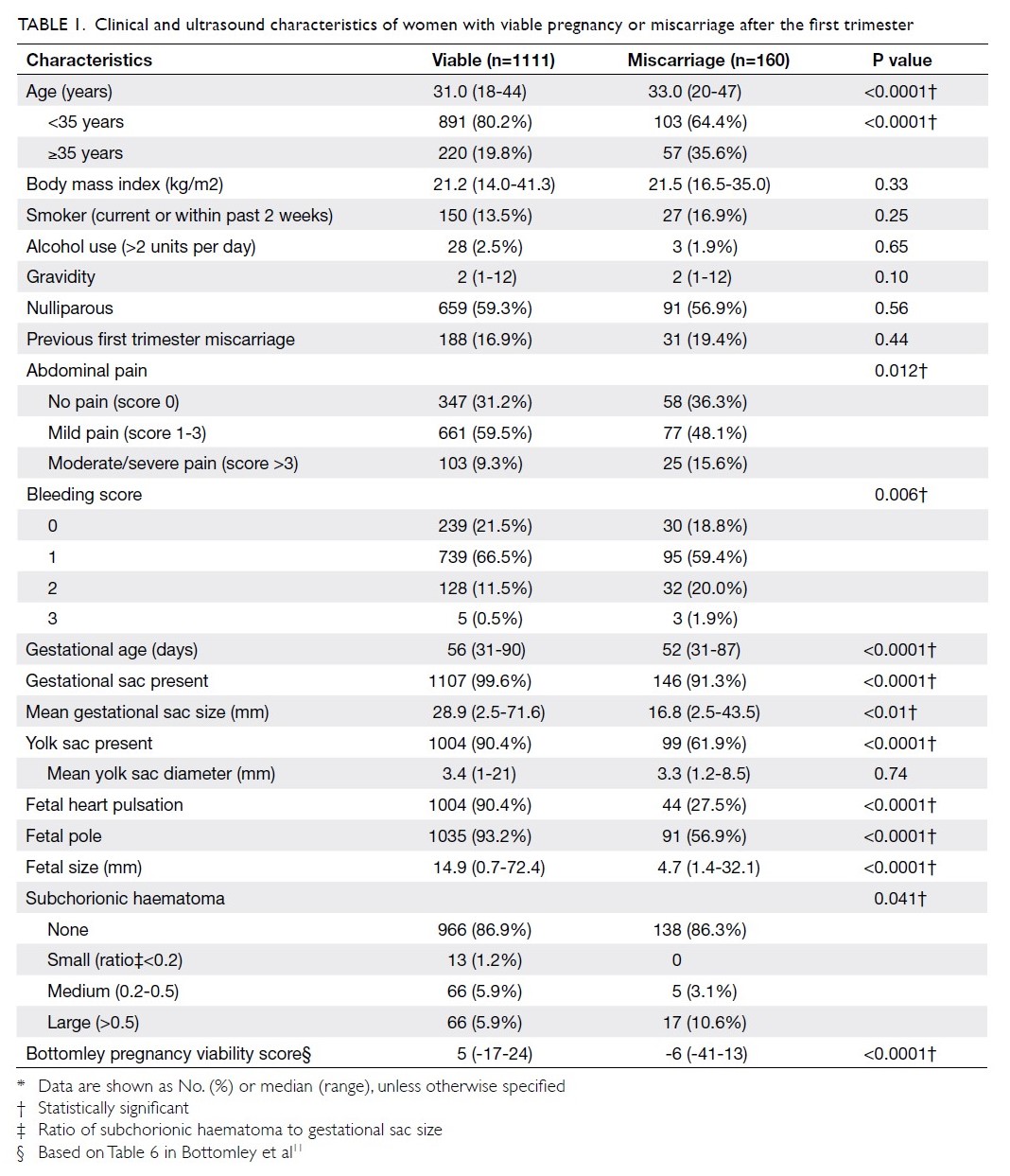
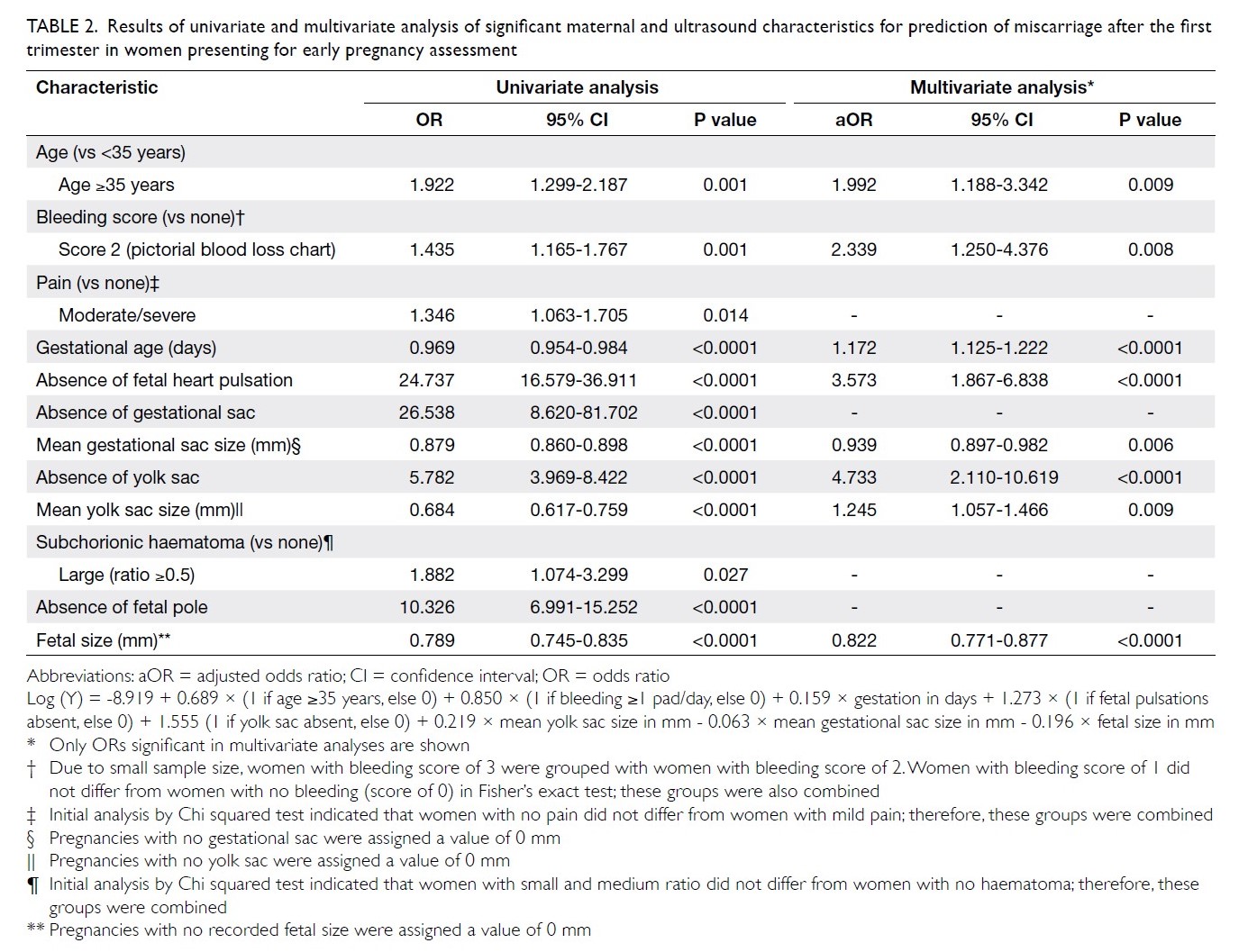
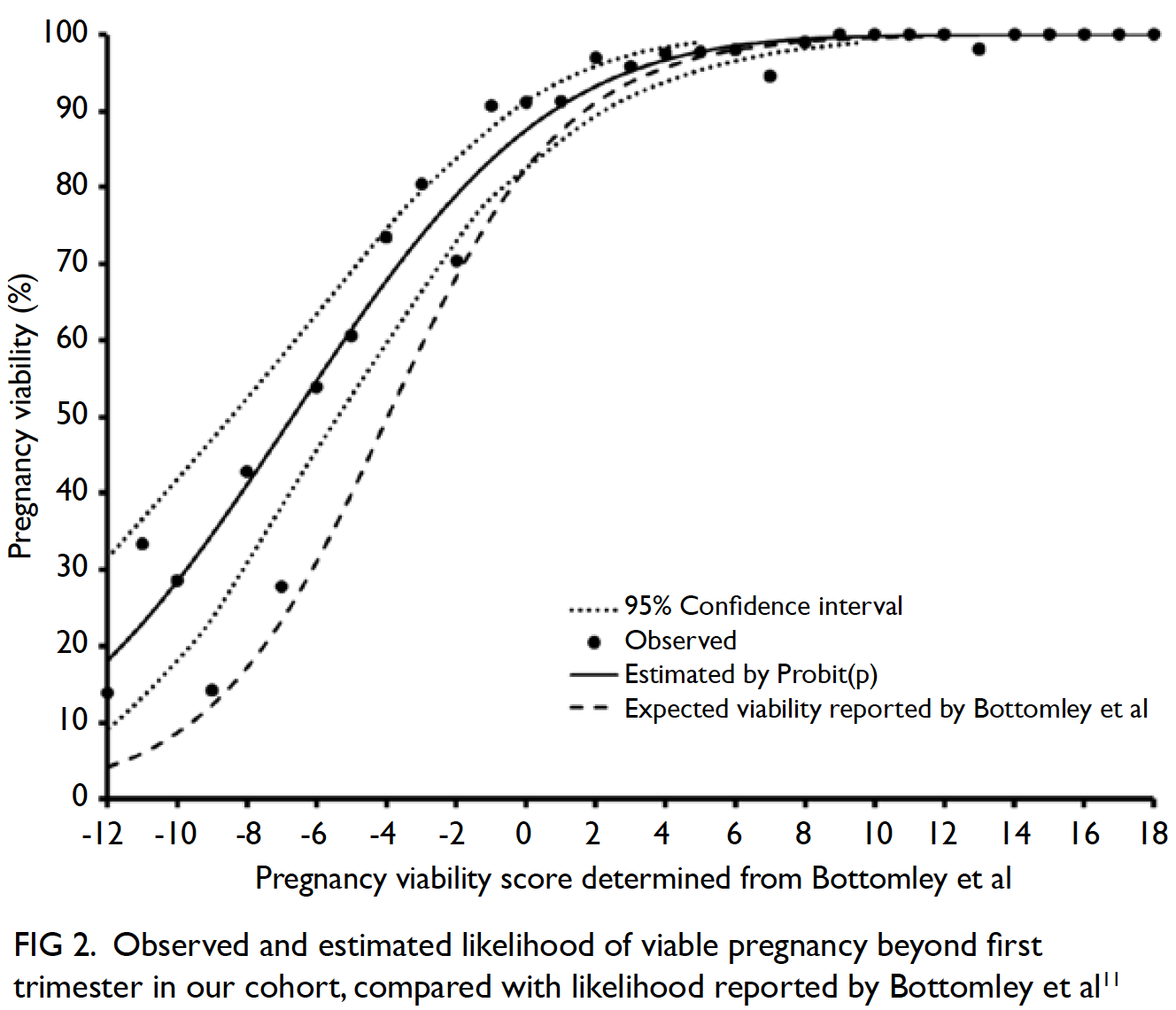
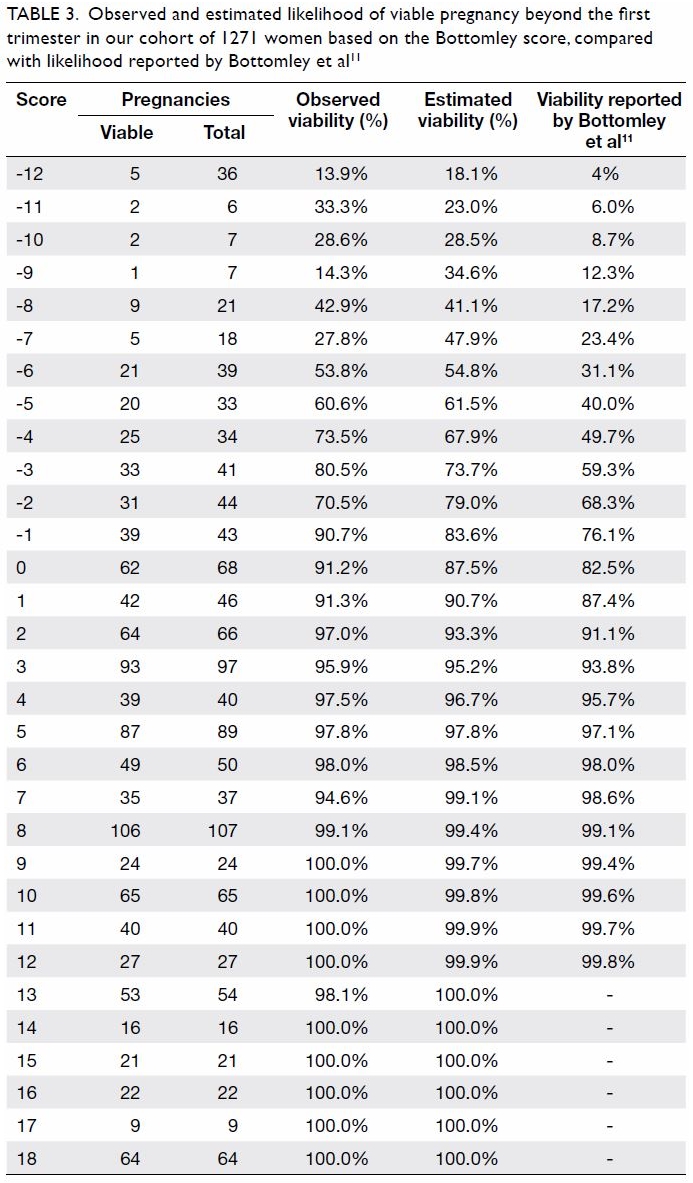
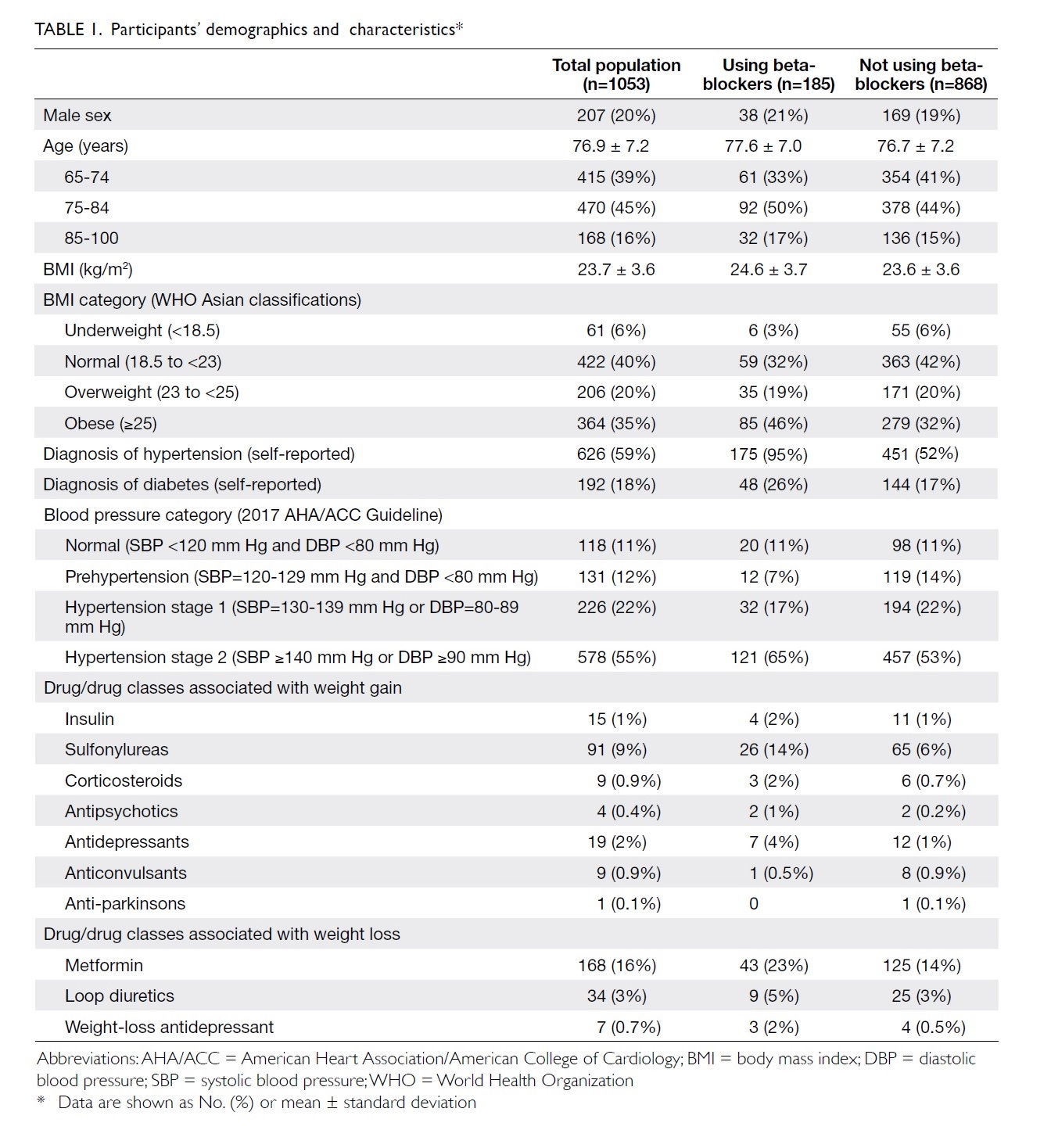
Blood transfusions in total knee arthroplasty: a retrospective analysis of a multimodal patient blood management programme
Hong Kong Med J 2020 Jun;26(3):201–7 | Epub 6 May 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Blood transfusions in total knee arthroplasty: a
retrospective analysis of a multimodal patient blood management programme
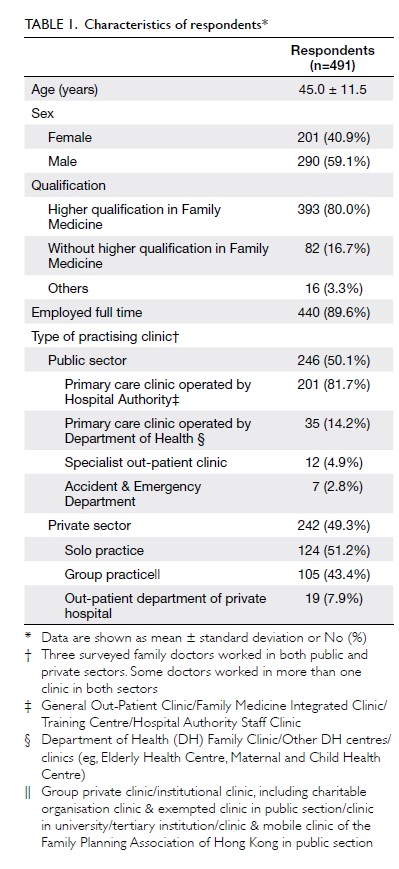
PK Chan, FHKCOS, FHKAM (Orthopaedic Surgery)1; YY Hwang, FHKCP, FHKAM (Medicine)2; Amy Cheung, FHKCOS, FHKAM (Orthopaedic Surgery)1; CH Yan, FHKCOS, FHKAM (Orthopaedic Surgery)1; Henry