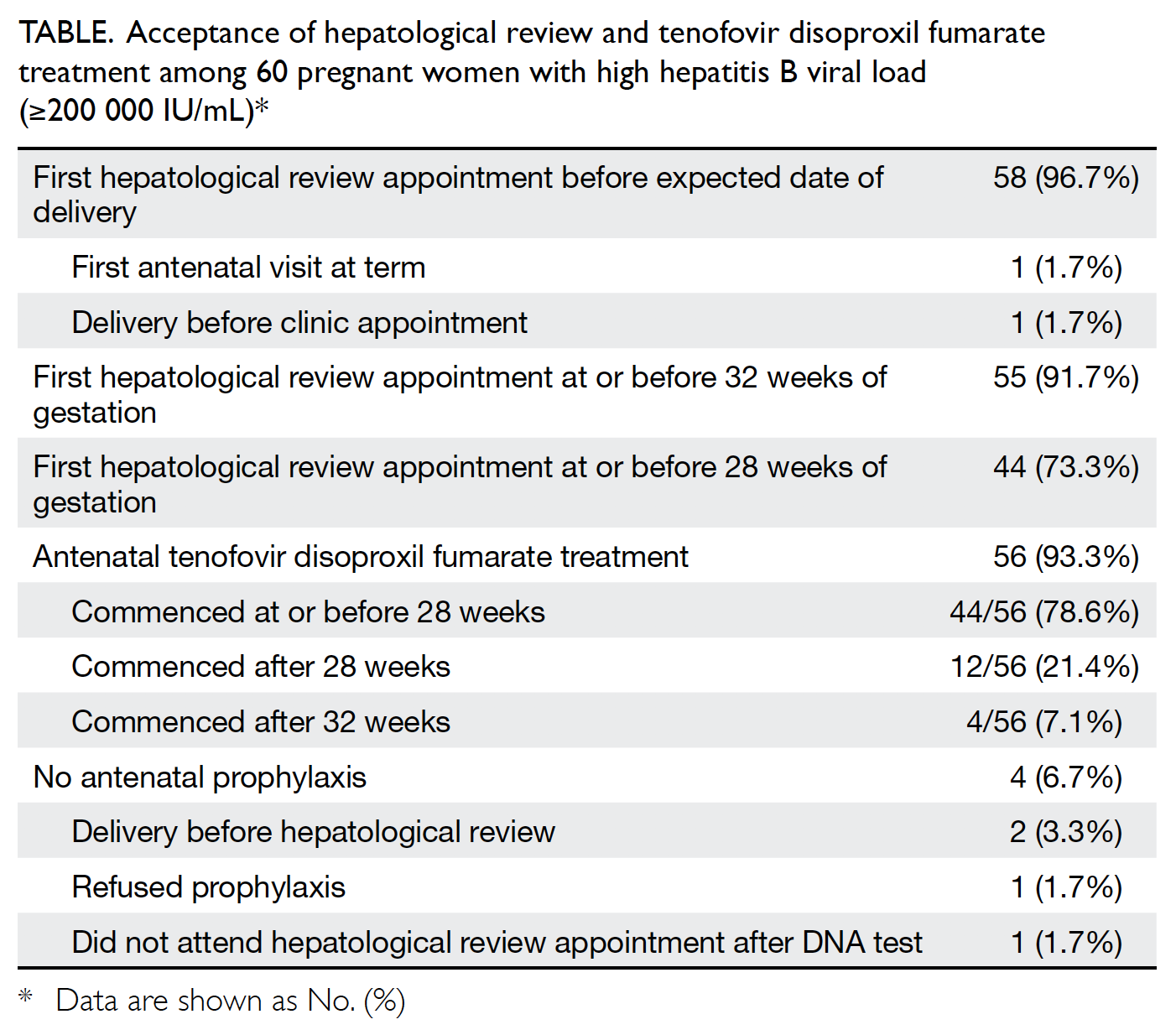
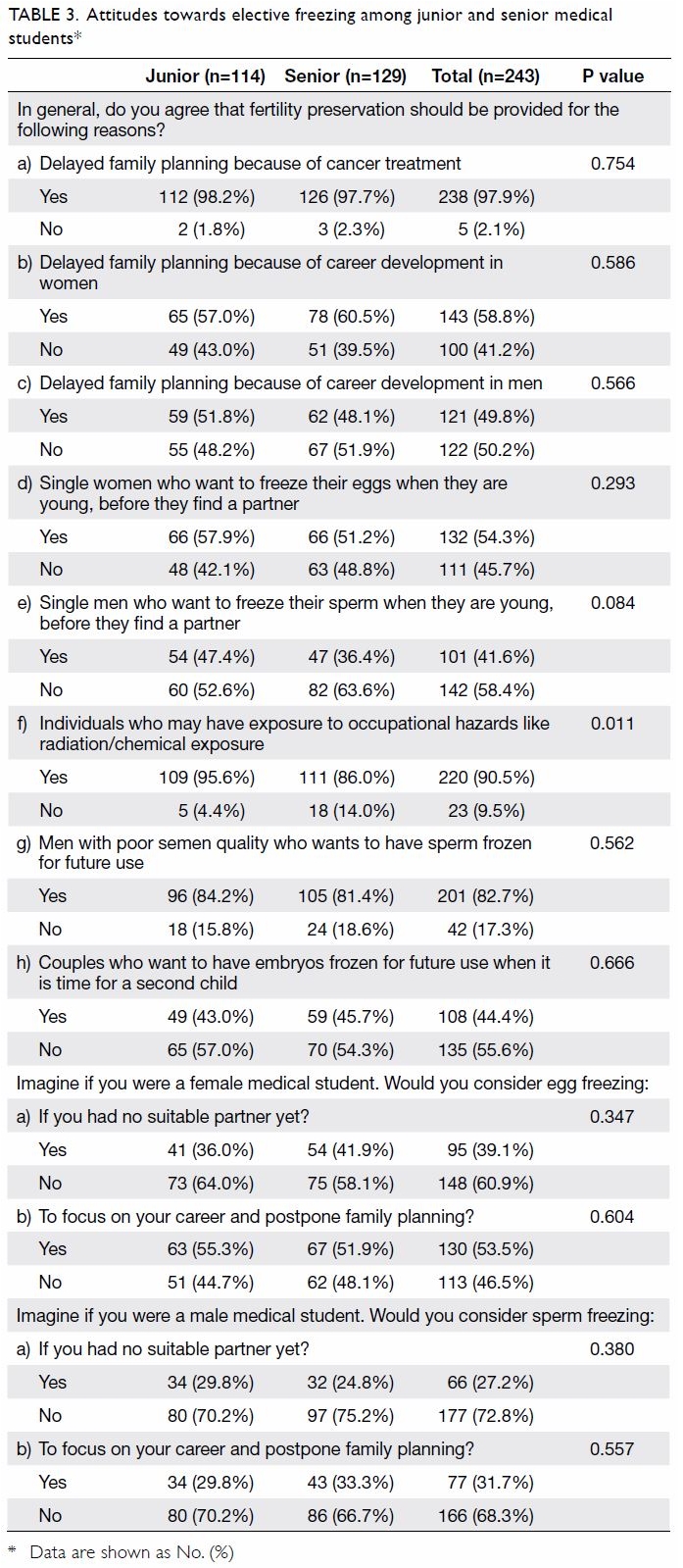
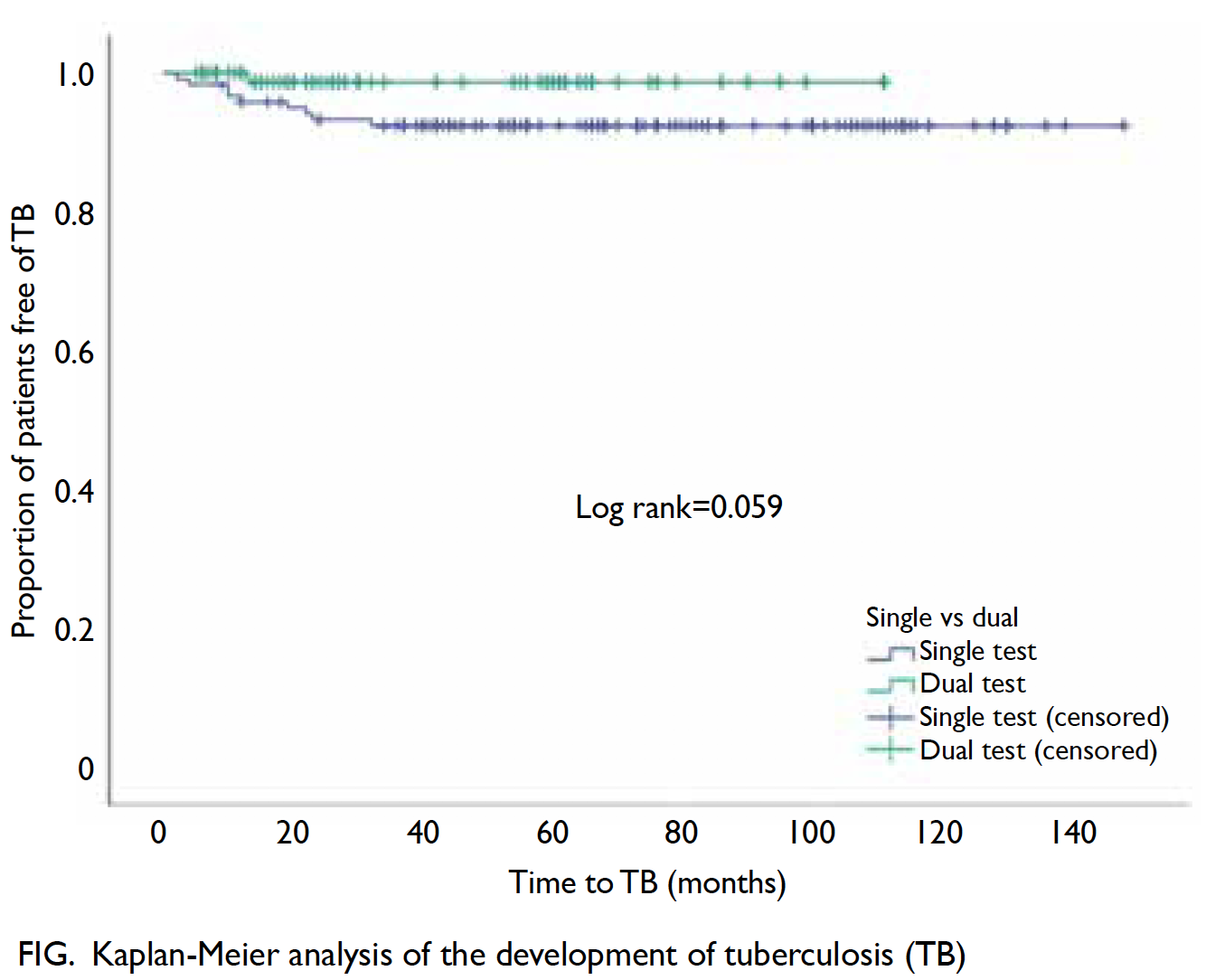
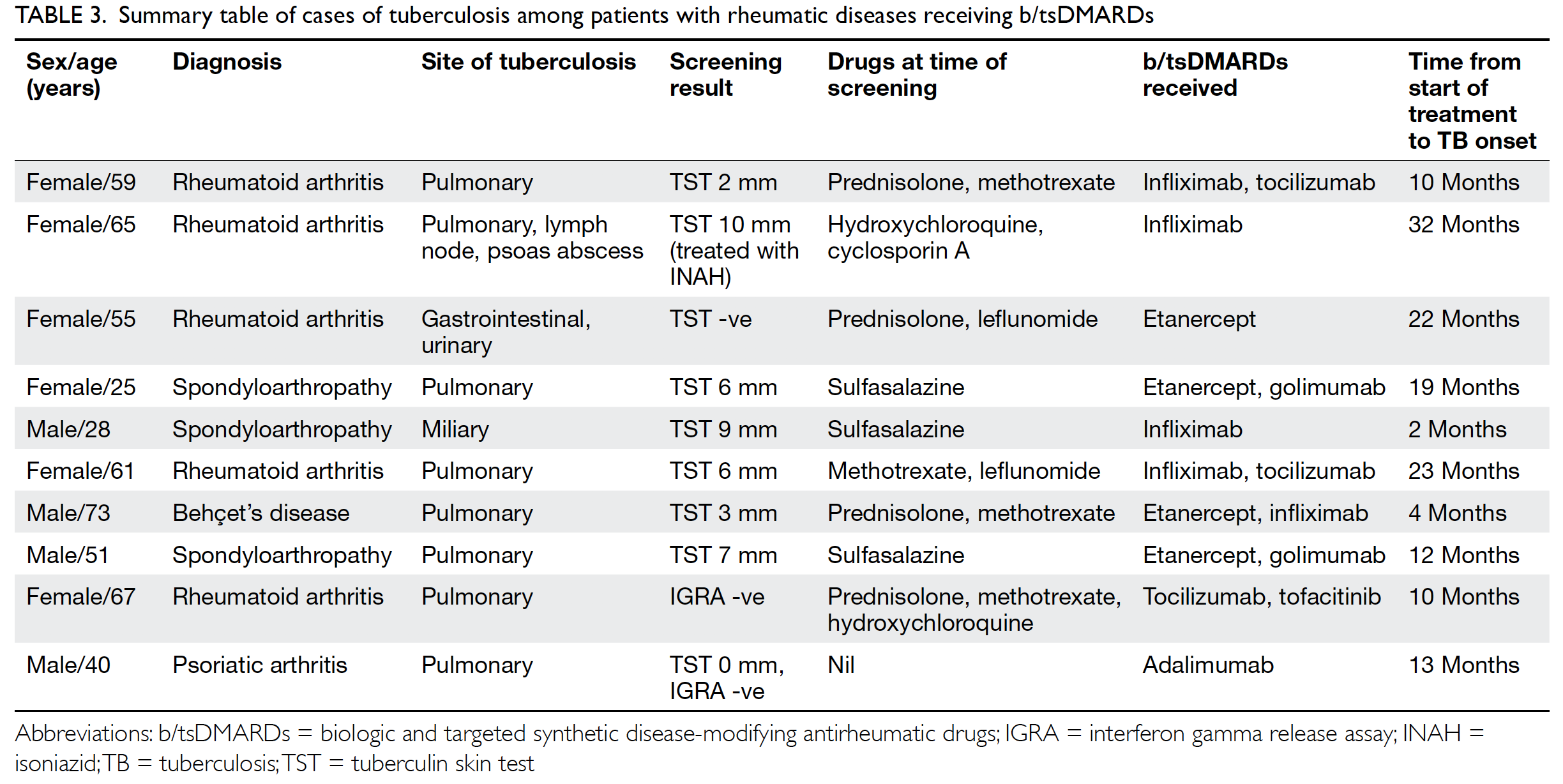
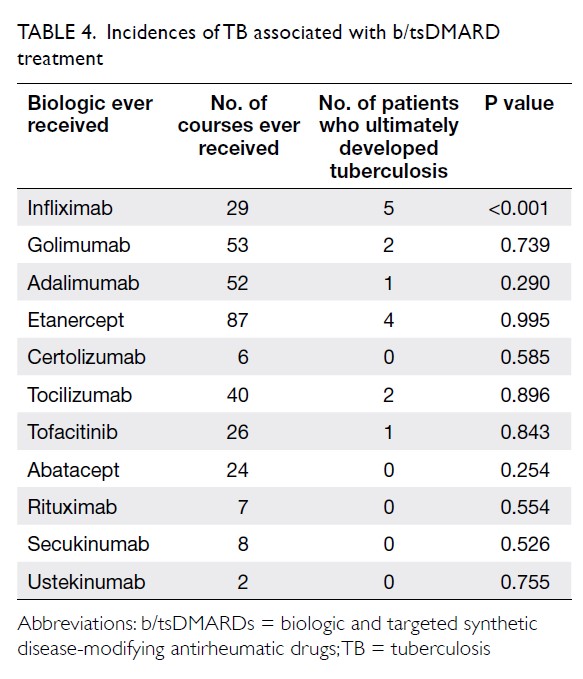
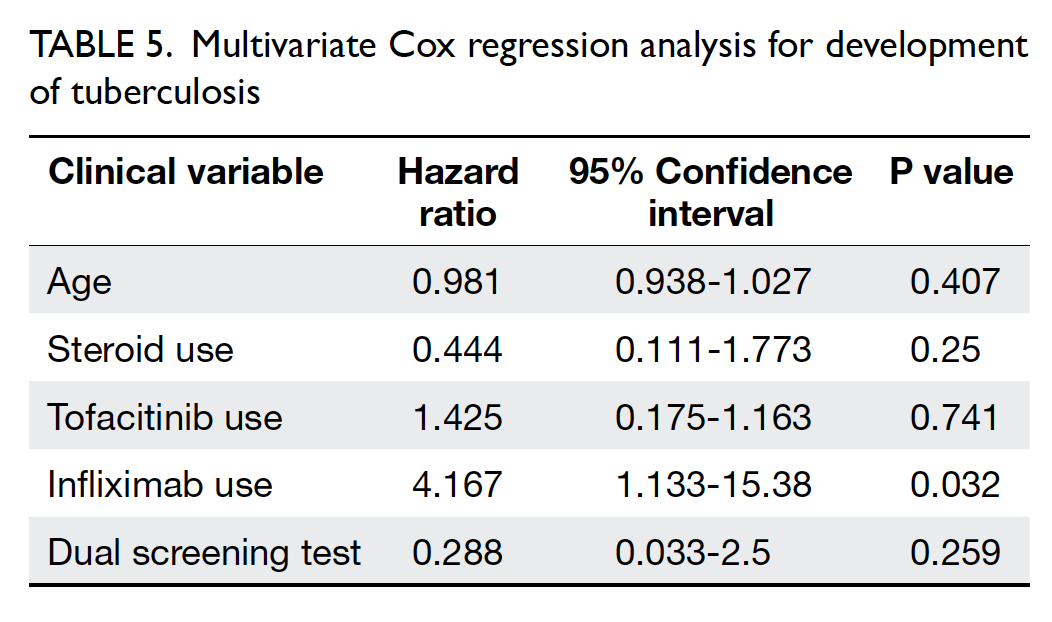
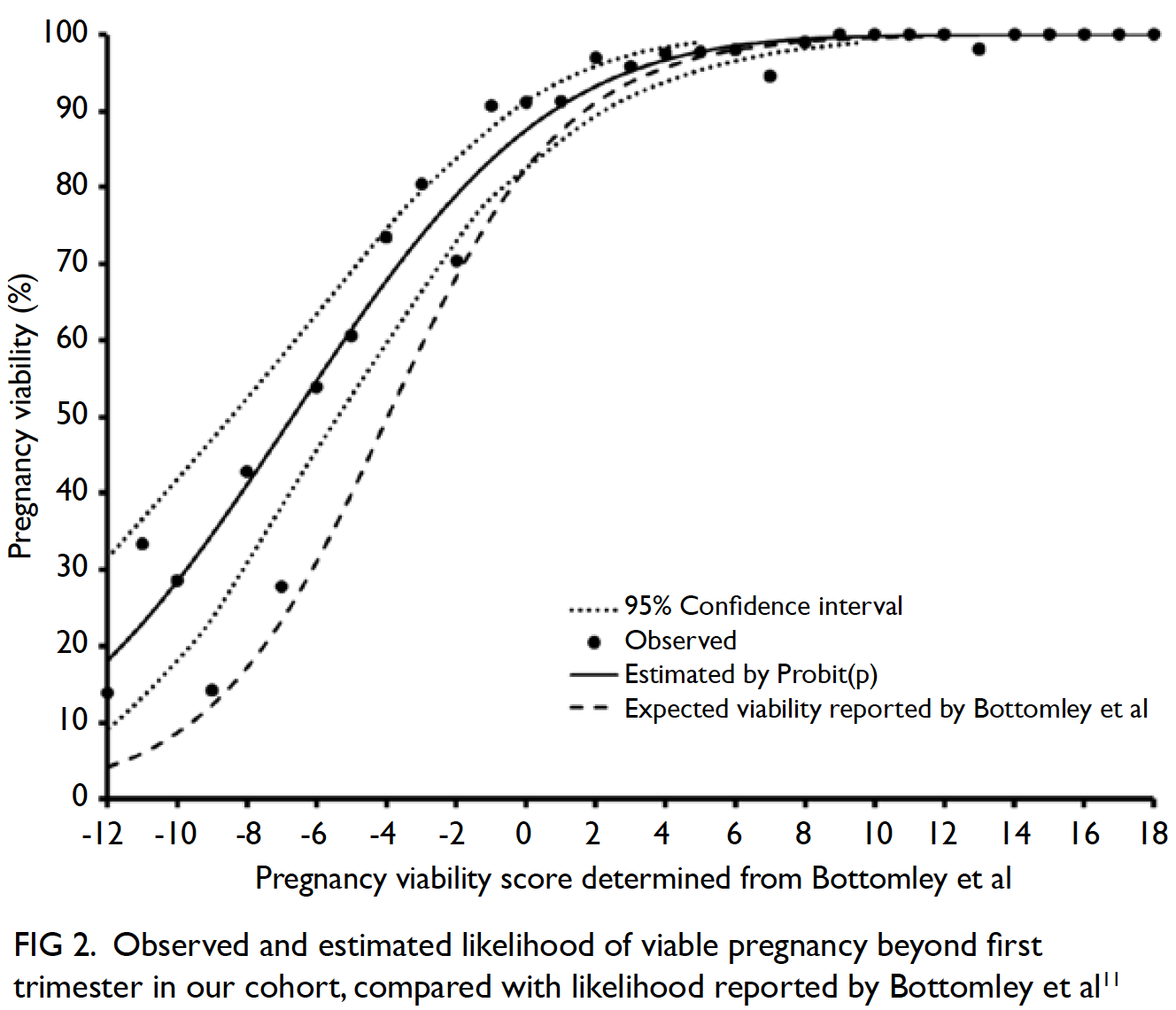
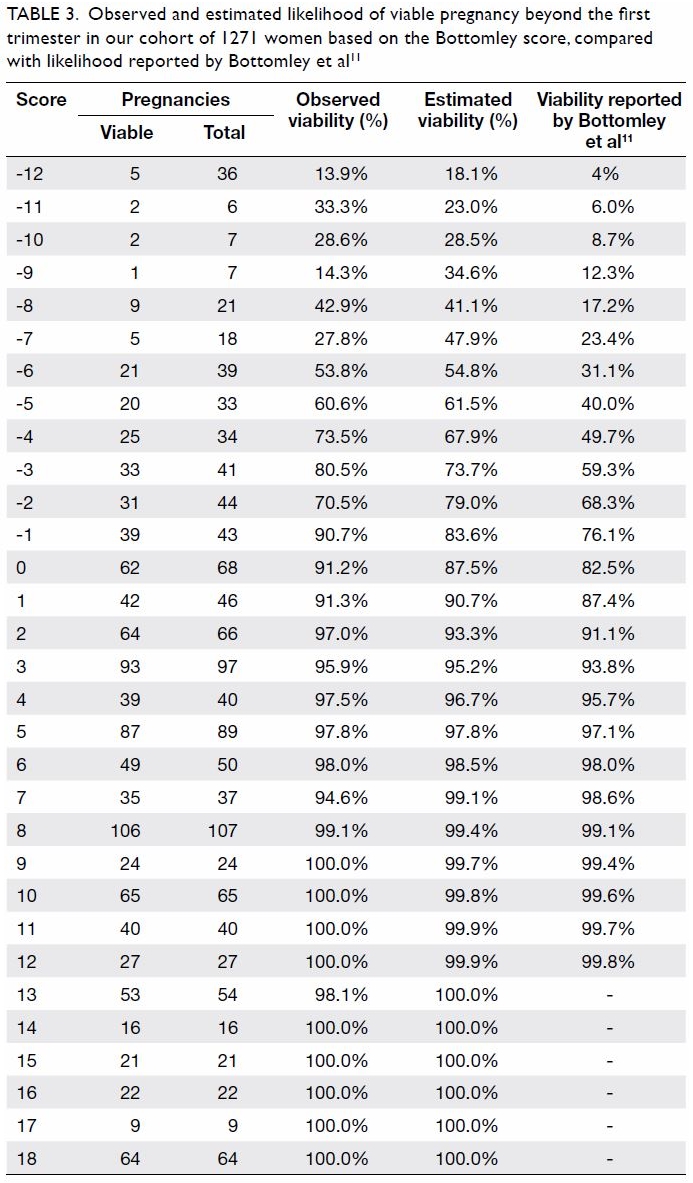
Universal haemoglobin A1c screening reveals high prevalence of dysglycaemia in patients undergoing total knee arthroplasty
Hong Kong Med J 2020 Aug;26(4):304–10 | Epub 7 Aug 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Universal haemoglobin A1c screening reveals
high prevalence of dysglycaemia in patients
undergoing total knee arthroplasty
Vincent WK Chan, FHKCOS, FHKAM (Orthopaedic Surgery)1; PK Chan, FHKCOS, FHKAM (Orthopaedic Surgery)1; YC Woo, FRCP (Lond), FHKAM (Medicine)2; Henry Fu, FHKCOS, FHKAM (Orthopaedic Surgery)1; Amy Cheung, FHKCOS, FHKAM (Orthopaedic Surgery)1; MH Cheung, FHKCOS, FHKAM (Orthopaedic Surgery)3; CH Yan, FHKCOS, FHKAM (Orthopaedic Surgery)3; KY Chiu, FHKCOS, FHKAM (Orthopaedic Surgery)3
1 Department of Orthopaedics and Traumatology, Queen Mary Hospital, Hong Kong
2 Department of Medicine, Queen Mary Hospital, Hong Kong
3 Department of Orthopaedics and Traumatology, The University of Hong Kong, Hong Kong
Corresponding author: Dr Vincent WK Chan (drvincentwkchan@gmail.com)
Abstract
Introduction: Diabetes mellitus is an established
modifiable risk factor for periprosthetic joint
infection (PJI). Haemoglobin A1c (HbA1c) is a
glycaemic marker that correlates with diabetic
complications and PJI. As diabetes and prediabetes
are frequently asymptomatic, and there is increasing
evidence to suggest a correlation between
dysglycaemia and osteoarthritis, it is reasonable
to provide HbA1c screening before total knee
arthroplasty (TKA). The aim of the present study
was to determine the prevalence of dysglycaemia
in patients who underwent TKA and investigate
whether HbA1c screening and optimisation of
glycaemic control before TKA affects the incidence
of PJI after TKA.
Methods: Patients who underwent primary TKA
before and after routine HbA1c screening was
