Hong Kong Med J 2020 Aug;26(4):318–22 | Epub 12 Aug 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Acceptance of antiviral treatment and enhanced
service model for pregnant patients carrying
hepatitis B
PW Hui, MD, FHKAM (Obstetrics and Gynaecology)1; Carmen Ng, MB, BS1; KW Cheung, MB, BS, FHKAM (Obstetrics and Gynaecology)1; CL Lai, MD, FHKAM (Medicine)
1 Department of Obstetrics and Gynaecology, Queen Mary Hospital, Hong Kong
2 Department of Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Dr PW Hui (apwhui@hku.hk)
Abstract
Introduction: A service model was established for
pregnant women with positive screening results for
hepatitis B surface antigen (HBsAg) at Queen Mary
Hospital in Hong Kong. All women were offered a
blood test for hepatitis B virus (HBV) DNA level
during the first antenatal visit. Women with HBV
DNA levels of ≥200 000 IU/mL received counselling
from hepatologists regarding treatment with
antenatal tenofovir disoproxil fumarate (TDF) 300 mg
daily.
Methods: This retrospective review included
women attending our antenatal clinic who exhibited
positive HBsAg screening results from 15 May 2017
to 31 December 2019. The proportions of women
with positive HBsAg, DNA test acceptance,
hepatological review, and TDF acceptance during
pregnancy were reviewed.
Results: In total, 375 (2.9%) of 13 082 pregnant
women had positive HBsAg screening results. Blood
tests for HBV DNA and hepatological reviews were
offered to 273 women who had not undergone
hepatological review prior to pregnancy; the
acceptance rate was 97.8%. Sixty (22.6%) pregnant
women were hepatitis B carriers with high viral loads of ≥200 000 IU/mL. Among 58 women with
high viral loads, 57 received antenatal counselling
regarding TDF and 56 (96.6%) agreed to take the
drug; 92.9% of these 56 women had commenced
TDF at or before 32 weeks of gestation.
Conclusions: This study indicated broad acceptance
of HBV DNA tests by pregnant women. Triage
allowed early review and commencement of
antiviral medication. This service model serves as a
framework for enhanced antenatal service to prevent
mother-to-child-transmission in public maternity
units.
New knowledge added by this study
- More than 70% of the pregnant women in our cohort did not have hepatitis B virus (HBV) viral load testing or regular hepatological surveillance before pregnancy.
- Antenatal testing of HBV DNA level and treatment with tenofovir disoproxil fumarate was widely accepted by pregnant women.
- More than 90% of pregnant women accepted tenofovir disoproxil fumarate treatment at or before 32 weeks of gestation.
- HBV DNA testing should be arranged in all pregnant women carrying hepatitis B; triage allows early review and commencement of antiviral medication.
- An enhanced service model involving multidisciplinary assessment and treatment by obstetricians and hepatologists is achievable in a public hospital in Hong Kong.
Introduction
The World Health Organization aims to eradicate
hepatitis B virus (HBV) by 2030 and prevention
of vertical transmission is a key element of its
eradication efforts.1 The risk of chronic infection
depends on the timing of infection acquisition
and is highest during the perinatal period, such
that chronic infection occurs in approximately
90% of newborns from HBV-infected mothers.2 The risk is dramatically reduced by administration
of hepatitis B immunoglobulin to newborns at
birth, in combination with a complete course of
hepatitis B vaccination.3 Despite a 75% to 90%
reduction in the carrier rate with these measures,
they have not resulted in complete eradication of
HBV infections. Among the maternal and obstetric
factors examined, a high maternal HBV DNA level
during pregnancy is the strongest risk factor leading
to immunoprophylaxis failure.4 5 6 7
The immunoprophylaxis failure rate in Hong
Kong is reportedly 1.1%, according to the results of
a local prospective multicentre observational study.5
Immunoprophylaxis failure occurs only in those
women with high viral load (ie, ≥6 log10 copies/mL
[≥171 821 IU/mL]; immunoprophylaxis failure rate
4.2%) or hepatitis B e-antigen (HBeAg)–positive
status (immunoprophylaxis failure rate 4.5%). The
use of antiviral treatment during the third trimester
in highly viraemic mothers to suppress viral load
has been advocated to reduce the risk of chronic
HBV infection in newborns.8 9 To achieve this, it is
essential to establish a management strategy that
includes HBV DNA assessment for identification
of high-risk patients, as well as initiation of prompt
antiviral treatment in the antenatal period.