Fu, FHKCOS, FHKAM (Orthopaedic Surgery)1; Timmy Chan, FHKCA, FHKAM (Anaesthesiology)3; WC Fung, BSc1; MH Cheung, FHKCOS, FHKAM (Orthopaedic Surgery)1; Vincent WK Chan, FHKCOS, FHKAM (Orthopaedic Surgery)1; KY Chiu, FHKCOS, FHKAM (Orthopaedic Surgery)1
1 Department of Orthopaedics and Traumatology, Queen Mary Hospital, The University of Hong Kong, Hong Kong
2 Department of Medicine, Queen Mary Hospital, Hong Kong
3 Department of Anaesthesiology, Queen Mary Hospital, Hong Kong
Corresponding author: Dr PK Chan (cpk464@yahoo.com.hk)
Abstract
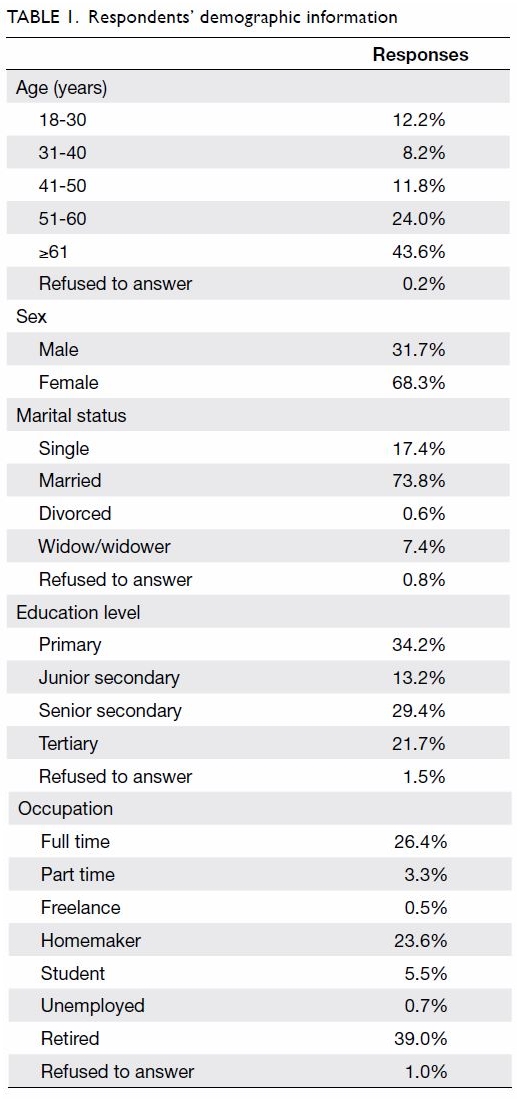
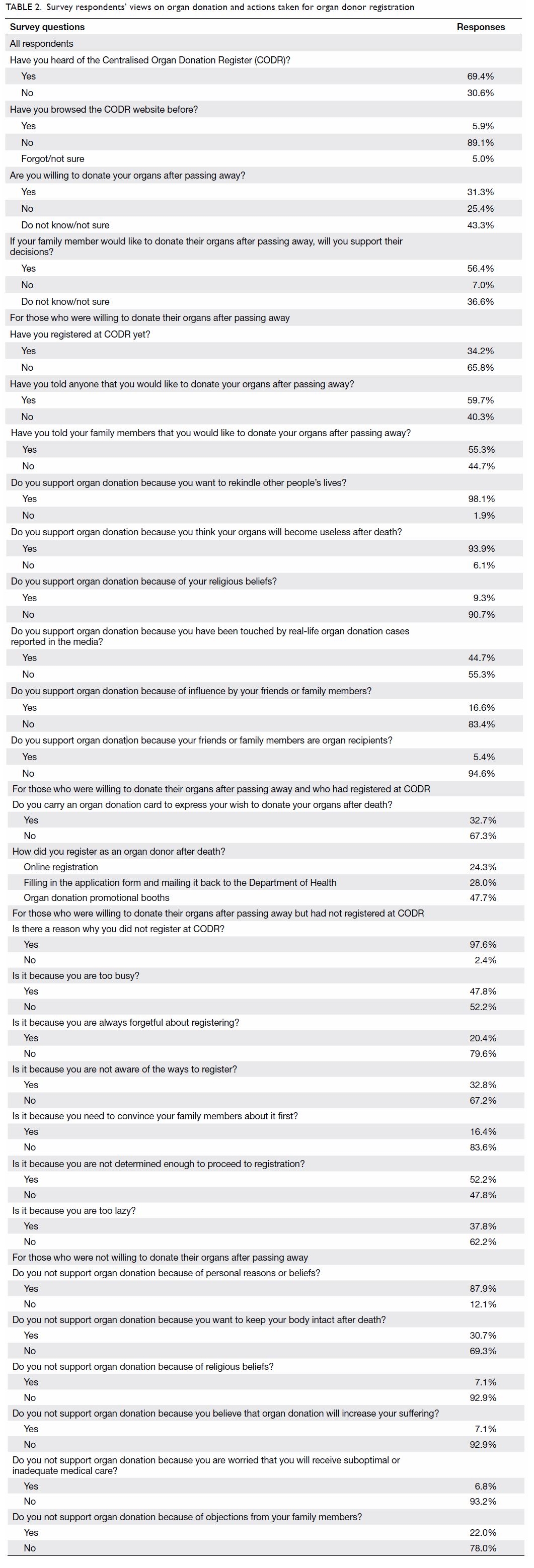
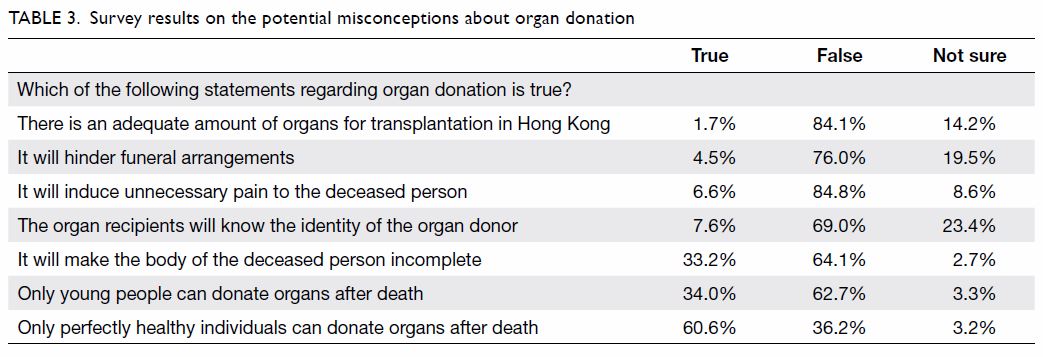
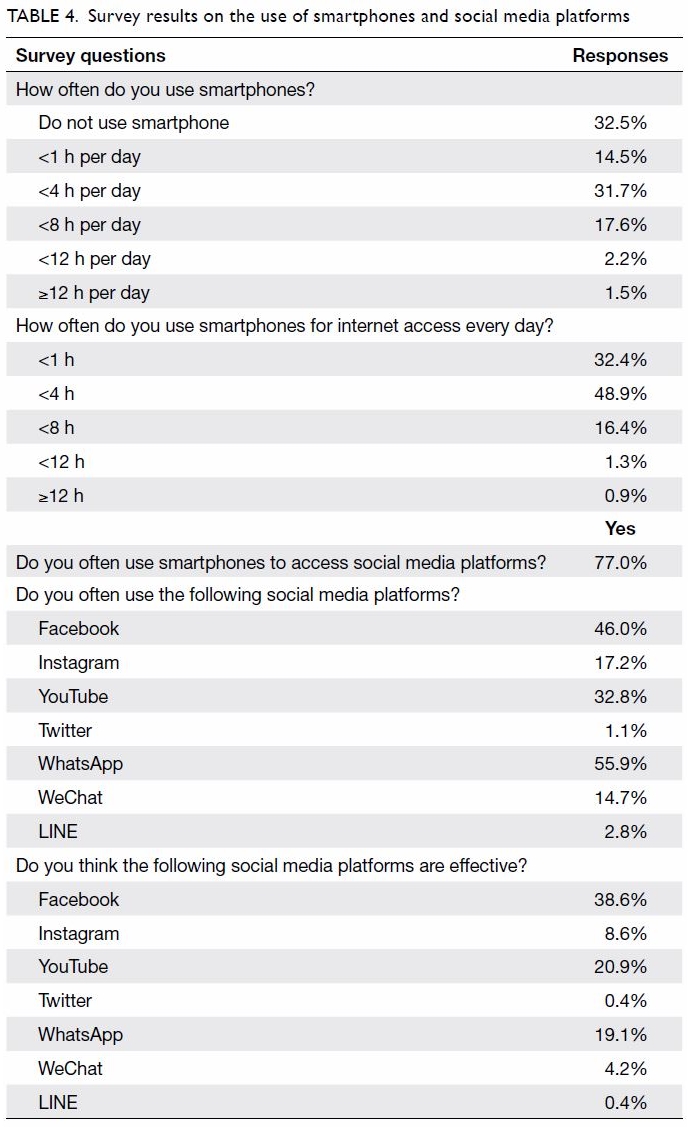
Purpose: Transfusion is associated with increased
perioperative morbidity and mortality in patients
undergoing total knee arthroplasty (TKA). Patient
blood management (PBM) is an evidence-based
approach to maintain blood mass via haemoglobin
maintenance, haemostasis optimisation, and blood
loss minimisation. The aim of the present study was
to assess the effectiveness of a multimodal PBM
approach in our centre.
Methods: This was a single-centre retrospective
study of patients who underwent primary TKA
in Queen Mary Hospital in Hong Kong in 2013 or
2018, using data from the Clinical Data Analysis and
Reporting System and a local joint registry database.
Patient demographics, preoperative haemoglobin,
length of stay, readmission, mean units of
transfusion, postoperative prosthetic joint infection,
and mortality data were compared between groups.