introduced in our unit were reviewed. Prediabetes
and diabetes were defined according to the American
Diabetes Association. Patients with HbA1c ≥7.5%
were referred to an endocrinologist for optimisation
of glycaemic control before TKA. The incidence PJI,
defined according to the Musculoskeletal Infection
Society criteria, was recorded.
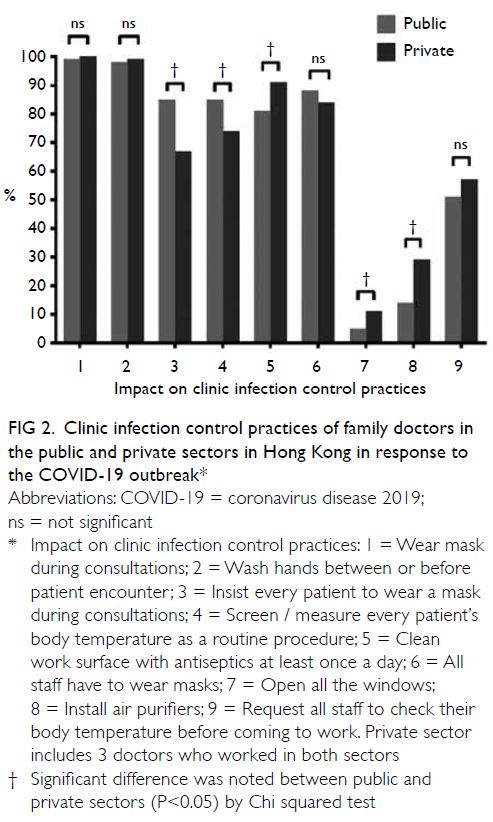
Results: A total of 729 patients (934 knees) had
HbA1c screening before TKA. Of them, 17 (2.3%)
and 184 (25.2%) patients had known prediabetes
and diabetes, respectively, and 265 (36.4%) and 12 (1.6%) had undiagnosed prediabetes and diabetes,
respectively. The incidence of PJI was significantly
lower in all patients who received HbA1c screening
compared with those who did not (0.2% vs 1.02%,
P=0.027).
Conclusion: Screening for HbA1c before TKA
provides a cost-effective opportunity to identify
undiagnosed dysglycaemia. Patients identified as
having dysglycaemia receive modified treatment,
significantly reducing the rate of PJI when compared
with historical controls.
New knowledge added by this study
- There is a high prevalence of undiagnosed diabetes and prediabetes in patients undergoing total knee arthroplasty (TKA) in Hong Kong.
- Universal haemoglobin A1c (HbA1c) screening before TKA can identify patients with undiagnosed dysglycaemia.
- HbA1c screening should be considered for all patients before TKA.
Introduction
Worldwide, the prevalence of diabetes mellitus
and the number of total knee arthroplasty (TKA)
surgeries performed is increasing; therefore, the
number of patients with dysglycaemia undergoing
TKA is expected to rise.1 2 3 The proportion of patients
undergoing TKA who have diabetes mellitus was
reported to be 20.6% in the US in 2018.4 Diabetes mellitus is associated with various adverse outcomes
after total joint arthroplasty, including periprosthetic
joint infection (PJI).5 6 7 8 9 10 Although the occurrence of
PJI is rare, it is a devastating complication after total
joint arthroplasty, resulting in significant morbidity
and even mortality. The economic burden to manage
PJI after total joint arthroplasty is projected to be