An enhanced service model for pregnant
women who had positive screening results for
hepatitis B surface antigen (HBsAg) was established
on 15 May 2017 at Queen Mary Hospital in Hong
Kong (Fig 1). All women were offered blood tests
for HBV DNA, performed by the Department of
Medicine, The University of Hong Kong, during
their first antenatal visits at Tsan Yuk Hospital or
Queen Mary Hospital. The cost of HK$400 per test
was borne by the patients; the laboratory results
were reviewed by hepatologists.
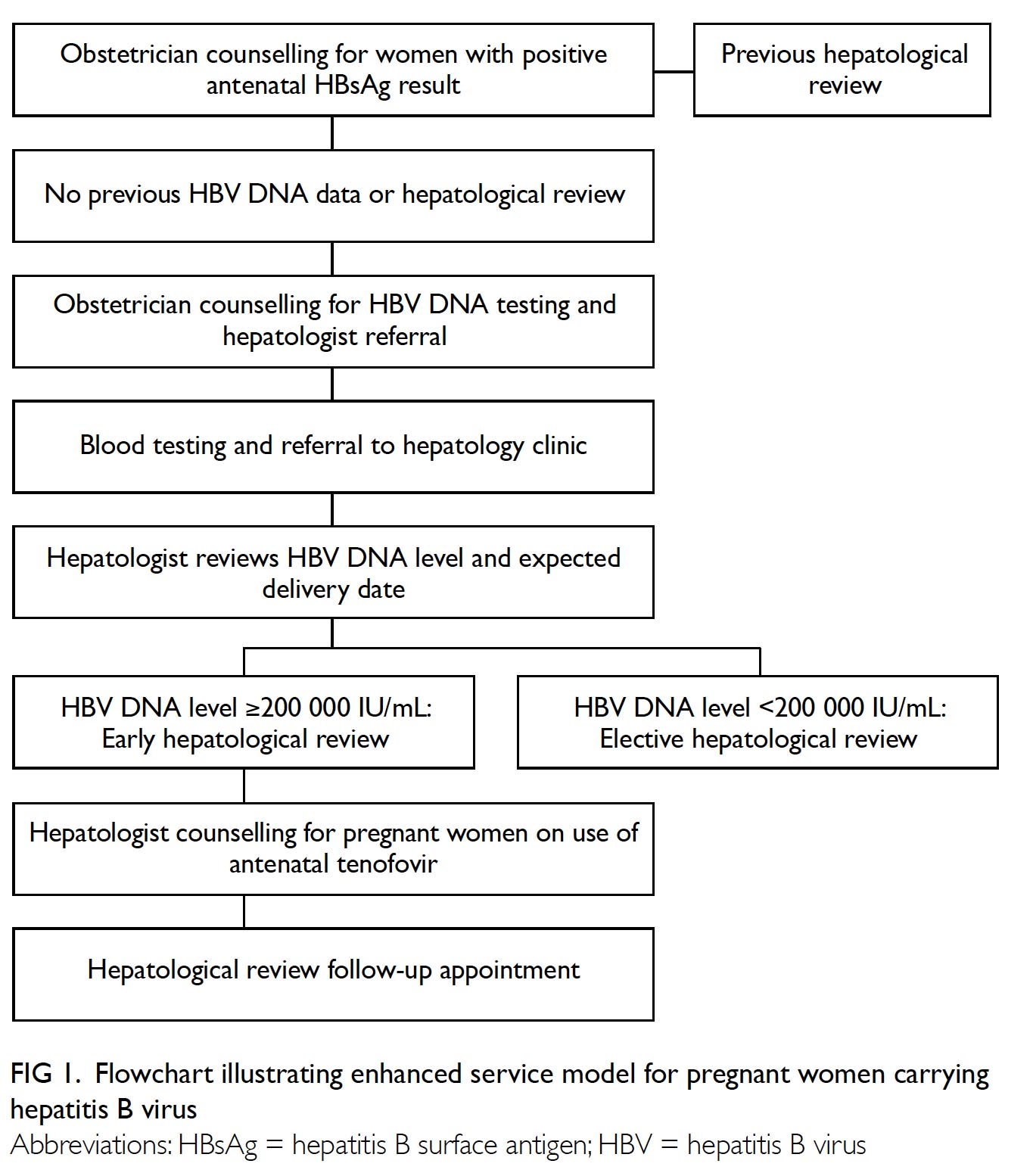
Figure 1. Flowchart illustrating enhanced service model for pregnant women carrying hepatitis B virus
Pregnant women with HBV DNA levels of
≥200 000 IU/mL were triaged by hepatologists for an
early clinic appointment, generally before 33 weeks
of gestation, to receive counselling regarding potential
antiviral treatment. Tenofovir disoproxil fumarate
(TDF) 300 mg daily was chosen for its potent
efficacy and risk of pre-existing or emergent resistant
mutants from previous lamivudine and telbivudine
treatments.10 11 12 Drug compliance and HBV DNA level
were monitored regularly. All other pregnant women
with viral loads of <200 000 IU/mL were also scheduled
for an elective long-term follow-up appointment.
Irrespective of viral load, all neonates born from
pregnant women with hepatitis B were administered
HBV vaccine and hepatitis B immunoglobulin within
12 hours of birth. The present study aimed to evaluate
the patients’ acceptance and outcome of this enhanced
service model for management of pregnant women
carrying hepatitis B.
Methods
This retrospective review included all women who
attended the antenatal clinic from 15 May 2017 to
31 December 2019. Information regarding hepatitis B
carrier status, HBV DNA blood test acceptance,
viral load, patient triage, and TDF acceptance
were retrieved from the antenatal record system
and clinical management system under the Hong