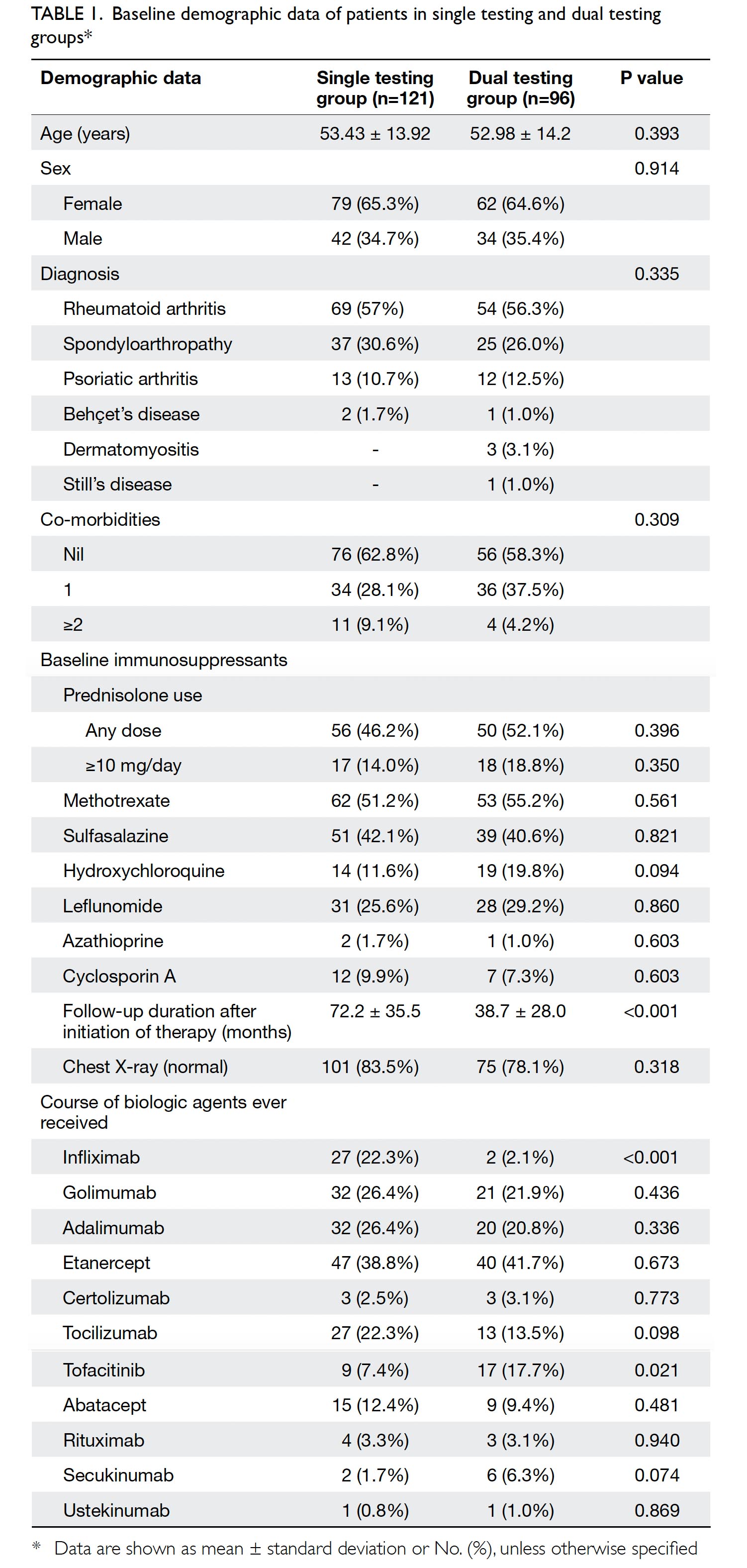
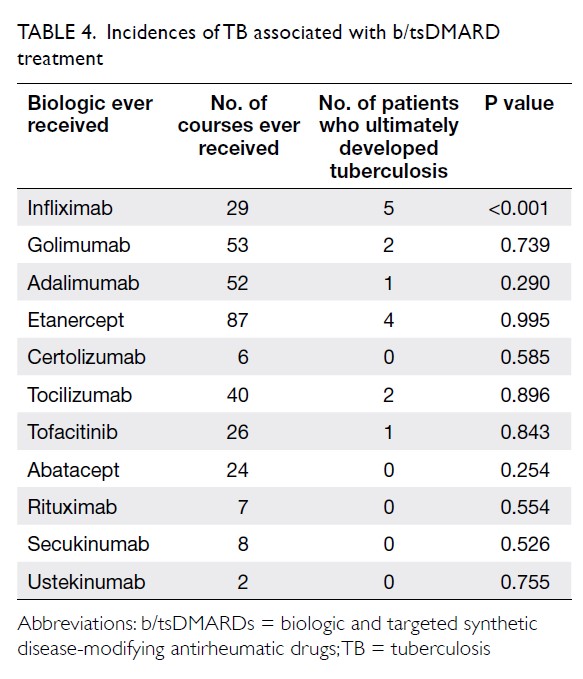
Results: In total, 262 and 215 patients underwent
primary TKA in 2013 and 2018, respectively. The
mean transfusion rate significantly decreased after
PBM implementation (2013: 31.3%; 2018: 1.9%,
P<0.001); length of stay after TKA also significantly
decreased (2013: 14.49±8.10 days; 2018: 8.77±10.14
days, P<0.001). However, there were no statistically
significant differences in readmission, early prosthetic joint infection, or 90-day mortality rates between the two groups.
Conclusion: Our PBM programme effectively
reduced the allogeneic blood transfusion rate
in patients undergoing TKA in our institution.
Thus, PBM should be considered in current TKA
protocols to reduce rates of transfusions and related
complications.
New knowledge added by this study
- Patient blood management effectively reduced the allogeneic blood transfusion rate in patients undergoing total knee arthroplasty in our institution; it also reduced the length of stay after total knee arthroplasty.
- Patient blood management should be considered in current total knee arthroplasty protocols to reduce rates of transfusions and related complications.
- Patient blood management in total knee arthroplasty could reduce healthcare expenditures among the ageing population in Hong Kong.
Introduction
Total knee arthroplasty (TKA) is the most effective
and efficacious surgical method to improve pain and
function for patients with end-stage osteoarthritis;
however, TKA has been associated with substantial
blood loss. In addition to visible blood loss from the
surgical field and wound drainage, hidden blood
loss occurred in patients undergoing TKA, which
resulted in mean blood loss of 1.5 L.1 Therefore, perioperative blood transfusion was needed in up to
38% of patients undergoing TKA.2
Blood transfusion is not risk-free. Often, no
adverse effects are encountered by patients who
undergo blood transfusion. However, adverse effects
occasionally occur, ranging from minor allergic
reactions to blood-borne infection and potentially
fatal acute immune haemolytic reaction. With the
implementation of best international practices to warrant blood transfusion safety by the Blood
Transfusion Service in Hong Kong, the transfusion
risk has significantly decreased in the past two
decades.3 However, absolute safety in transfusion
cannot be achieved because of the window period
for detecting infections, possibility of emerging
infections, and potential human errors related to the
process of transferring collected blood from donors
to transfusion recipients. Notably, researchers in
Hong Kong reported the first two cases (worldwide)
of transmission of Japanese encephalitis virus,
via blood transfusion to immunocompromised
hosts, in 2018.4 In addition to the general risks
associated with transfusion, blood transfusion has
been independently associated with poor surgical
outcome. Specifically, patients who underwent
transfusion exhibited an eight-fold to 10-fold excess
risk of adverse outcomes, defined as postoperative
complications in the American College of Surgeons
National Surgical Quality Improvement Project.5
With respect to total hip or knee arthroplasty, a
dose-dependent relationship between transfusion
and risk of surgical site infection was observed.6
With increasing understanding regarding the
benefits and risks of blood transfusion, as well as
alternative approaches for patients who experience
blood loss, the concept of patient blood management
(PBM) was developed. The World Health
Organization defines PBM as ‘a patient-focused,
evidence-based and systematic approach to optimise
the management of patients and transfusion of blood products for quality and effective patient care. It is
designed to improve patient outcomes through the
safe and rational use of blood and blood products
and by minimising unnecessary exposure to blood
products…’.7 The three major components of PBM
are as follows: (1) optimisation of the patient’s own
blood mass; (2) minimisation of blood loss; and (3)
optimisation of physiological tolerance to anaemia.8
This new standard of care is now well-established
in some centres in the US, Austria, and Western
Australia, as well as nationally in the Netherlands.
However, PBM remains an uncommon practice in
Asia.
We introduced the PBM programme for
patients undergoing TKA in our institution,
beginning in 2014. Various components were
introduced gradually (in phases) from 2014 to 2018.
The key measures of PBM in preoperative, intra-operative,
and postoperative periods were fully
implemented in 2018. To the best of our knowledge,
our PBM programme is a pioneer PBM programme
in Hong Kong. The aim of the present study was
to assess the effectiveness of the multimodal PBM
approach in our university-based centre.
Methods
This single-centre retrospective study was approved
by the Institutional Review Board of the University
of Hong Kong/Hospital Authority Hong Kong West
Cluster (Ref UW 19-600). The requirement for
patient consent was waived by the review board.
We retrospectively collected blood transfusion
data regarding patients who underwent unilateral
primary TKA in our centre from 1 January 2013 to
31 December 2018. Patients who underwent one-stage
bilateral primary TKA or revision TKA were
excluded from our study. Patients with acquired or
congenital coagulopathy, as well as those currently
taking anticoagulants, were included in our study;
notably, these patients were at greater risk of
perioperative blood loss and transfusion.
The primary outcome measure was the mean
yearly transfusion rate, which was defined as the
number of patients who received transfusion after
TKA (during the same hospitalisation episode)
divided by the number of patients who underwent
TKA during the period from 1 January to 31
December; this result was multiplied by 100. The
mean units of blood given per transfusion episode
in 1 year was defined as the cumulative total number
of blood units transfused to patients after TKA in 1
year divided by the number of transfusion episodes
in that year. Transfusion data were retrieved from
the Clinical Data Analysis and Reporting System,
a database developed by the Hong Kong Hospital
Authority for research purposes; this database
contains medical information recorded by the Hong
Kong Hospital Authority since 1993.
Secondary outcome measures were mean
length of hospital stay after surgery, the rate of
unexpected readmission through the Emergency
Department after discharge from the hospital, the
proportion of patients who had early prosthetic
joint infection requiring revision surgery within 90
days after index surgery, and the 90-day mortality
rate. These data and other patient demographic data
(eg, age and sex), perioperative data (eg, American
Society of Anesthesiologists [ASA] physical status),
and preoperative haemoglobin level were retrieved
from our patient records, as well as a local joint
replacement registry.
Patient blood management was a relatively new
concept in Hong Kong when it was first implemented
in our institution in 2014. Initially, this programme
was a standalone surgeon-initiated programme
without external support; it was implemented in
sequential stages based on PBM guidelines provided
by the National Blood Authority in Australia.9
The sequential stages of PBM implementation
in our centre are described in Table 1. The PBM
strategies included modern surgical, anaesthetic,
and perioperative care. In 2014, PBM was initiated
with instructions regarding proper indications
for transfusion, single-unit transfusion policy,10
and restrictive transfusion policy with transfusion
triggered at haemoglobin ≤8 g/dL in healthy
individuals.11 In 2015, the traditional practice of
routine placement of a surgical drain during TKA
(associated with a higher transfusion rate12) was
stopped; the use of topical tranexamic acid (injection
of 1 g tranexamic acid into knee joint at the end of the
surgical procedure, shown to reduce postoperative
blood loss and transfusion rate13) was implemented
to reduce perioperative blood loss. In 2016,
preoperative anaemia screening and optimisation
were initiated via collaboration with haematologists.