over US$1.62 million by 2020.11 Despite advances in total joint arthroplasty, the risk of PJI remains and
likely cannot be eliminated. Therefore, enhancing
preoperative screening and optimisation of various
risk factors for PJI is of the utmost importance.
Glycated haemoglobin A1c (HbA1c) is a readily
accessible glycaemic control marker and, according
to the American Diabetes Association, HbA1c is
also a predictor for diabetes-related complications.12
Previous studies have found that preoperative
HbA1c >7.5% or 8% is associated with an increased
risk of PJI and wound complications after TKA.13 14 15
Therefore, optimising HbA1c levels to below these
suggested thresholds might be a feasible strategy
to reduce PJI. Moreover, patients with prediabetes
and diabetes are frequently asymptomatic in the
early stages and up to 50% of patients present with
complications at the time of diagnosis.16 Diabetes
mellitus is also associated with the development of
osteoarthritis.17 18 Preoperative assessment for TKA
provides an ideal opportunity for diabetes screening.
In our centre, we introduced universal HbA1c
screening 2 to 3 months before surgery for all patients
undergoing TKA, regardless of their diabetic status,
in March 2017. Patients with HbA1c level ≥7.5%
are referred to an endocrinologist for optimisation
of glycaemic control before proceeding to TKA
surgery.
The aim of the present study was to determine
the prevalence of prediabetes and diabetes in patients
who underwent TKA and investigate whether the
introduction of universal HbA1c screening and
optimisation of glycaemic control affected the rate
of PJI after TKA.
Methods
All patients who underwent primary TKA at Queen
Mary Hospital, Hong Kong, from December 2014 to
May 2019 were reviewed. Patients were diagnosed as
prediabetes or diabetes according to the American
Diabetes Association definitions, wherein a HbA1c
level of 5.7% to 6.4% is defined as prediabetes,
and a HbA1c level ≥6.5% is defined as diabetes.10
Patients were classified as undiagnosed prediabetes
or diabetes if there was no previous diagnosis or
diabetic status in the patient’s medical record.