Kong Hospital Authority. The HBV DNA assays
were performed in Department of Medicine, The
University of Hong Kong.
Each patient’s HBV carrier status was
determined by an HBsAg test performed during
pregnancy. Hepatitis B virus DNA level was
considered high for viral loads of ≥200 000 IU/mL
and low for viral loads of <200 000 IU/mL. The
rate of antenatal acceptance of TDF was defined as
the proportion of women taking TDF in the group
with high viral loads who had been counselled by
hepatologists. Descriptive statistics are reported.
Results
Of 13 082 women who attended the antenatal
clinic from 15 May 2017 to 31 December 2019,
375 pregnant women had positive HBsAg screening
results; the carrier rate was 2.9%. In total, 102 (27.2%)
women had undergone HBV DNA testing or received
regular hepatological follow-up before pregnancy.
Blood tests for HBV DNA and hepatological reviews
were offered to 273 pregnant women. Two women
refused further assessment and four women did
not attend the blood test visit. Reasons for refusal
or non-attendance were not documented. Of the
four women who did not attend the blood test visit,
two were reminded of the need for a blood test at
subsequent antenatal visits, but did not complete the
tests. Overall, the acceptance rate for hepatological
review was 97.8% (267/273). Among the 267 women
who accepted hepatological reviews, blood tests were
not performed because of pregnancy termination
due to trisomy 21 (n=1) and miscarriage (n=1). Thus,
the final cohort comprised 265 pregnant women
who had HBV viral load results available for triage
assessment. The median gestational age at the time
of HBV testing was 17 weeks.
Sixty (22.6%) pregnant women were HBV
carriers with viral loads of ≥200 000 IU/mL; highest
level was 688 000 000 IU/mL. The median age of
women with high viral loads was not substantially
lower than that of women with low viral loads
(33 years vs 35 years; Wilcoxon rank sum test; not
significant).