Patients with preoperative haemoglobin <11 g/dL
were examined for causes of anaemia, in accordance
with Network for Advancement of Transfusion
Alternatives guidelines14; their erythropoiesis
and preoperative haemoglobin characteristics
were optimised before TKA was performed. For
example, patients with iron-deficiency anaemia
were checked for gastrointestinal blood loss and
prescribed iron supplementation; their haemoglobin
levels were rechecked after supplementation to
confirm achievement of ≥11 g/dL before TKA.
To further reduce intra-operative blood loss,
combined intravenous tranexamic acid (15 mg/kg
administered intravenously at the induction of
anaesthesia) and topical tranexamic acid were
implemented; these are reportedly effective for
reduction of blood loss.15 In 2017, a more stringent
restrictive transfusion policy was adopted with
transfusion triggered at haemoglobin ≤7 g/dL in
healthy individuals. Moreover, active warming (a modern anaesthetic technique used during intra-operative
care) was implemented to avoid intra-operative
hypothermia16; this hypothermia has been
associated with greater volume of blood loss and the
need for transfusion.17 18 By the beginning of 2018, all
above PBM strategies were fully implemented.
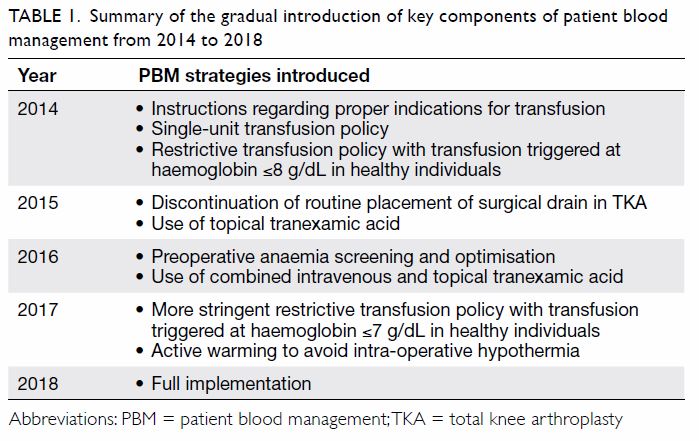
Table 1. Summary of the gradual introduction of key components of patient blood management from 2014 to 2018
In addition to PBM strategies, other changes
in patient selection and perioperative management
were implemented between 2013 and 2018. First, the
degree of medical co-morbidities may have differed
because of the establishment of another joint
replacement centre in July 2016; this new centre
provided joint replacement surgeries for patients
with improved medical fitness, among patients with
end-stage osteoarthritis in our hospital. Therefore,
the medical co-morbidities of the patients in 2013
and 2018 were compared on the basis of ASA status.
Second, because of technological advances in the
design of TKA prostheses over time, there were