Patients with HbA1c level ≥7.5% were referred to
an endocrinologist for optimisation of glycaemic
control before proceeding to TKA.
Patients who underwent primary TKA from
December 2014 to February 2017 did not receive
universal HbA1c screening. These patients were
included in the study as historical controls, to
compare the PJI rate with patients who received
HbA1c screening before undergoing TKA from
March 2017 to May 2019. These 27-month periods
immediately prior to and after the initiation of
HbA1c screening were chosen to match as closely
as possible the duration, comparable indications,
perioperative management, surgical technique, and
wound care protocol for better comparisons.
All patients received one dose of prophylactic
antibiotic on the induction of anaesthesia and no
further doses of antibiotics postoperatively. All
PJIs were defined according to the Workgroup of
the Musculoskeletal Infection Society diagnostic
criteria.19
The primary outcome of this study was
the prevalence of undiagnosed prediabetes and
diabetes in patients undergoing TKA, identified by
universal HbA1c screening. The secondary outcome
was the difference in the PJI rate between patients
undergoing TKA who received HbA1 screening and
historical control patients undergoing TKA who did
not receive HbA1c screening.
Fisher’s exact test was used for statistical
analysis of categorical variables, and Student’s t test
was used for continuous variables. We used SPSS
(Windows version 26.0; IBM Corp, Armonk [NY],
US) for all analyses. A P value <0.05 was considered
statistically significant.
Results
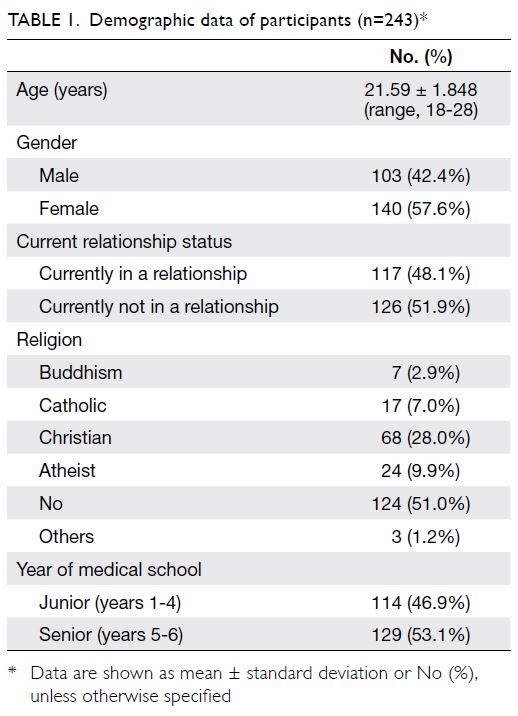
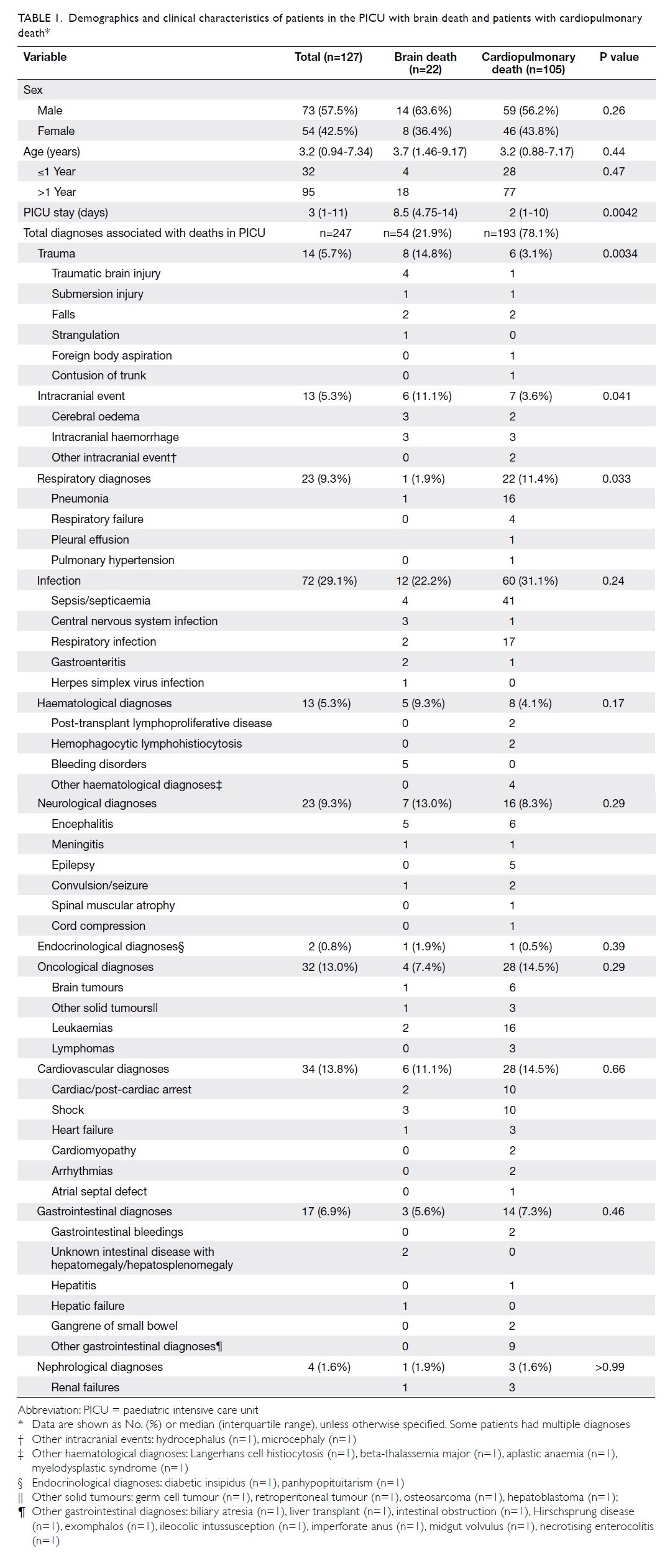
A total of 1566 patients (2017 knees) who underwent
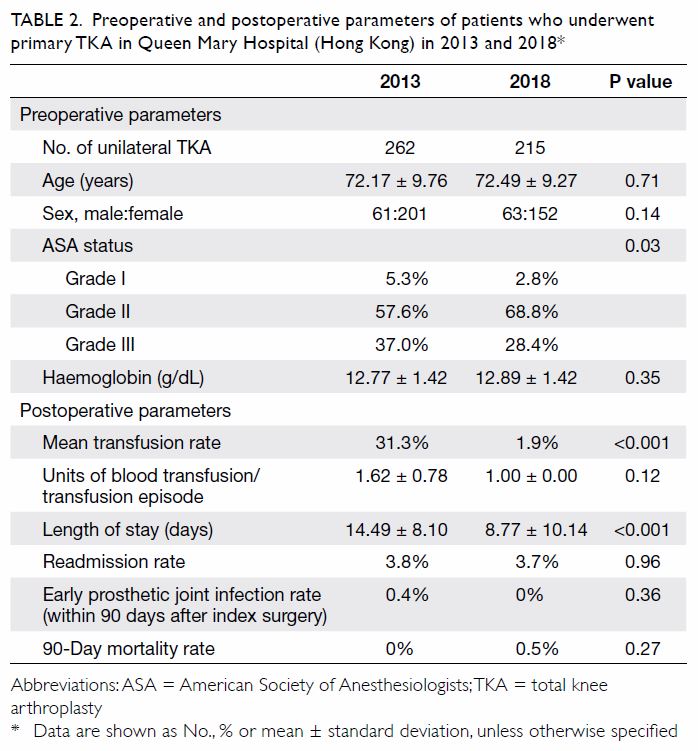
primary TKA were included for analysis. Of them, 729 patients (934 knees) received HbA1c screening
before TKA surgery and 837 patients (1083 knees) did
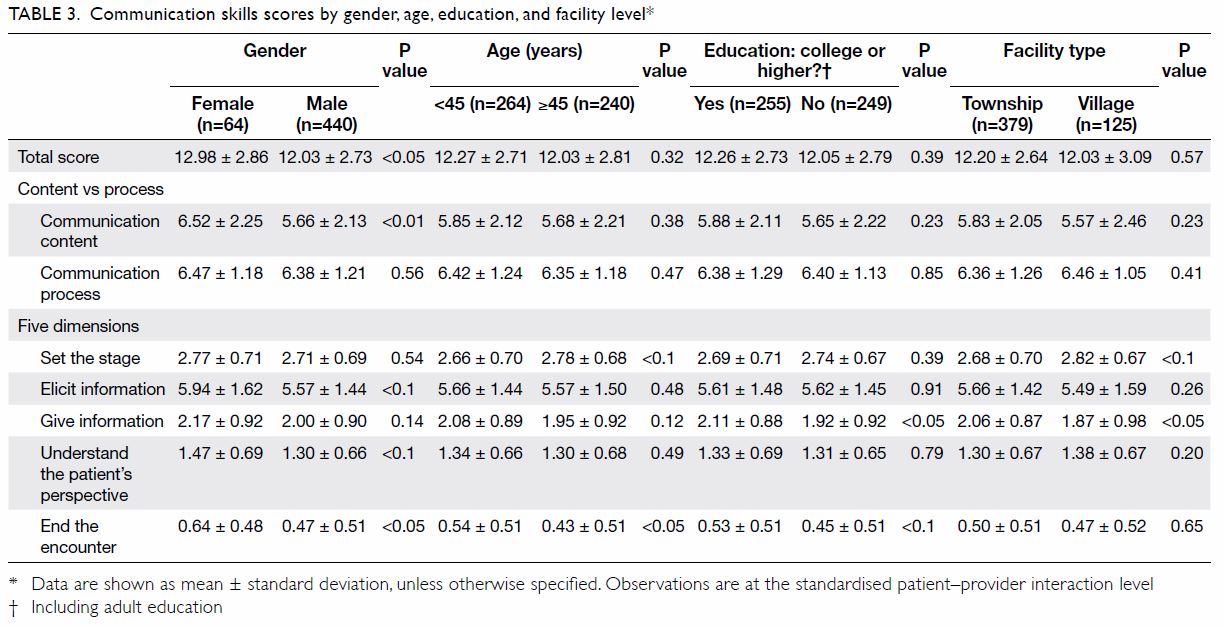
not. The baseline demographics for both groups of
patients, including age, sex, body mass index (BMI),
the prevalence of known diabetes and diagnosis
for TKA are shown in Table 1. The BMI of patients
who received HbA1c screening was significantly
higher than that of patients who did not (28.4±4.7 vs
27.1±4.5, P=0.0001). Other baseline characteristics
were not significantly different between the
two groups.