Fifty eight of the 60 patients with high viral
loads were scheduled for hepatological review before
the expected date of delivery. First hepatological
review appointments were scheduled for 55 (91.7%)
women and 44 (73.3%) women at or before 32 and
28 weeks of gestation, respectively. Among the three
women who scheduled their first hepatological
review appointment after 32 weeks of gestation,
two delayed the referral submission and one had
attended the antenatal clinic at an advanced stage of
gestation (Fig 2).
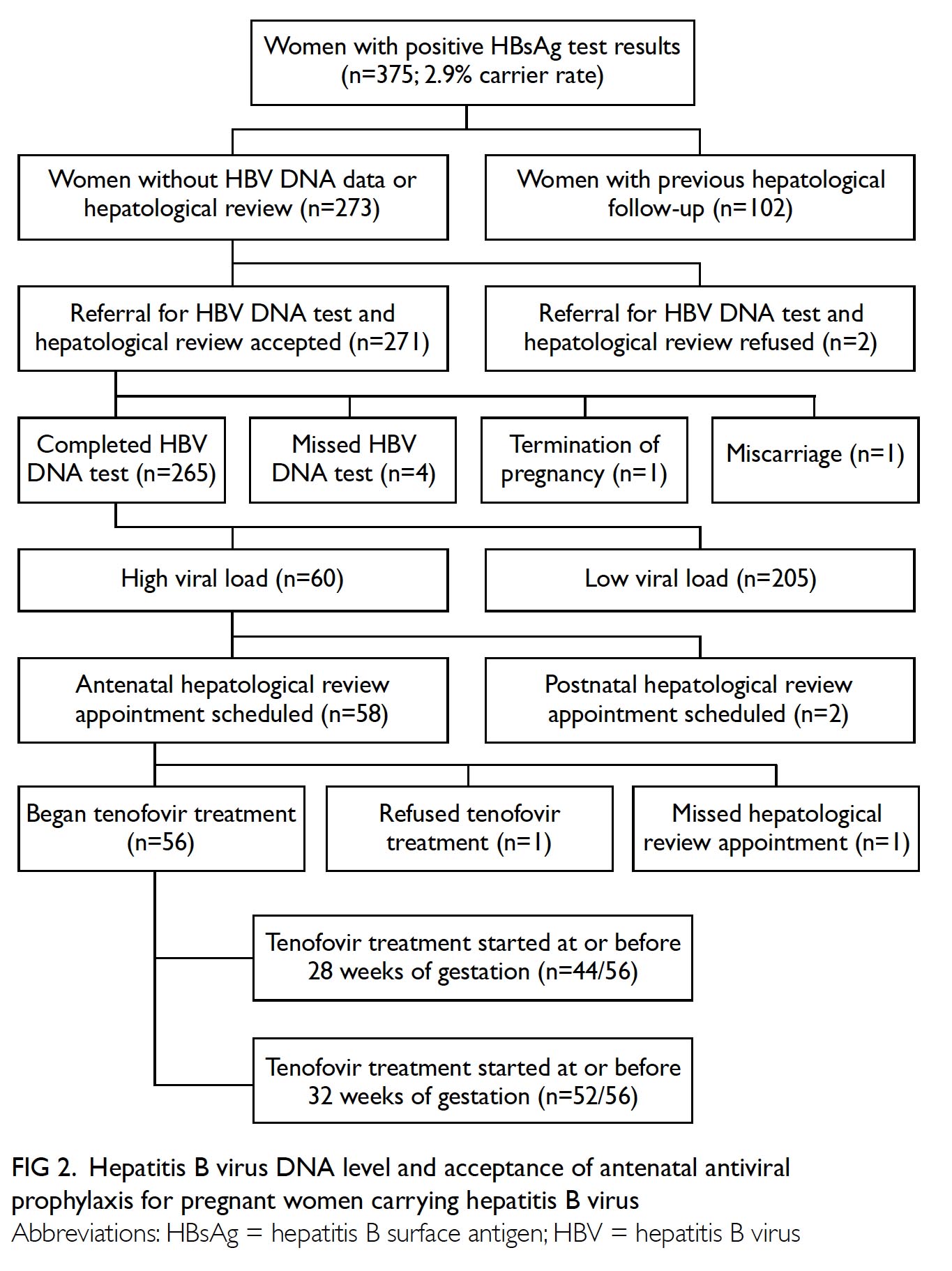
Figure 2. Hepatitis B virus DNA level and acceptance of antenatal antiviral prophylaxis for pregnant women carrying hepatitis B virus
One patient did not attend a hepatological
review appointment before 28 weeks of gestation.