differences in the numbers of modern-design TKA
prostheses between 2013 and 2018; these modern-design
TKA prostheses aimed to improve knee
kinematics, rather than reduce transfusion rate.
However, these prostheses were unlikely to bias
our transfusion rate results, according to a prior
assessment of factors predictive of transfusion rate
in patients undergoing TKA.19
In 2013, no PBM strategies had been
implemented in our institution, whereas all strategies
had been fully implemented by 2018. The Chi
squared test was used to compare the transfusion
rate between patients who underwent TKA in 2013
and those who underwent TKA in 2018; differences
with P<0.05 were considered statistically significant.
As mentioned above, medical co-morbidities of the
patients in 2013 and 2018 were compared on the
basis of ASA status.
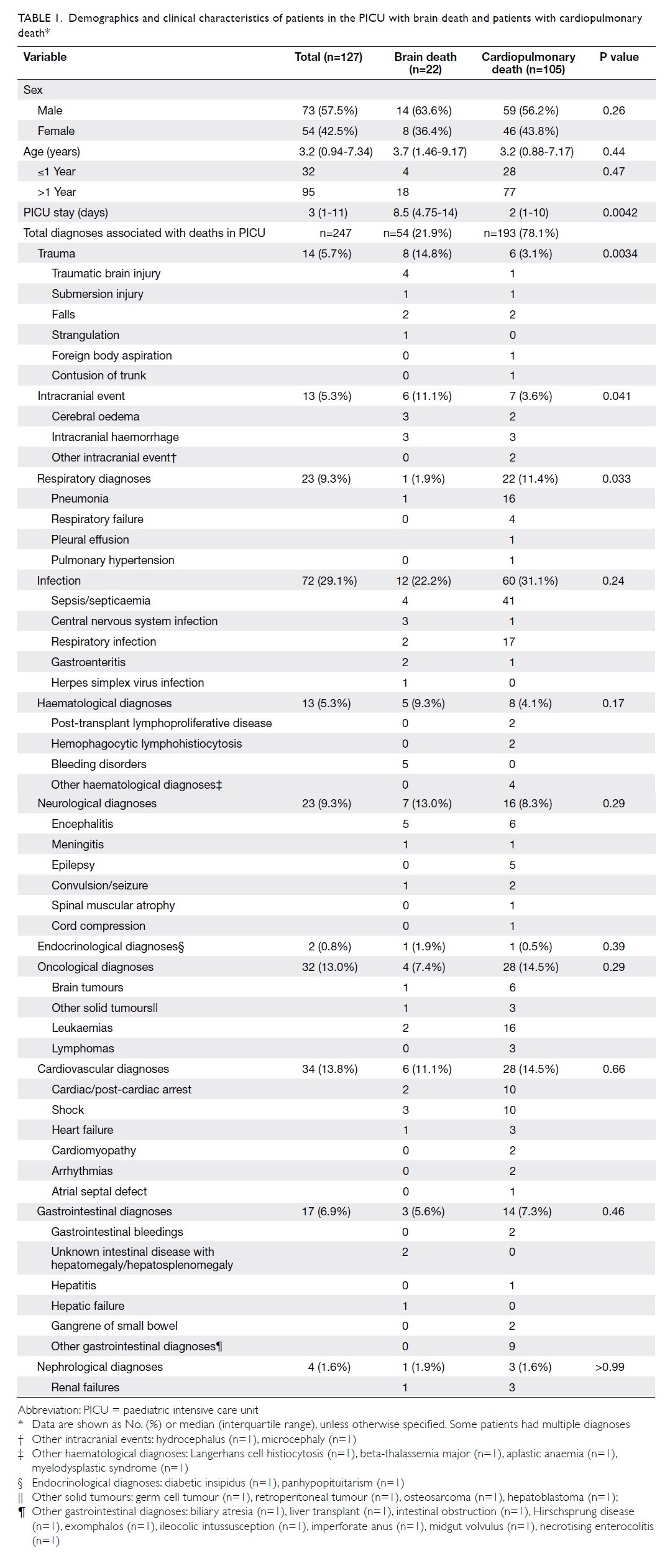
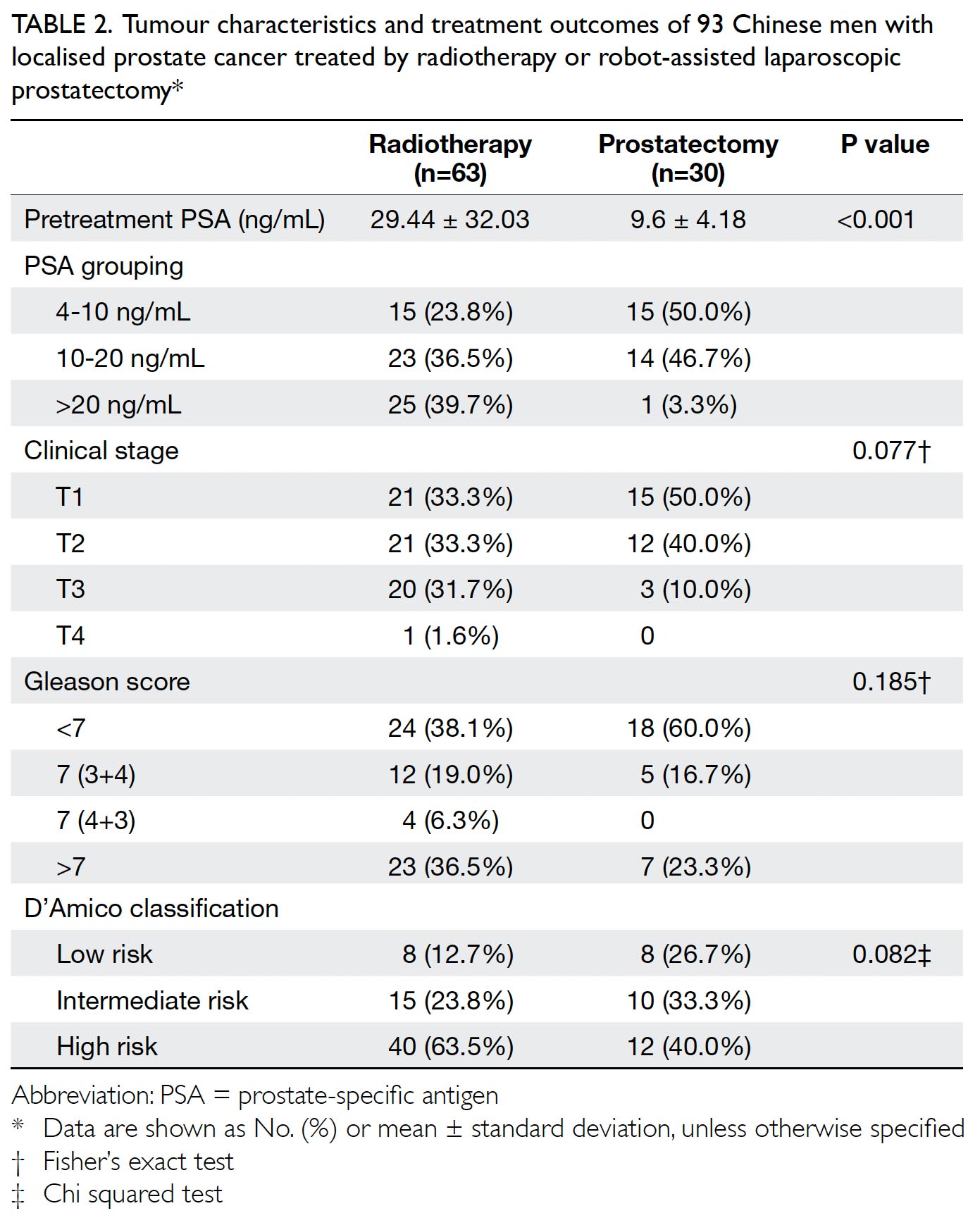
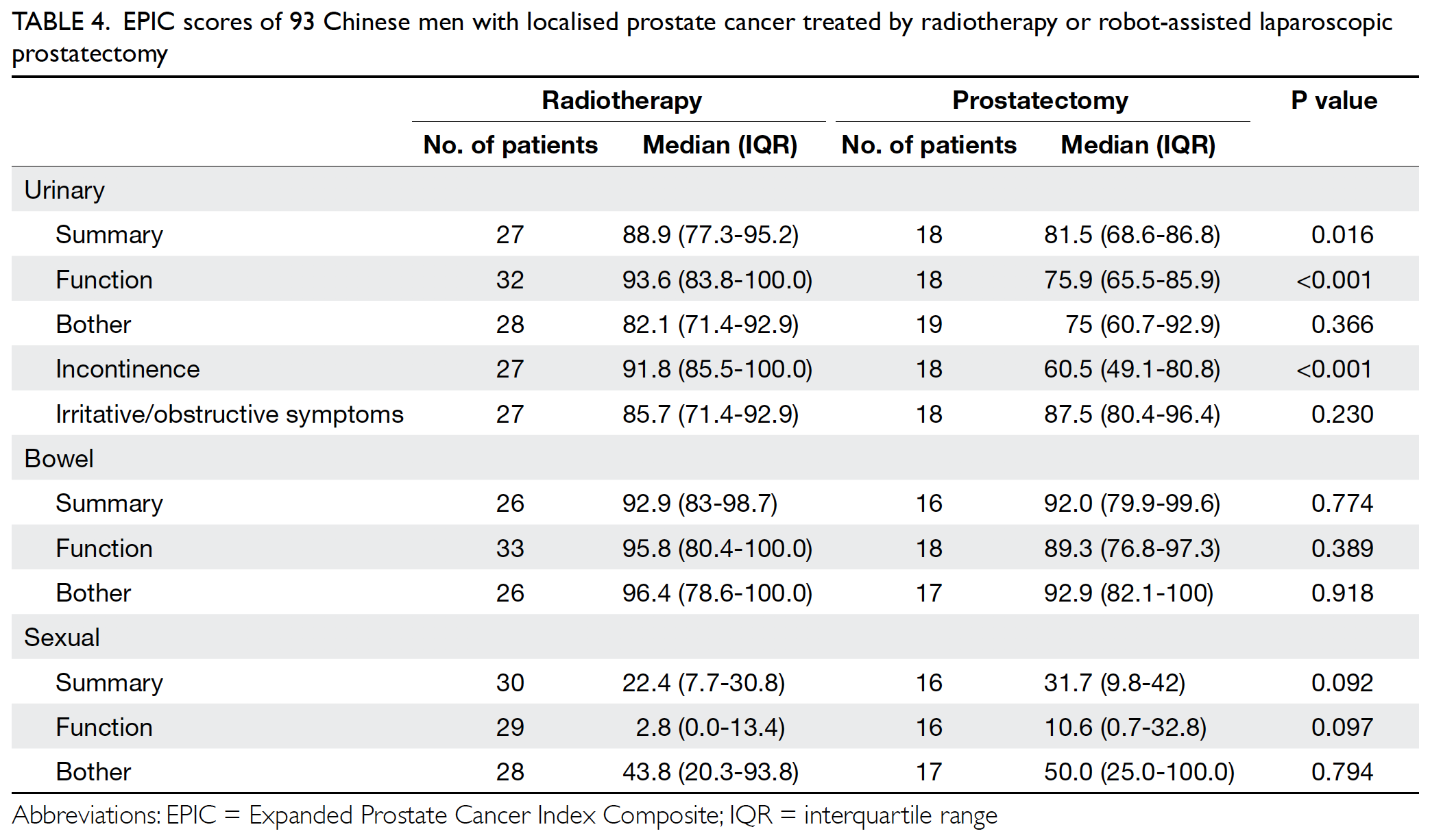
Results
In total, 262 and 215 patients underwent primary
TKA in our centre in 2013 and 2018, respectively
(Table 2). There were no significant differences in
mean age (2013: 72.17±9.76 years; 2018: 72.49±9.27
years, P=0.71) or sex (male:female) ratio (2013, 61:201; 2018, 63:152, P=0.14) between the groups. The
preoperative haemoglobin level was also similar
between the groups (2013: 12.77±1.42 g/dL;
2018: 12.89±1.42 g/dL, P=0.35). However, there was
a significant difference in ASA distribution between
the groups (P=0.03). There was a comparatively
greater proportion of patients with ASA Grade II
status in 2018 (2013: 57.6%; 2018: 68.8%).
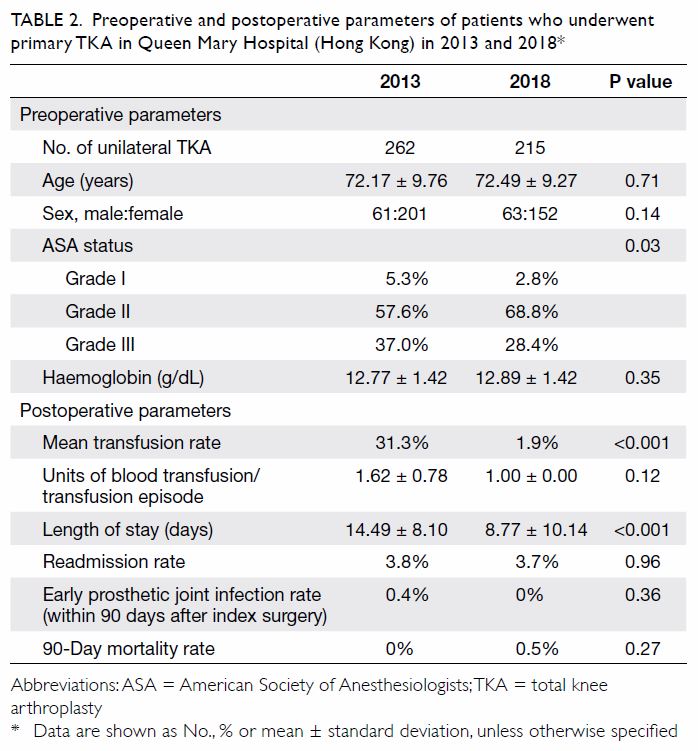
Table 2. Preoperative and postoperative parameters of patients who underwent primary TKA in Queen Mary Hospital (Hong Kong) in 2013 and 2018
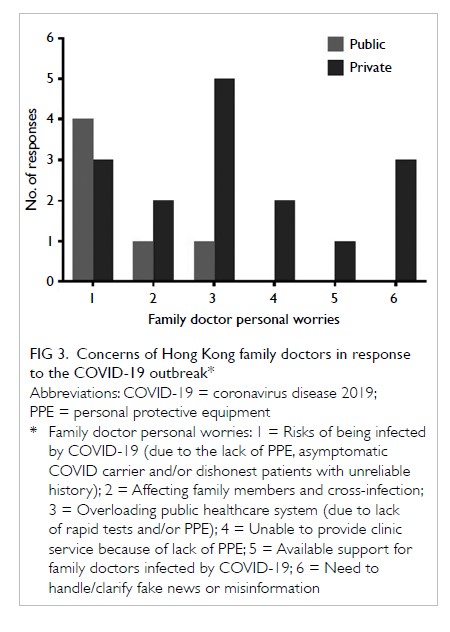
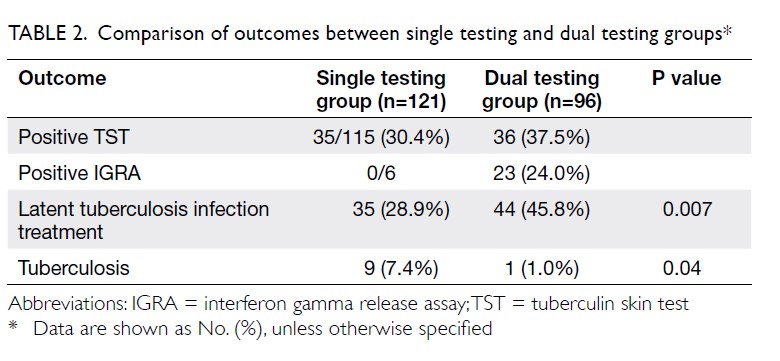
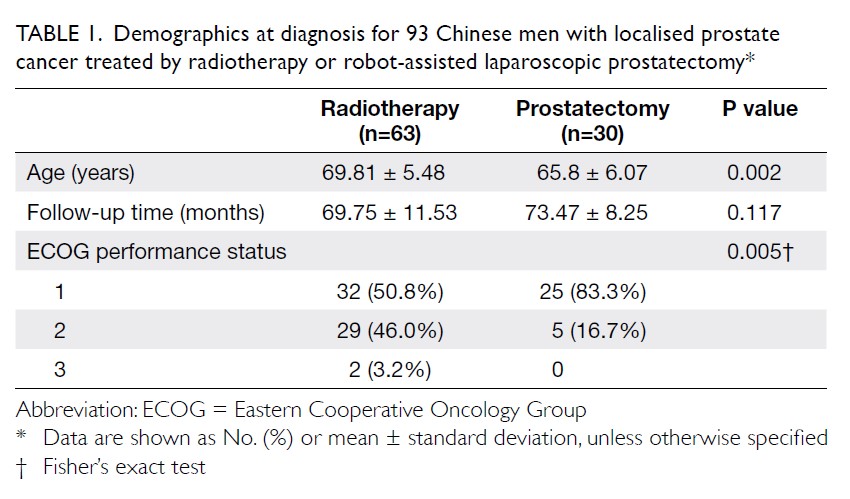
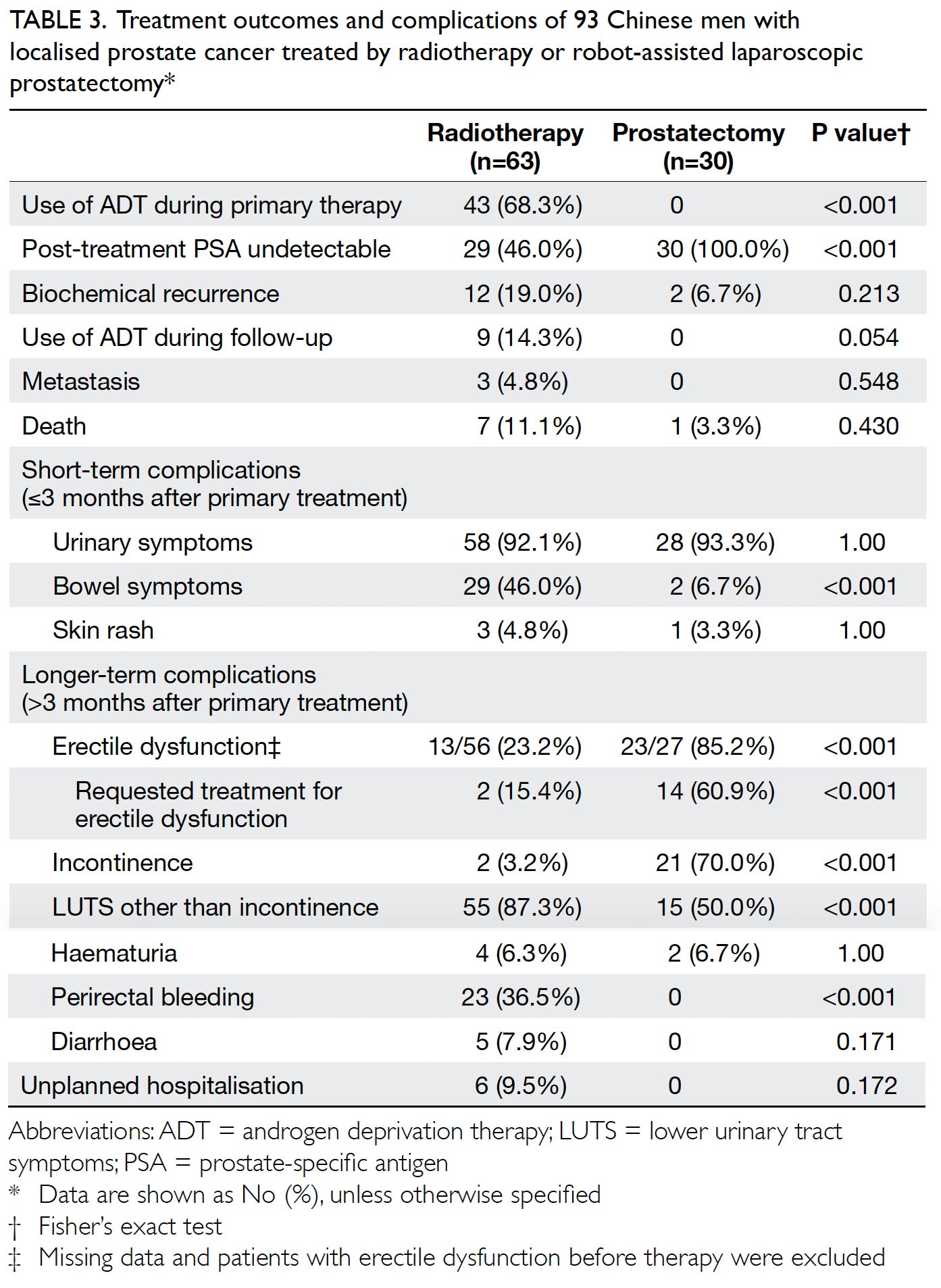
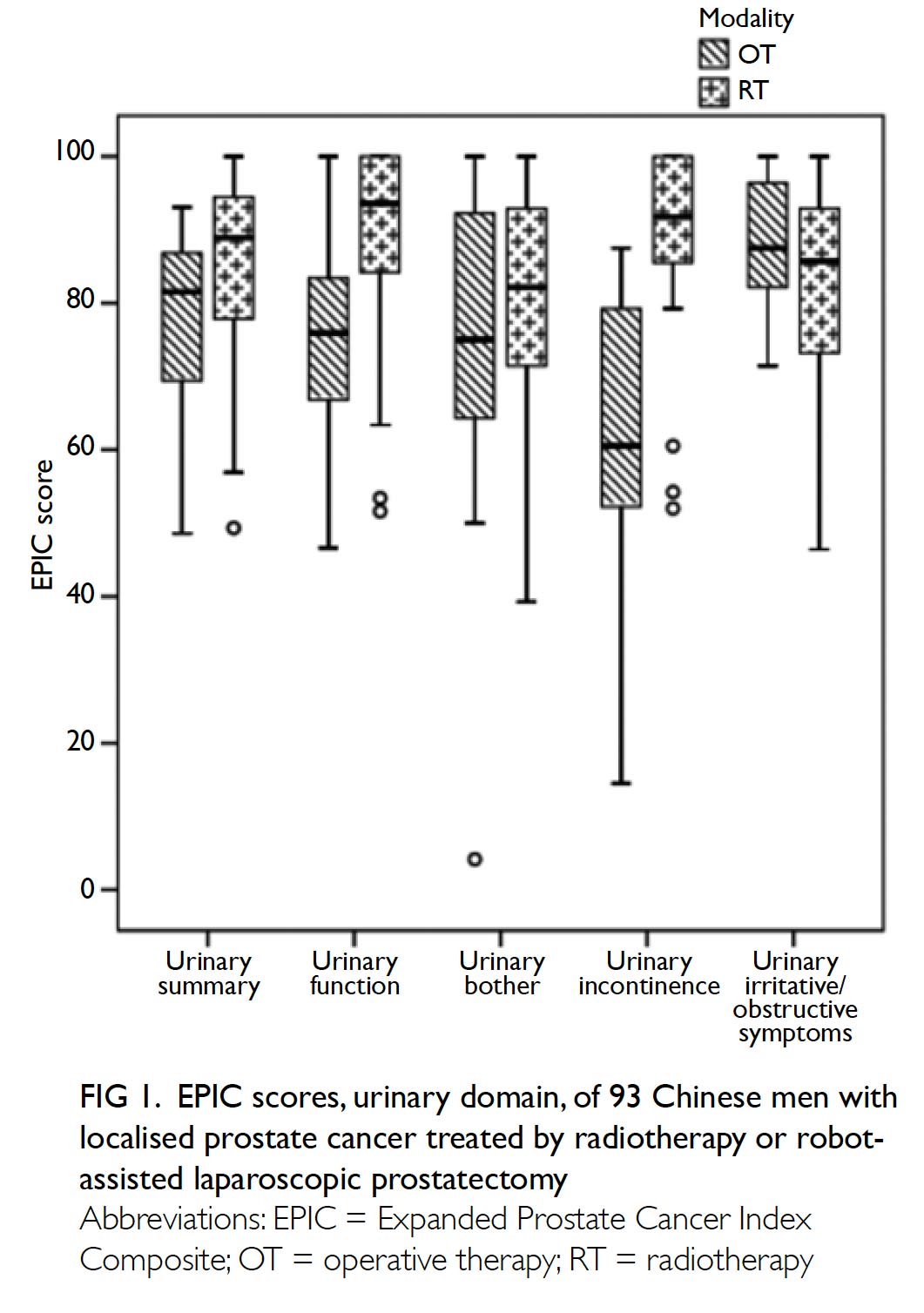
The primary outcome was the mean transfusion
rate in 1 year. There was a significant difference in the