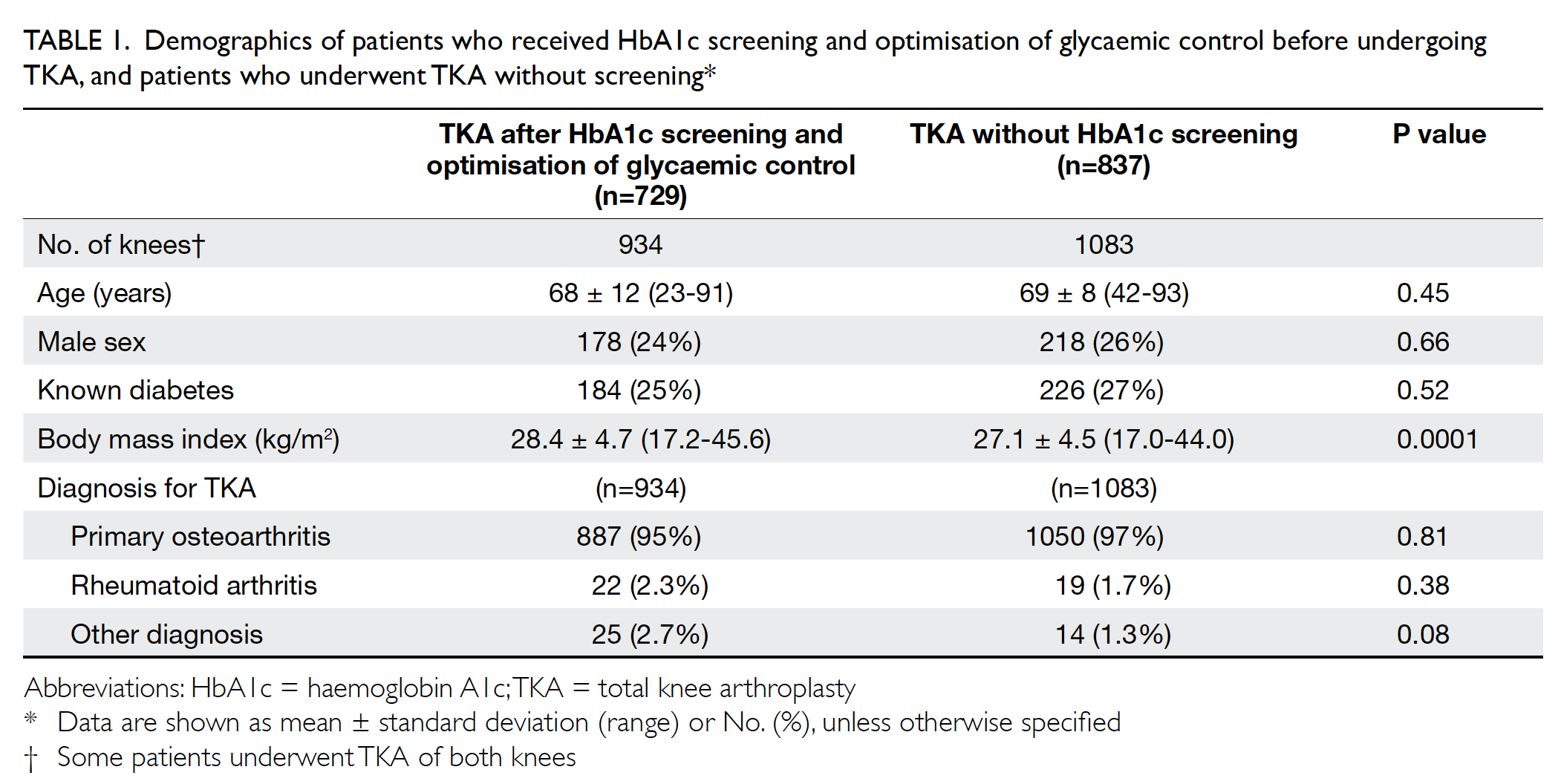
Table 1. Demographics of patients who received HbA1c screening and optimisation of glycaemic control before undergoing TKA, and patients who underwent TKA without screening
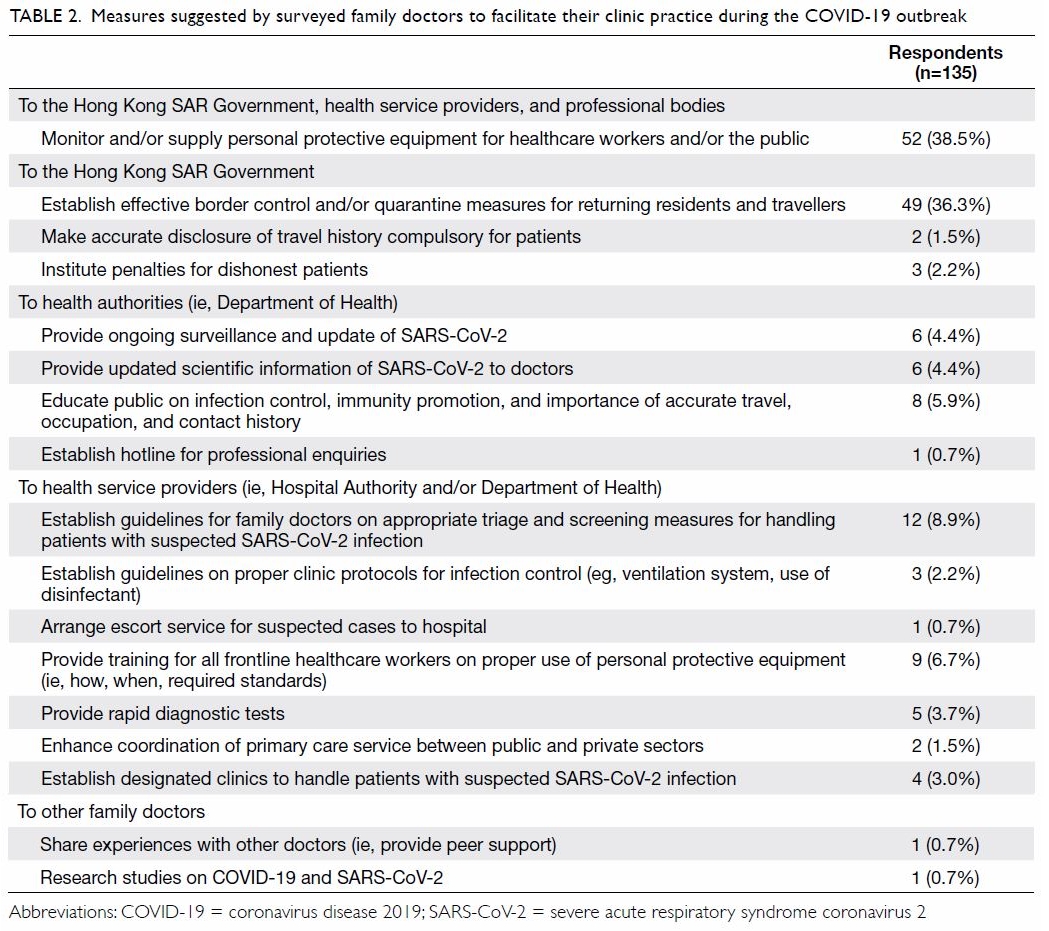
Of the patients who received HbA1c screening,
17 (2.3%) patients were referred to an endocrinologist
for optimisation of glycaemic control before TKA
and all 17 were seen within 4 months. All 17 of these
patients had TKA performed 3 to 18 months after
HbA1c level was controlled to <7.5%.
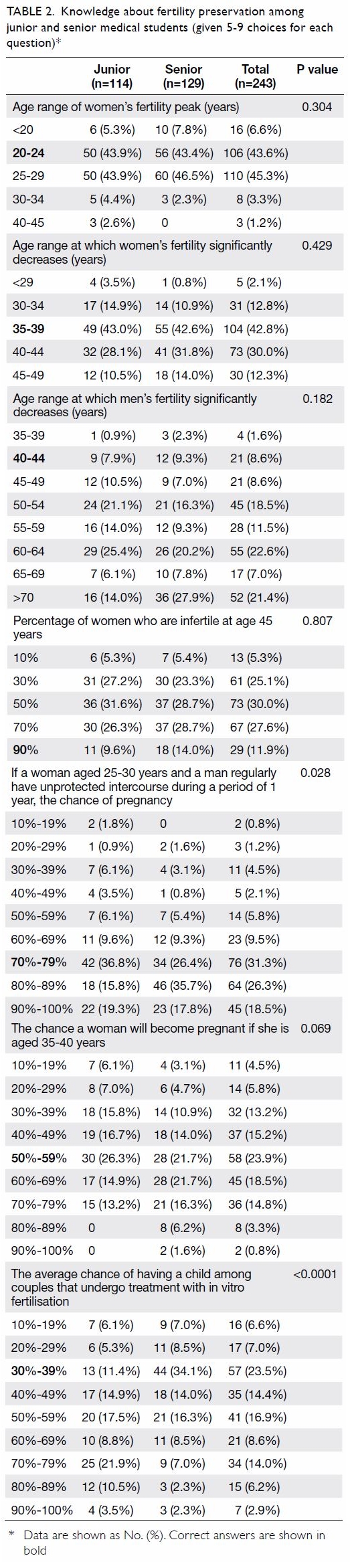
Concerning the results for universal HbA1c
screening, the overall prevalence of diabetes and
prediabetes was 26.9% and 38.7%, respectively.