The remaining 57 women with high viral loads
received antenatal counselling regarding TDF and
56 women agreed to take the drug, thereby
constituting an antenatal acceptance rate of 96.6% (56/58). The acceptance rates of TDF among women with high viral loads are shown in the Table.
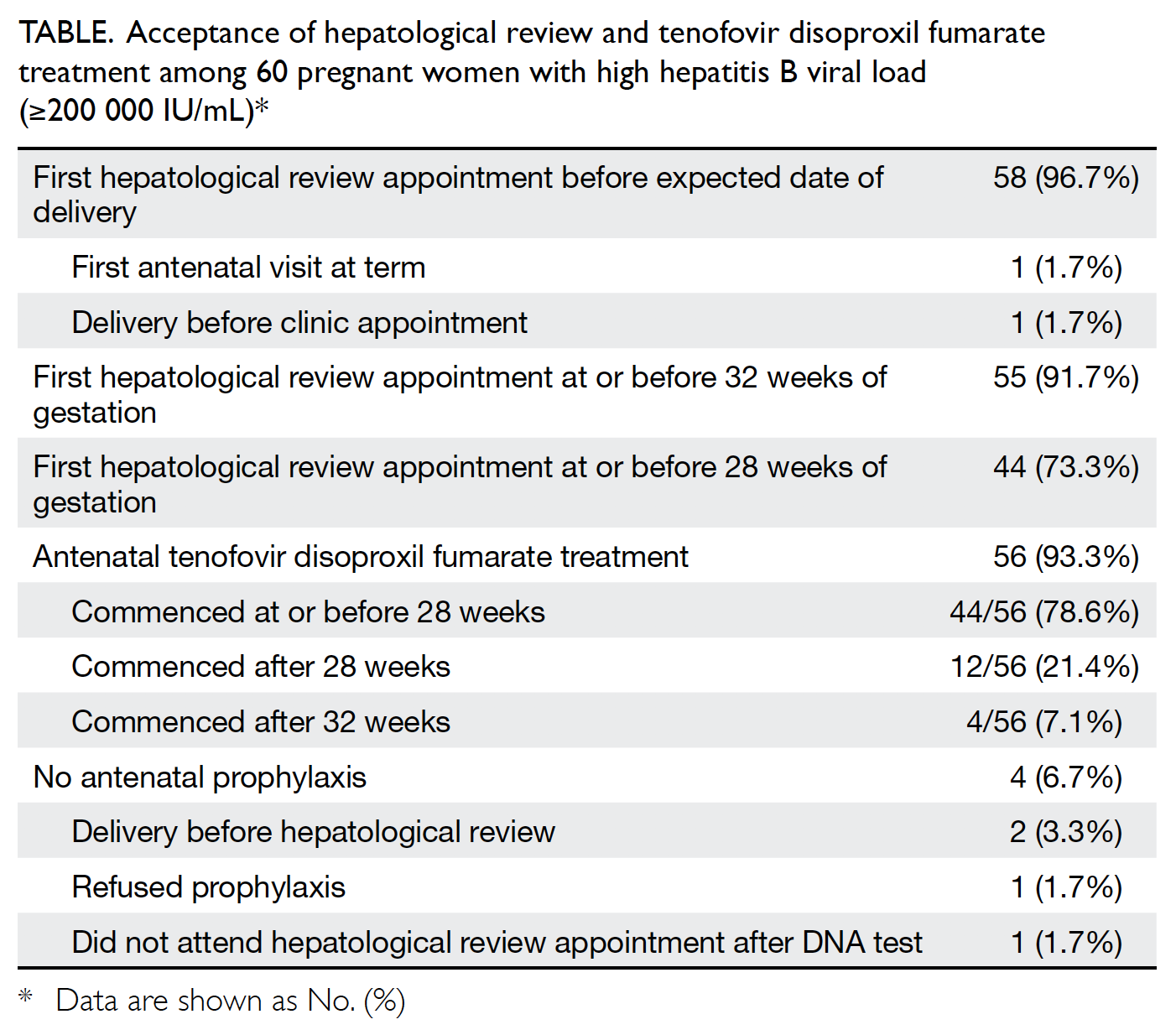
Table. Acceptance of hepatological review and tenofovir disoproxil fumarate treatment among 60 pregnant women with high hepatitis B viral load (≥200 000 IU/mL)
Treatment with TDF commenced at a median
gestational age of 26 weeks (range, 20-38 weeks).