mean transfusion rate after primary TKA between
2013 and 2018 (2013: 31.3%; 2018: 1.9%, P<0.001);
however, there was no significant difference in the
mean units of blood transfused per transfusion
episode (2013: 1.62±0.78; 2018: 1.00±0.00, P=0.12).
Moreover, the mean annual transfusion rate after
primary TKA exhibited stepwise reduction as PBM
strategies were implemented during the period from
2014 to 2018 (Fig).
Regarding secondary outcomes, the mean
length of hospitalisation was significantly lower
in 2018 (2013: 14.49±8.10 days; 2018: 8.77±10.14
days, P<0.001). However, there was no difference
in the unexpected readmission rate through the
Emergency Department (2013: 3.8%; 2018: 3.7%,
P=0.96), the proportion of patients who exhibited
early prosthetic joint infection within 90 days after
index surgery (2013: 0.4%; 2018: 0%, P=0.36), or the
proportion of patients with 90-day mortality (2013:
0%; 2018: 0.5%, P=0.27).
Discussion
Blood transfusion is a life-saving therapy, but is a limited resource. In recent years, there have been
recurrent blood shortages in Hong Kong, and the
Hong Kong Red Cross has issued an urgent appeal for
blood donors on several occasions.20 21 22 23 The amount of
blood stored in blood banks is determined by supply
and demand. To increase blood supply, additional
blood donors are needed. The Annual Report of Hong
Kong Red Cross in 2018/2019 revealed that 4% of
blood donors were aged >60 years, and the largest
group of blood donors were aged 41-50 years (23.7%
of donors).24 With the increasing number of older
people in Hong Kong, more blood donors are needed
from older age-groups. In addition, healthcare
professionals should be judicious in prescribing
transfusion, and should consider methods to
minimise transfusion. Our study demonstrated the
effectiveness of implementing PBM in our centre. In
addition to comparing the mean transfusion rate in
2013 and 2018, this study included an audit of the
mean annual transfusion rate after primary TKA
from 2014 to 2018 (Fig).