Patients with a known diagnosis of diabetes and
prediabetes consisted of 25.2% and 2.3%, respectively,
while undiagnosed diabetes and prediabetes
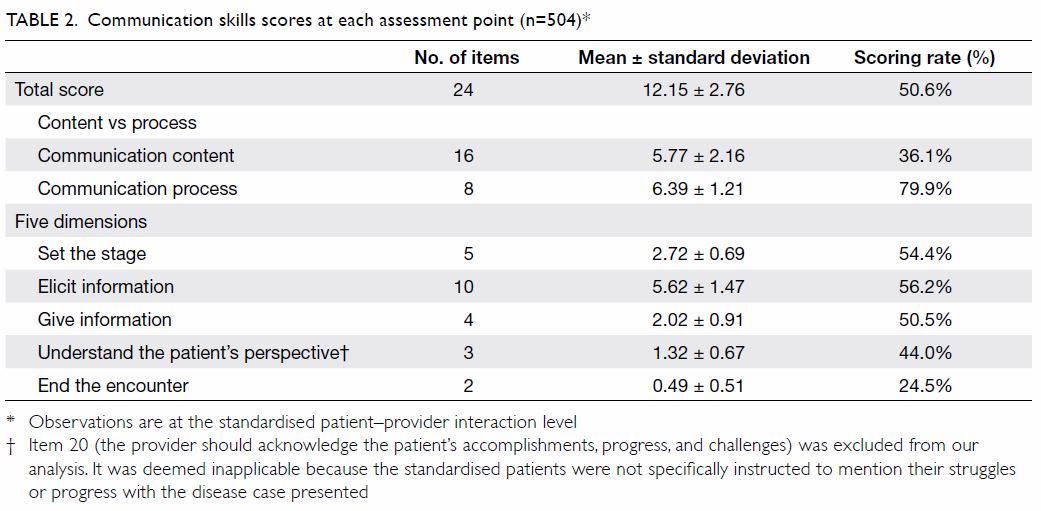
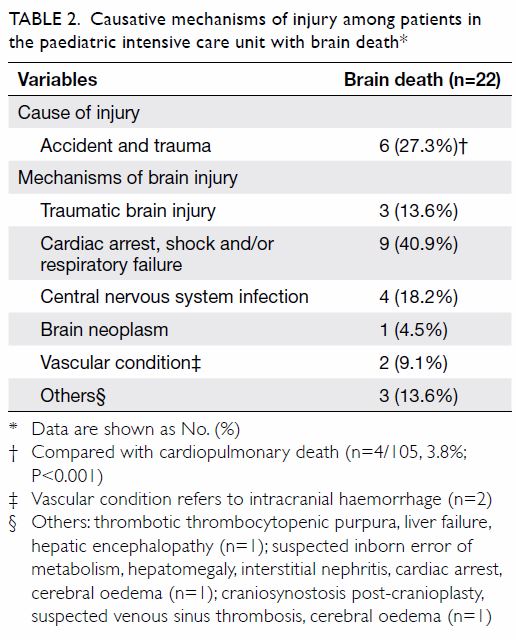
consisted of 1.6% and 36.4% as shown in Table 2.
Therefore, a total of 38% of patients scheduled for
primary TKA have undiagnosed dysglycaemia that
were only detected with HbA1c screening. Mean
(±standard deviation) HbA1c levels for patients with
undiagnosed diabetes, undiagnosed prediabetes,
known diabetes, known prediabetes, and those
without diabetes were 6.7%±0.15 (range, 6.5-7%),
5.9%±0.20 (range, 5.7-6.4%), 6.6%±0.62 (range,
4.6-8.6%), 6.1%±0.45 (range, 5.4-6.4%), and
5.4%±0.19 (range, 4.8-5.6%), respectively, as shown
in Table 2.
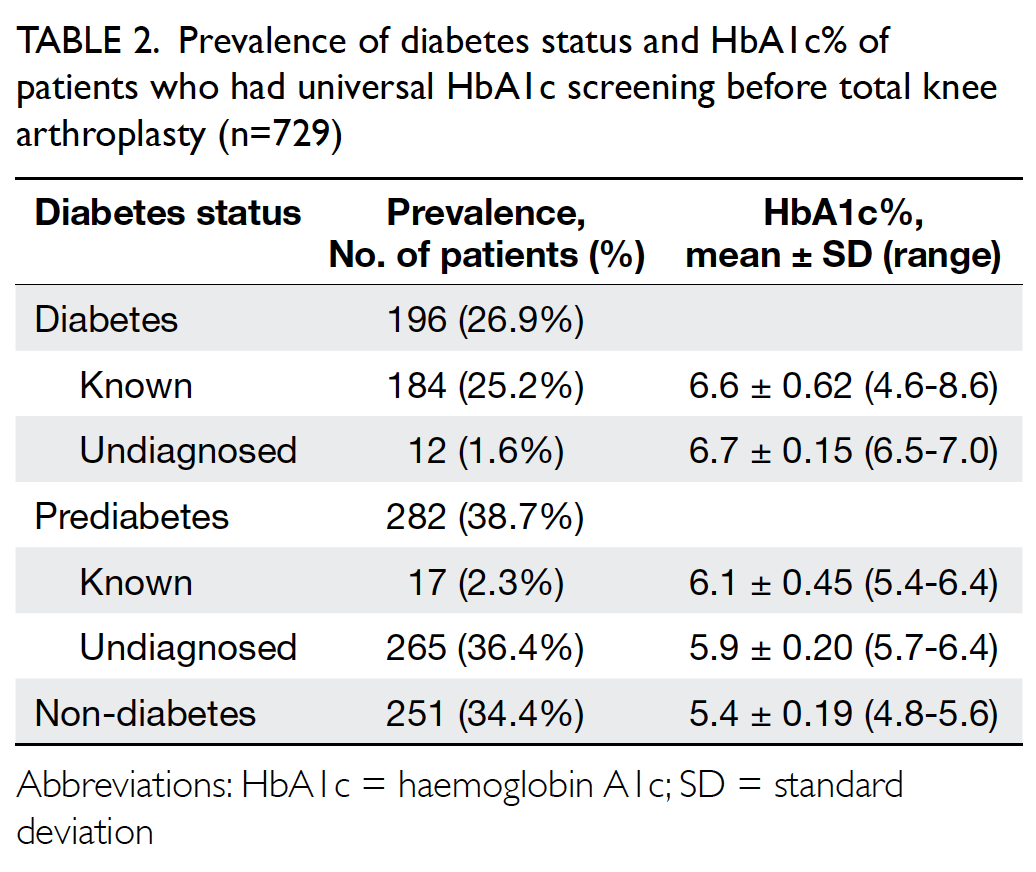
Table 2. Prevalence of diabetes status and HbA1c% of patients who had universal HbA1c screening before total knee arthroplasty (n=729)
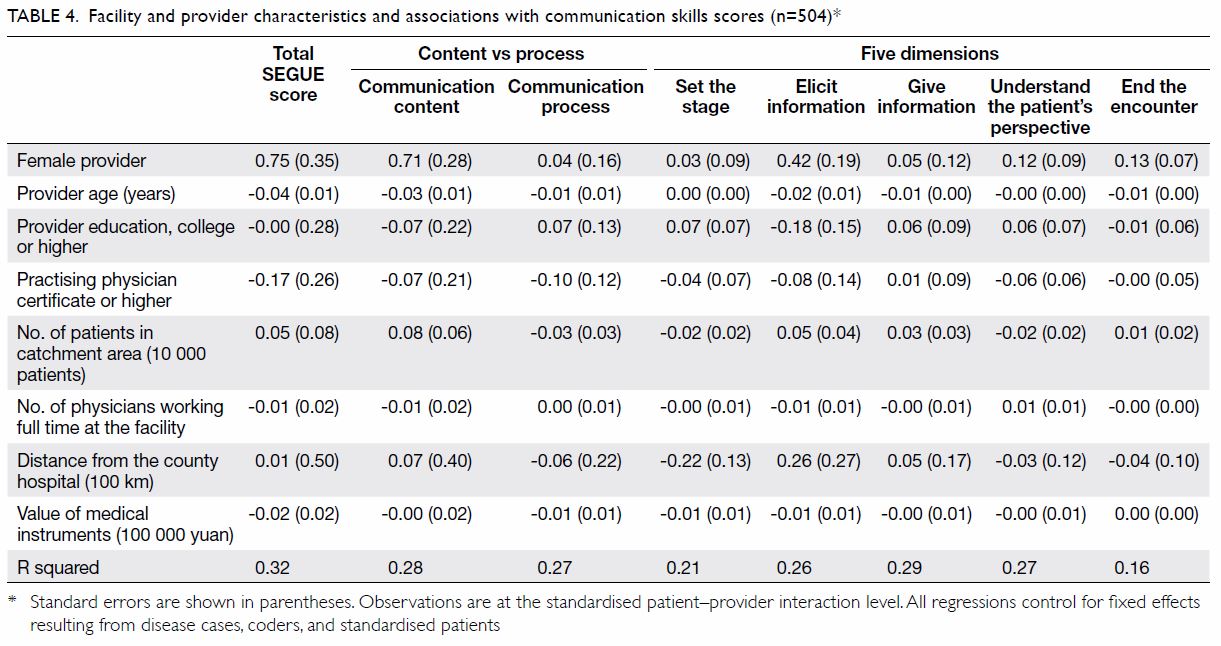
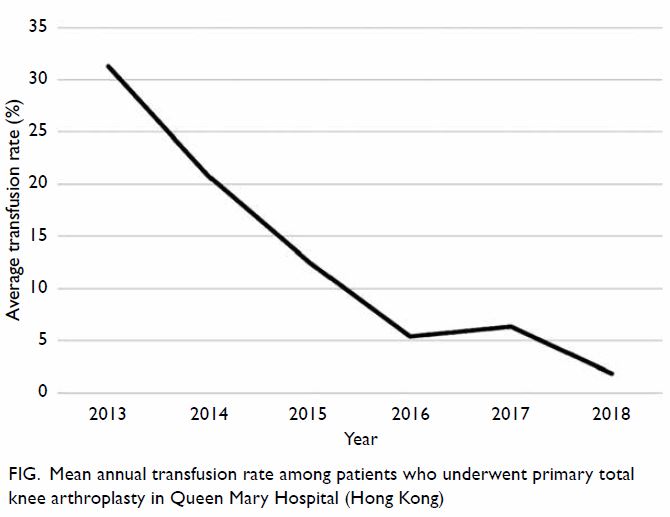
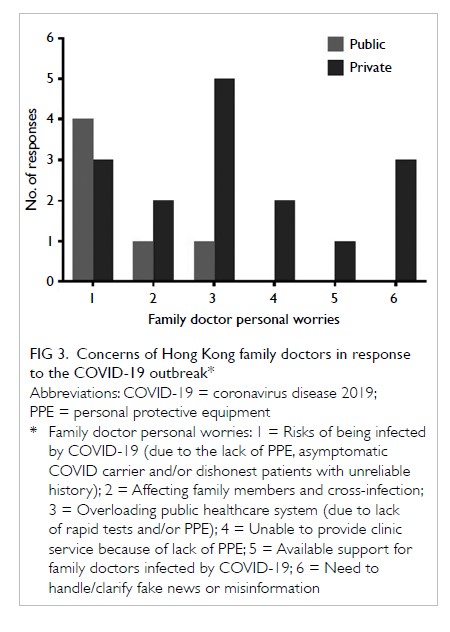
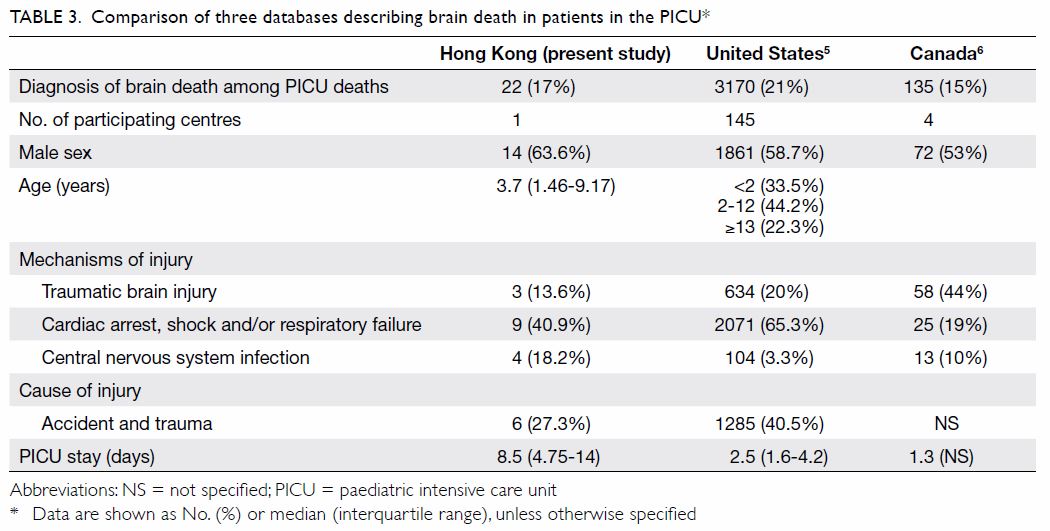
The PJI rate for patients who received HbA1c
screening before undergoing TKA was significantly lower than that for the historical control group
(0.2% vs 1.0%; P=0.027) [Table 3]. Further
comparisons found that the PJI rate for patients with
dysglycaemia was not significantly higher than that
for patients without dysglycaemia in the HbA1c
screening group (0.33% vs 0%; P>0.05). The rate of
PJI was not significantly different between patients
with and without diabetes in the historical control
group (1.03% vs 1.02%; P>0.05).