Overall, 44 (78.6%) women received 300 mg daily
TDF at or before 28 weeks of gestation. Among
the 12 women who began TDF treatment after
28 weeks of gestation, four had deferred blood tests
for assessment of viral load, six had late antenatal
clinic appointments, one did not attend the initial
appointment at 28 weeks, and one attended the
clinic at 33 weeks of gestation. Overall, 52 (92.9%)
women commenced TDF treatment at or before
32 weeks of gestation.
Discussion
Screening for HBV carrier status is a universal
antenatal test in Hong Kong. Women who have
positive screening results are counselled regarding the risk of vertical transmission. In 1983, a neonatal
HBV vaccination programme was introduced in
Hong Kong to cover vaccination for first newborns of
carrier mothers. This became universal in November
1988; hepatitis B immunoglobulin and hepatitis B
vaccine have since been administered to all babies
born to mothers carrying HBV. These measures focus
mainly on postnatal neonatal immunoprophylaxis;
however, they lack a robust system for actively
reducing the antenatal risk of vertical transmission,
as well as a referral system that ensures long-term
hepatological follow-up for carrier mothers.
The present study demonstrated an effective
and acceptable approach involving HBV DNA testing
during triage of obstetric patients for prevention of
perinatal HBV transmission. Data from the Centre
of Health Protection have shown that the HBsAg
prevalence in pregnant women is decreasing, from
>10% in the early 1990s to 5.0% in 2017.13 The HBV
carrier rate in the present study (2.9%) was lower
than that previously reported in Hong Kong. Our
cohort included women with HBsAg who were tested
from May 2017 to December 2019; the low carrier
rate in the present study might reflect a continuous
reduction in overall HBsAg prevalence, due to
universal neonatal vaccination.14 However, more than
70% of the pregnant women in our cohort did not
have HBV viral load testing or regular hepatological
surveillance before pregnancy. This is an important
public health concern, because HBV carriers with
high viral loads are at higher risk of mother-to-child
transmission of HBV, as well as development of liver cirrhosis and hepatocellular carcinoma. With proper
antenatal education and general awareness, nearly
98% of the obstetric patients in our population
were willing to undergo self-financed HBV DNA
testing.
Viral load is a key factor that influences
immunoprophylaxis failure4; a higher risk of
immunoprophylaxis failure has been demonstrated
in women with viral loads of ≥200 000 IU/mL.
Positive HBeAg screening results, maternal age
<35 years, and body mass index ≤21 kg/m2 have
been associated with a higher mean viral load.15
Our study showed that 22.6% of women had viral
loads of ≥200 000 IU/mL, which was comparable
to previous findings. Although age was not a
statistically significant factor in the present study, a
previous study showed that women with high viral
loads were younger than women with low viral
loads.5 The prevention of perinatal transmission is
of considerable importance in achieving complete
eradication of HBV. Incorporation of HBV DNA
testing during pregnancy is a key element that can
facilitate identification of at-risk pregnant women
who may benefit from antenatal antiviral prophylaxis.