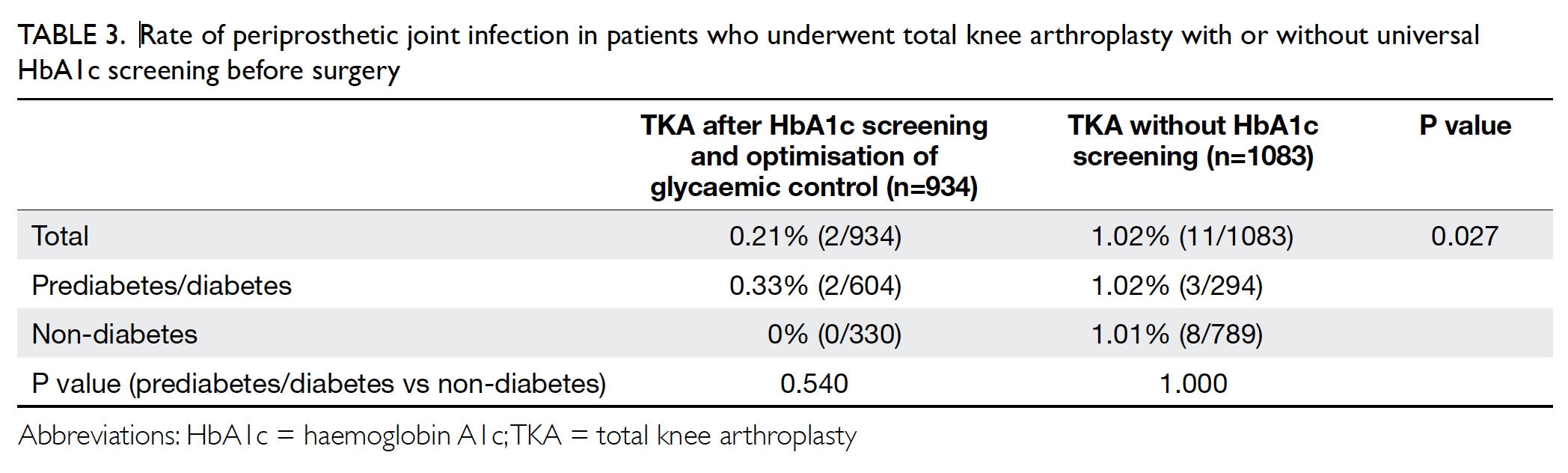
Table 3. Rate of periprosthetic joint infection in patients who underwent total knee arthroplasty with or without universal HbA1c screening before surgery
Discussion
The main finding of the present study is that a
substantial proportion (38.0%) of patients undergoing
primary TKA had undiagnosed prediabetes or
diabetes. This finding is consistent with an earlier
study in the US, which reported 33.6% of patients had
undiagnosed dysglycaemia before total hip or knee
arthroplasty.20 Universal HbA1c screening allows for earlier diagnosis of prediabetes and diabetes
and timely intervention. Because diabetes mellitus
is an established risk factor for PJI,5 6 7 8 9 identifying
patients with prediabetes and diabetes allows better
preoperative communication and risk expectation
with the patient before surgery. Moreover, initiating
medical treatment to optimise blood glucose control
may reduce postoperative hyperglycaemia, of clinical
significance, which is an independent risk factor for
wound complications and PJI.21 22 23
Undiagnosed prediabetes was found in 36.4%
of our TKA patients. These patients might have
remained undiagnosed for a long period, as most
were asymptomatic. Nathan et al24 reviewed the
natural history of prediabetes and found that 25%
of patients with prediabetes progress to diabetes
over the subsequent 3 to 5 years. Therefore, early
detection and treatment of prediabetes is important
to prevent the development of diabetes and its
complications. Early lifestyle changes and medical
treatment for prediabetes reduce the chance of
progressing to diabetes.25 26
In the present study, the PJI rate for patients
who received HbA1c screening before undergoing
TKA was significantly lower than that for the
historical control group (0.2% vs 1.0%; P=0.027).
However, only 17 (2.3%) of the screened patients
required endocrinologist referral; therefore, the
observed reduction in PJI is likely the result of
multiple factors. Antibiotic-loaded cement can
reduce PJI after total joint arthroplasty27 28; therefore,
we routinely use antibiotic-loaded cement for
patients with dysglycaemia, who are considered
at higher risk of PJI. Further measures are used to
prevent postoperative hyperglycaemia in patients
with dysglycaemia, such as closer monitoring
of glucose level, choice of intravenous fluid, and
providing a diabetic diet during their in-patient
stay. In addition to screening for dysglycaemia and
direct optimisation of glycaemic control, employing
a more preventive perioperative care might have
contributed to the observed lower rate of PJI in all
patients who received HbA1c screening.
Patients with diabetes and prediabetes are at increased risk of transient hyperglycaemia and
increase glycaemic variability.29 30 Acute glucose
fluctuation increases oxidative stress at the
cellular level increasing diabetic microvascular
and macrovascular complications.29 31 32 Moreover,
a recent retrospective review using point-of-care
glucose measurement showed that higher
postoperative glucose variability after total joint
arthroplasty is associated with adverse outcomes,
including surgical site infection and PJI.33 Therefore,
identifying patients with prediabetes and diabetes
before surgery allows closer postoperative
surveillance and glycaemic control, which might
improve the patients’ clinical outcomes.
Universal HbA1c screening for diabetes
among patients undergoing primary TKA fulfils
many of the criteria for effective screening set out
by Wilson and Jungner34 in 1968, including being
an important and prevalent health issue, having an
acceptable screening test and treatment, and having
a recognised early asymptomatic stage. Quan et al3
reviewed the complete census of public health
records in Hong Kong and reported that the overall
incidences of diabetes and prediabetes in 2014
were 10.29% and 8.9%, respectively. In the present
study, we found that the prevalences of diabetes and
prediabetes in Hong Kong patients undergoing TKA
were 26.9% and 38.7% respectively, which are much
higher than in the general local population. This
is explained by the linkage between diabetes and
osteoarthritis, together with the relatively older age
of patients undergoing TKA.17 18 Moreover, the mean
BMI in both patient groups is above the cut-off value
for obesity in the Hong Kong Chinese population,35
and these patients are therefore considered at high
risk for developing type 2 diabetes and cardiovascular
disease by the World Health Organization.36 Thus,
preoperative assessment for TKA provides an ideal
occasion for opportunistic screening for diabetes.
Blood HbA1c level is a useful marker in
monitoring glucose control and correlates with
diabetic complications.12 37 Multiple studies have
shown that high preoperative HbA1c is associated
with PJI and wound complications after TKA, with proposed HbA1c thresholds from 7.5% to 8%.13 14 15
Other glycaemic markers, such as preoperative
fasting glucose, fructosamine, postoperative
hyperglycaemia, and glucose variability, are also
associated with an increased risk of adverse clinical
outcomes, including PJI.21 22 23 31 38 Future studies are
needed to clarify the role of each marker, and the use
of continuous glucose monitoring devices can reveal
the postoperative glucose profile in patients with
and without diabetes mellitus after TKA.
The rate of PJI after total joint arthroplasty
is 0.5% to 2%, and PJI remains the leading cause
of revision arthroplasty, comprising up to 25%
of all TKA failures.39 40 41 42 Preventing PJI will have a
substantial impact on clinical outcomes and the
economic burden on our healthcare system. The
cost of a single HbA1c test in local laboratories
ranges from HK$290 to HK$480. We found that
38% of patients scheduled for primary TKA had
undiagnosed dysglycaemia. Therefore, the cost to
identify each case of undiagnosed dysglycaemia
would be HK$870 to HK$1440, and these patients
can receive appropriate and timely treatment. In
contrast, treating a single PJI would cost HK$530 000
to HK$830 000.43 Using 7.5% as the HbA1c threshold
for referral, we found that only 2.3% of the screening
population required assessment and optimisation
of glycaemic control by an endocrinologist. Hence,
our HbA1c screening and optimisation of glycaemic
control did not result in excessive use of medical
services.
To the best of our knowledge, this is the
first study to compare the PJI rate of patients who
underwent TKA with or without preoperative
universal HbA1c screening. Our findings from a
Hong Kong Chinese population add to the body of
evidence supporting universal HbA1c screening for
patients undergoing TKA. Although few patients
in the present study required endocrinologist
assessment, identifying undiagnosed dysglycaemia
allows early and appropriate intervention. Knowing
the diabetic status of patients undergoing TKA also
alters the perioperative treatment of these patients,
including the use of antibiotic-loaded cement,
the choice of intravenous fluid, and postoperative
glucose monitoring. Because primary TKA is an
elective surgery, the risk factors for adverse outcomes
should be thoroughly assessed and optimised, to
improve patient safety and maximise the benefit of
the surgery.
There are several limitations to this study.
This was a retrospective study involving Hong
Kong Chinese patients undergoing TKA at a single
institution. Genetic and social differences affect the
prevalence of diabetes,44 and the perioperative care
for dysglycaemic patients varies between different
institutions; therefore caution is advised when
generalising the results to other populations. Other medical co-morbidities that affect the risk of PJI were
not controlled for, such as rheumatological diseases,
obesity, malnutrition, preoperative anaemia, history
of steroid administration, and malignancy.7 45 46 In the
present study, diagnosis and identification of PJI was
based on analysis of medical records in the public
healthcare system. Patients treated elsewhere, such
as in the private healthcare sector, were not included
in this study. Similarly, patients in the historical
control that had dysglycaemia diagnosed and
managed by private practitioners would be labelled as
non-diabetes. Moreover, diabetes and prediabetes
were defined using only HbA1c, and fasting blood
glucose and oral glucose tolerance tests were not
performed, potentially leading to an underestimation
of dysglycaemia. Finally, although all TKA procedures
and perioperative care routines were performed
consistently by the same surgical team, advances in
surgical technique and perioperative patient care
may have created bias when historical data are used
as controls. Future prospective, comparative studies
with larger sample sizes and multivariate analyses
are required to clarify the role of universal diabetes
screening and optimisation of the risks of PJI after
total joint arthroplasty.
Conclusion
Universal HbA1c screening for patients before
undergoing TKA provides a valuable opportunity
to identify undiagnosed dysglycaemia. Patients
identified as having dysglycaemia receive modified
treatments, including preoperative optimisation of
glycaemic control, resulted in a significantly lower
rate of PJI when compared with historical controls.
Author contributions
All authors contributed to the concept of the study, analysis
or interpretation of the data, drafting of the manuscript, and
critical revision of the manuscript for important intellectual
content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Declaration
The results of this study were presented in part as a free paper on adult joint reconstruction at the Hong Kong Orthopaedic
Association Annual Congress in 2019.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the University of Hong Kong/Hospital Authority Hong Kong West Cluster Institutional Review Board (Ref UW 20-157). The need for informed
consent from the patients was waived by Institutional Review
Board, owing to the retrospective nature of the study.
References
1. Klonoff DC. The increasing incidence of diabetes in the
21st century. J Diabetes Sci Technol 2009;3:1-2. Crossref
2. Memtsoudis SG, Della Valle AG, Besculides MC, Gaber L,
Laskin R. Trends in demographics, comorbidity profiles,
in-hospital complications and mortality associated with
primary knee arthroplasty. J Arthroplasty 2009;24:518-27. Crossref
3. Quan J, Li TK, Pang H, et al. Diabetes incidence and
prevalence in Hong Kong, China during 2006-2014. Diabet
Med 2017;34:902-8. Crossref
4. Shohat N, Goswami K, Tarabichi M, Sterbis E, Tan TL,
Parvizi J. All patients should be screened for diabetes before
total joint arthroplasty. J Arthroplasty 2018;33:2057-61. Crossref
5. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP.
The impact of glycemic control and diabetes mellitus on
perioperative outcomes after total joint arthroplasty. J
Bone Joint Surg Am 2009;91:1621-9. Crossref
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ,
Morrey BF. Elevated postoperative blood glucose and
preoperative hemoglobin A1C are associated with
increased wound complications following total joint
arthroplasty. J Bone Joint Surg Am 2013;95:808-14, S1-2. Crossref
7. Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD;
INFORM Team. Patient-related risk factors for
periprosthetic joint infection after total joint arthroplasty:
a systematic review and meta-analysis. PloS One
2016;11:e0150866. Crossref
8. Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors
for periprosthetic joint infection and postoperative
mortality following total hip arthroplasty in Medicare
patients. J Bone Joint Surg Am 2012;94:794-800. Crossref
9. Schwarz EM, Parvizi J, Gehrke T, et al. 2018 International
Consensus Meeting on Musculoskeletal Infection:
Research Priorities from the General Assembly Questions.
J Orthop Res 2019;37:997-1006. Crossref
10. Meding JB, Reddleman K, Keating ME, et al. Total knee
replacement in patients with diabetes mellitus. Clin
Orthop Relat Res 2003;(416):208-16. Crossref
11. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J.
Economic burden of periprosthetic joint infection in the
United States. J Arthroplasty 2012;27(8 Suppl):61-5.e1. Crossref
12. American Diabetes Association. Standards of medical care
in diabetes–2013. Diabetes Care 2013;36 Suppl 1:S11-66. Crossref
13. Cancienne JM, Werner BC, Browne JA. Is there an
association between hemoglobin A1C and deep
postoperative infection after TKA? Clin Orthop Relat Res
2017;475:1642-9. Crossref
14. Tarabichi M, Shohat N, Kheir MM, et al. Determining the
threshold for HbA1c as a predictor for adverse outcomes
after total joint arthroplasty: a multicenter, retrospective
study. J Arthroplasty 2017;32:S263-S267.e1. Crossref
15. Han HS, Kang SB. Relations between long-term glycemic
control and postoperative wound and infectious
complications after total knee arthroplasty in type 2
diabetics. Clin Orthop Surg 2013;5:118-23. Crossref
16. UK Prospective Diabetes Study (UKPDS). VIII. Study
design, progress and performance [editorial]. Diabetologia
1991;34:877-90. Crossref
17. Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract
2016;122:198-206. Crossref
18. Schett G, Kleyer A, Perricone C, et al. Diabetes is
an independent predictor for severe osteoarthritis:
results from a longitudinal cohort study. Diabetes Care
2013;36:403-9. Crossref
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition
for periprosthetic joint infection: from the Workgroup of
the Musculoskeletal Infection Society. Clin Orthop Relat
Res 2011;469:2992-4. Crossref
20. Capozzi JD, Lepkowsky ER, Callari MM, Jordan ET,
Koenig JA, Sirounian GH. The prevalence of diabetes
mellitus and routine hemoglobin A1c screening in
elective total joint arthroplasty patients. J Arthroplasty
2017;32:304-8. Crossref
21. Jämsen E, Nevalainen P, Eskelinen A, Huotari K,
Kalliovalkama J, Moilanen T. Obesity, diabetes, and
preoperative hyperglycemia as predictors of periprosthetic
joint infection: a single-center analysis of 7181 primary hip
and knee replacements for osteoarthritis. J Bone Joint Surg
Am 2012;94:e101. Crossref
22. Kheir MM, Tan TL, Kheir M, Maltenfort MG, Chen AF.
Postoperative blood glucose levels predict infection
after total joint arthroplasty. J Bone Joint Surg Am
2018;100:1423-31. Crossref
23. Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL,
Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia
predictive of periprosthetic joint infection or death
following primary total joint arthroplasty? J Arthroplasty
2015;30:1197-202. Crossref
24. Nathan DM, Davidson MB, DeFronzo RA, et al.
Impaired fasting glucose and impaired glucose tolerance:
implications for care. Diabetes Care 2007;30:753-9. Crossref
25. Knowler WC, Barrett-Connor E, Fowler SE, et al.
Reduction in the incidence of type 2 diabetes with lifestyle
intervention or metformin. N Engl J Med 2002;346:393-
403. Crossref
26. Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained
reduction in the incidence of type 2 diabetes by lifestyle
intervention: follow-up of the Finnish Diabetes Prevention
Study. Lancet 2006;368:1673-9. Crossref
27. Wang J, Zhu C, Cheng T, et al. A systematic review and
meta-analysis of antibiotic-impregnated bone cement
use in primary total hip or knee arthroplasty. PLoS One
2013;8:e82745. Crossref
28. Sebastian S, Liu Y, Christensen R, Raina DB, Tägil M,
Lidgren L. Antibiotic containing bone cement in
prevention of hip and knee prosthetic joint infections: a
systematic review and meta-analysis. J Orthop Translat
2020;23:53-60. Crossref
29. Timmons JG, Cunningham SG, Sainsbury CA, Jones GC.
Inpatient glycemic variability and long-term mortality
in hospitalized patients with type 2 diabetes. J Diabetes
Complications 2017;31:479-82. Crossref
30. Hanefeld M, Sulk S, Helbig M, Thomas A, Kohler C.
Differences in glycemic variability between normoglycemic
and prediabetic subjects. J Diabetes Sci Technol 2014;8:286-
90. Crossref
31. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin
A1c-independent risk factor for diabetic complications.
JAMA 2006;295:1707-8. Crossref
32. Nusca A, Tuccinardi D, Albano M, et al. Glycemic variability
in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev 2018;34:e3047. Crossref
33. Shohat N, Restrepo C, Allierezaie A, Tarabichi M, Goel R,
Parvizi J. Increased postoperative glucose variability is
associated with adverse outcomes following total joint
arthroplasty. J Bone Joint Surg Am 2018;100:1110-7. Crossref
34. Wilson JM, Jungner YG. Principles and practice of
screening for disease [in Spanish]. Bol Oficina Sanit Panam
1968;65:281-393.
35. Ko GT, Tang J, Chan JC, et al. Lower BMI cut-off value to
define obesity in Hong Kong Chinese: an analysis based on
body fat assessment by bioelectrical impedance. Br J Nutr
2001;85:239-42. Crossref
36. WHO Expert Consultation. Appropriate body-mass index
for Asian populations and its implications for policy and
intervention strategies. Lancet 2004;363:157-63. Crossref
37. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of
hyperglycemia in type 2 diabetes, 2018. A consensus report
by the American Diabetes Association (ADA) and the
European Association for the Study of Diabetes (EASD).
Diabetes Care 2018;41:2669-701. Crossref
38. Shohat N, Tarabichi M, Tischler EH, Jabbour S, Parvizi J.
Serum fructosamine: a simple and inexpensive test for
assessing preoperative glycemic control. J Bone Joint Surg
Am 2017;99:1900-7. Crossref
39. Bozic KJ, Ries MD. The impact of infection after total hip
arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am 2005;87:1746-51. Crossref
40. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of
revision total knee arthroplasty in the United States. Clin
Orthop Relat Res 2010;468:45-51. Crossref
41. Delanois RE, Mistry JB, Gwam CU, Mohamed NS,
Choksi US, Mont MA. Current epidemiology of revision
total knee arthroplasty in the United States. J Arthroplasty
2017;32:2663-8. Crossref
42. Sculco TP. The economic impact of infected total joint
arthroplasty. Instr Course Lect 1993;42:349-51.
43. Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN,
Bozic KJ. Periprosthetic joint infection: the economic
impact of methicillin-resistant infections. J Arthroplasty
2010;25(6 Suppl):103-7. Crossref
44. Golden SH, Yajnik C, Phatak S, Hanson RL, Knowler WC.
Racial/ethnic differences in the burden of type 2 diabetes
over the life course: a focus on the USA and India.
Diabetologia 2019;62:1751-60. Crossref
45. Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related
risk factors for postoperative mortality and periprosthetic
joint infection in Medicare patients undergoing TKA. Clin
Orthop Relat Res 2012;470:130-7. Crossref
46. Zhu Y, Zhang F, Chen W, Liu S, Zhang Q, Zhang Y. Risk
factors for periprosthetic joint infection after total joint
arthroplasty: a systematic review and meta-analysis. J
Hosp Infect 2015;89:82-9. Crossref