Ideally, both liver function test and HBeAg should be
assessed in pregnant women to determine their HBV
disease status; antiviral treatment may be initiated
for maternal indications. Although the presence of
HBeAg suggests a high risk of immunoprophylaxis
failure, HBeAg was not routinely assessed during
triage in the present cohort because it was not
regarded as an indicator of the need for antiviral
treatment to prevent vertical transmission.9 16 17
Additionally, HBV DNA quantification provides
a continuous assessment of risk according to viral
load, compared to the dichotomous result of HBeAg
screening. Therefore, HBV DNA level should be
used to identify women who should receive antiviral
treatment.18
Tenofovir disoproxil fumarate is the drug
of choice for antenatal prophylaxis because of
its potent effect and the possibility of mutants
resistant to lamivudine and telbivudine, due to prior
treatment with those drugs.11 12 19 Breastfeeding is
not contra-indicated for women who are taking TDF.
A highly promising rate of antenatal acceptance of
TDF (96.6%) was observed among women who had
undergone antenatal hepatological review. This
indicates the need for surveillance and the usefulness
of patient education during the antenatal period.
Although the optimal timing of antiviral
treatment remains controversial, randomised
controlled trials show that initiation of TDF during
the period between 28 and 32 weeks of gestation is
effective in reduction of immunoprophylaxis failure.
Earlier initiation of antiviral treatment is unnecessary,
because the immunoprophylaxis failure rate is not
appreciably reduced. Postponement of treatment to a point later than 32 weeks of gestation may result in
an insufficient duration of treatment and suboptimal
viral load reduction at delivery.10 20 Guidelines
from the American Association for the Study of
Liver Diseases and the Asian Pacific Association
for the Study of the Liver recommend initiation
of treatment at 28 to 32 weeks of gestation.9 21 The
present study demonstrated a feasible framework
for triage of nearly all pregnant women with high
viral loads before their dates of delivery. Nearly 93%
were able to initiate antiviral prophylaxis at or before
32 weeks of gestation; this could only be attained with
an appropriate hepatological review appointment
during pregnancy. The arrangement could be further
improved if women could attend antenatal visits
earlier during pregnancy, blood test logistics could
be simplified, and resources could be allocated more
effectively.
Conclusion
To the best of our knowledge, the multidisciplinary
efforts of obstetricians and hepatologists have
enabled Queen Mary Hospital to become the
first public hospital in Hong Kong with enhanced
antenatal management for pregnant women carrying
hepatitis B. The proportion of women with high viral
loads was comparable to the proportions in previous
studies. Our results indicated the usefulness of
HBV DNA blood tests in pregnant women and
high acceptance of antenatal antiviral treatment.
Triage according to HBV DNA level allowed early
hepatological review and commencement of antiviral
medication, thereby reducing the viral load at the
time of delivery and minimising the risk of vertical
transmission. This service model was adopted as a
framework for implementation of a fully funded
enhanced antenatal service to prevent mother-to-child
transmission of HBV in public maternity units,
commencing 1 January 2020.
Author contributions
Concept or design: PW Hui.
Acquisition of data: C Ng, PW Hui.
Analysis or interpretation of data: C Ng, PW Hui.
Drafting of the manuscript: PW Hui.
Critical revision of the manuscript for important intellectual content: KW Cheung, CL Lai.
Acquisition of data: C Ng, PW Hui.
Analysis or interpretation of data: C Ng, PW Hui.
Drafting of the manuscript: PW Hui.
Critical revision of the manuscript for important intellectual content: KW Cheung, CL Lai.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Mr John Yuen for performing HBV DNA analysis.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Institutional Review Board
of the University of Hong Kong / Hospital Authority West
Cluster (Ref UW 20-092).
References
1. World Health Organization. Global Health Sector
Strategy on viral hepatitis 2016-2021, towards ending viral
hepatitis. 2016. Available from: http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1. Accessed 2 Feb 2020.
2. Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC.
The influence of age on the development of the hepatitis B
carrier state. Proc Biol Sci 1993;253:197-201. Crossref
3. Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of
hepatitis B immunisation in newborn infants of mothers
positive for hepatitis B surface antigen: systematic review
and meta-analysis. BMJ 2006;332:328-36. Crossref
4. Cheung KW, Seto MT, Wong SF. Towards complete
eradication of hepatitis B infection from perinatal
transmission: review of the mechanisms of in utero
infection and the use of antiviral treatment during
pregnancy. Eur J Obstet Gynecol Reprod Biol 2013;169:17-23. Crossref
5. Cheung KW, Seto MT, Kan AS, et al. Immunoprophylaxis
failure of infants born to hepatitis B carrier mothers
following routine vaccination. Clin Gastroenterol Hepatol
2018;16:144-5. Crossref
6. Wen WH, Chang MH, Zhao LL, et al. Mother-to-infant
transmission of hepatitis B virus infection: significance
of maternal viral load and strategies for intervention. J
Hepatol 2013;59:24-30. Crossref
7. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic
factors associated with failure to passive-active
immunoprophylaxis in infants born to HBsAg-positive
mothers. J Viral Hepat 2012;19:e18-25. Crossref
8. European Association for the Study of the Liver. EASL
2017 Clinical Practice Guidelines on the management of
hepatitis B virus infection. J Hepatol 2017;67:370-98.
9. Terrault NA, Lok AS, McMahon BJ, et al. Update on
prevention, diagnosis, and treatment of chronic hepatitis
B: AASLD 2018 hepatitis B guidance. Hepatology
2018;67:1560-99. Crossref
10. Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis
B transmission in mothers with high viral load. N Engl J
Med 2016;374:2324-34. Crossref
11. Hyun MH, Lee YS, Kim JH, et al. Systematic review with
meta-analysis: the efficacy and safety of tenofovir to
prevent mother-to-child transmission of hepatitis B virus.
Aliment Pharmacol Ther 2017;45:1493-505. Crossref
12. Hu YH, Liu M, Yi W, Cao YJ, Cai HD. Tenofovir rescue
therapy in pregnant females with chronic hepatitis B.
World J Gastroenterol 2015;21:2504-9. Crossref
13. Viral Hepatitis Control Office, Special Preventive
Programme, Centre for Health Protection, Department of
Health, Hong Kong SAR Government. Surveillance of Viral
Hepatitis in Hong Kong—2017 Update Report. Available
from: https://www.chp.gov.hk/files/pdf/viral_hepatitis_report.pdf. Accessed 2 Feb 2020.
14. Lao TT, Sahota DS, Chan PK. Three decades of neonatal
vaccination has greatly reduced antenatal prevalence of
hepatitis B virus infection among gravidae covered by the
program. J Infect 2018;76:543-9. Crossref
15. Cheung KW, Seto MTY, So PL, et al. Optimal timing
of hepatitis B virus DNA quantification and clinical
predictors for higher viral load during pregnancy. Acta
Obstet Gynecol Scand 2019;98:1301-6. Crossref
16. Hu Y, Xu C, Xu B, et al. Safety and efficacy of telbivudine in
late pregnancy to prevent mother-to-child transmission of
hepatitis B virus: a multicenter prospective cohort study. J
Viral Hepat 2018;25:429-37. Crossref
17. Jourdain G, Ngo-Giang-Huong N, Harrison L, et al.
Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med 2018;378:911-23. Crossref
18. Cheung KW, Lao TT. Hepatitis B—Vertical transmission
and the prevention of mother-to-child transmission [in
press]. Best Pract Res Clin Obstet Gynaecol. In press.
19. Cheung KW, Seto MT, Lao TT. Prevention of perinatal
hepatitis B virus transmission. Arch Gynecol Obstet
2019;300:251-9. Crossref
20. Yang X, Zhong X, Liao H, Lai Y. Efficacy of antiviral
therapy during the second or the third trimester for
preventing mother-to-child hepatitis B virus transmission:
a systematic review and meta-analysis. Rev Inst Med Trop
Sao Paulo 2020;62:e13. Crossref
21. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical
practice guidelines on the management of hepatitis B: a
2015 update. Hepatol Int 2016;10:1-98. Crossref