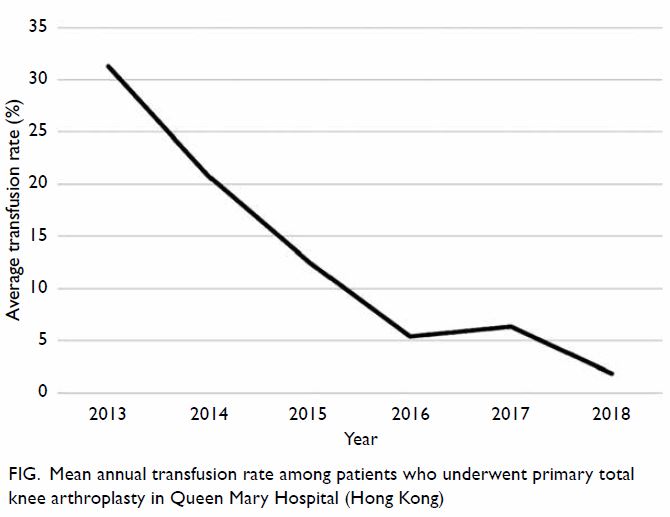
Figure. Mean annual transfusion rate among patients who underwent primary total knee arthroplasty in Queen Mary Hospital (Hong Kong)
Globally, PBM is not a new concept. In May 2010,
the World Health Organization formally recognised
the importance of PBM and recommended its use
to the 193 member states.25 Subsequently, PBM has been successfully implemented in Western
countries, especially Australia26 and the US.27 The
Asia-Pacific PBM Expert Consensus Meeting
Working Group assessed the status of PBM in Asia.28
In Singapore, PBM was implemented nationally,
beginning in 2013. The Ministry of Health and Blood
Services Group actively promoted PBM at public
hospitals in the first few years; regular national
audits of PBM-related efforts have been performed
since 2017 to promote appropriate use of red blood
cell transfusion and implementation of preoperative
anaemia screening for elective surgeries. Patient
blood management programmes were successfully
implemented in National University Hospital and
Singapore General Hospital.28 29 In Korea, PBM was
implemented through a professional initiative by the
Korean Research Society of Transfusion Alternatives
of the Republic of Korea in 2006; the Korean Patient
Blood Management Research Group was formed to
promote greater PBM use in 2016. The efforts of the
Korean Patient Blood Management Research Group
resulted in achievement of several PBM milestones
in 2016.28 Notably, PBM was included in the Korean
Transfusion Guidelines; a new steering committee
was also formed, comprising leading physicians from
various specialties. Patient blood management was
successfully implemented in a number of Korean
hospitals, which led to a reduction in transfusion
rate.30 31 32 In Malaysia, PBM has been promoted at the
local hospital level.28 In the Department of Maternal
and Fetal Medicine at the Sultan Haji Ahmad Shah
Hospital, women at high risk of anaemia are screened
for iron deficiency anaemia in early pregnancy; iron-deficient
women are provided oral or intravenous
iron supplementation.
At our institution, PBM was first implemented
in 2014 as a surgeons’ initiative in the Division of
Joint Replacement Surgery. In addition to good
surgical techniques, good perioperative care is an
important determinant of surgical outcomes. Patient
blood management is a component of our overall
perioperative management protocol in the modern
enhanced recovery after surgery programme. With
implementation of PBM strategies and measures in
the enhanced recovery after surgery programme,
the length of hospitalisation was shortened in 2018,
compared with 2013. Despite the shorter length of
stay, there were no differences in the unexpected
readmission rate through the Emergency
Department, the proportion of patients who had
early prosthetic joint infection within 90 days after
index surgery, or the proportion of patients who had
90-day mortality.
To the best of our knowledge, there have been
few studies regarding PBM in patients undergoing
TKA in Hong Kong. In 2015, Lee et al33 reported
their pioneering experience with implementation
of PBM in patients undergoing TKA; their PBM protocols included typing and screening only for
patients with preoperative haemoglobin of <11 g/dL,
and restrictive transfusion triggered at haemoglobin
8 g/dL. When they compared outcomes before
and after introduction of the PBM programme, the
transfusion rate (before: 10.3%; after: 3.1%, P=0.046)
and cross-match rate (before: 100%; after: 3.1%,
P<0.001) both decreased. We implemented PBM in
our institution, beginning in 2014. Modern surgical,
anaesthetic, and perioperative techniques in PBM
were gradually introduced from 2014 to 2018. Our
PBM protocols are more comprehensive than those
of Lee et al, because our protocols were designed in
accordance with the PBM guidelines provided by the
National Blood Authority in Australia.9 Therefore, our
transfusion rate in TKA in 2018 decreased to 1.9%.
Prosthetic joint infection is a severe
complication in arthroplasty; affected patients often
require revision surgery. Notably, patients who
underwent treatment for prosthetic joint infection
exhibited a significant, independent risk of increased
mortality, due to the direct adverse effect of infection
and the indirect effect of poor underlying health
condition.34 In a recent meta-analysis involving
21 770 patients who underwent total hip and knee
arthroplasty, patients who received allogeneic blood
transfusion had a significantly greater risk of surgical-site
infection (pooled odds ratio: 1.71, P=0.02).35
Recently, a dose-dependent relationship was
observed between allogeneic transfusion and surgical
site infection after total hip or knee arthroplasty.6
Therefore, PBM was expected to reduce the incidence
of surgical site infection in our centre. However,
because of the relatively small sample size in our
study and the relatively low incidence of prosthetic
joint infection (approximately 1%), a difference in
the incidence of prosthetic joint infection between
2013 and 2018 could not be identified. When PBM
was fully implemented in our centre (2018), the
transfusion rate after primary TKA was 1.9%; this
was comparable to international reported values.
Specifically, when PBM strategies were implemented
in the US, the transfusion rates were approximately
4.5%.36 When PBM is implemented by high-volume
surgeons with an eight-step checklist to reduce
bleeding, the transfusion rate after TKA could be as
low as 0.0044%.37 Therefore, a transfusion rate of 0%
is achievable.
As the transfusion rate decreased in patients
undergoing TKA, there were also benefits to the
healthcare system. Blood transfusion involves many
costs associated with blood transfer from donors
to recipients (eg, collection, screening, storage,
transportation, and prescription of donated blood).
We do not have data regarding the cost of packed
red blood cells in Hong Kong; however, the cost was
estimated to be approximately 1130 USD/unit in a
study conducted in the US.36 Therefore, reduced transfusions through implementation of PBM can
result in lower healthcare expenditures, which are of
considerable importance because of the increasing
demand for TKA among the aging population in
Hong Kong.
There were some limitations in this study. First,
it was a retrospective study; thus, compliance with
PBM strategies could not be fully verified. However,
as each strategy was introduced throughout the
course of the study, there was gradual reduction in
the transfusion rate. Therefore, compliance with the
strategies was presumably optimal. Second, because
different strategies were implemented successively,
the strategies with the greatest contribution to the
reduced transfusion rate could not be identified.
Third, because this was not a prospective randomised
placebo-controlled interventional trial, a causal
relationship between PBM strategies and reduction
in transfusion rate could not be established.
However, our study provided an assessment of real-world
implementation of PBM strategies within a
large hospital; thus, it comprises pioneering research
in Hong Kong. Fourth, some potential cofounding
factors may not have been identified or controlled
in the present analysis. For example, the type of
prosthesis used was not analysed as a separate factor.
However, preoperative haemoglobin levels (the
most significant predictor of blood transfusion19)
were compared between both groups. To the best
of our knowledge, there remains minimal relevant
literature regarding the effect of TKA prosthesis on
the transfusion rate.
In conclusion, our results demonstrated the
effectiveness of PBM implementation on transfusion
rate in patients undergoing TKA. From 2014 to 2018,
there was a stepwise reduction in transfusion rate
after TKA; this was similar to findings in previously
published research. This is one of the few studies
in Hong Kong to review PBM in surgical practice.
Although we focused on patients undergoing TKA,
the principles of PBM could be useful for other
medical or surgical specialties.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of data, analysis or interpretation of data, drafting
of the manuscript, and critical revision of the manuscript for
important intellectual content.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgements
We thank Dr CK Lee, Chief Executive and Medical Director, Hong Kong Red Cross Blood Transfusion Service, Hong
Kong, for providing advice on the patient blood management
programme. We also acknowledge and express our gratitude to the following departments of our hospital for the support in this multidisciplinary project: Nursing Division, Department of Orthopaedics and Traumatology; Operation Theatre Services; and Blood Transfusion Committee.
Declaration
This research has been presented in part as an oral presentation
at the Hong Kong Hospital Authority Convention 2018.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong
West Cluster (Ref UW 19-600). The requirement for patient
consent was waived by the review board.
References
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee 2000;7:151-5. Crossref
2. Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clin Orthop Relat Res 1991;(269):98-101. Crossref
3. Lee CK. Risk minimization in transfusion transmitted infection in Hong Kong [thesis]. University of Hong Kong; 2017.
4. Cheng VC, Sridhar S, Wong SC, et al. Japanese encephalitis virus transmitted via blood transfusion, Hong Kong, China. Emerg Infect Dis 2018;24:49-57. Crossref
5. Ferraris VA, Hochstetler M, Martin JT, Mahan A, Saha SP. Blood transfusion and adverse surgical outcomes: the good and the bad. Surgery 2015;158:608-17. Crossref
6. Everhart JS, Sojka JH, Mayerson JL, Glassman AH, Scharschmidt TJ. Perioperative allogeneic red blood-cell transfusion associated with surgical site infection after total hip and knee arthroplasty. J Bone Joint Surg Am 2018;100:288-94. Crossref
7. World Health Organization. WHO Global Forum for Blood Safety: patient blood management. Available from: https://www.who.int/bloodsafety/events/gfbs_01_pbm/en/ Accessed 5 Jan 2020.
8. Isbister JP. The three-pillar matrix of patient blood management-an overview. Best Pract Res Clin Anaesthesiol 2013;27:69-84. Crossref
9. National Blood Authority Australia. Patient Blood Management Guidelines. Available from: https://www.blood.gov.au/pbm-guidelines. Accessed 9 Jan 2020.
10. National Blood Authority Australia. Single Unit Transfusion Guide. Available from: https://www.blood.gov.au/single-unit-transfusion. Accessed 9 Jan 2020.
11. Hardy JF. Current status of transfusion triggers for red blood cell concentrates. Transfus Apher Sci 2004;31:55-66. Crossref
12. Zhang Q, Liu L, Sun W, et al. Are closed suction drains necessary for primary total knee arthroplasty? A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11290. Crossref
13. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee 2013;20:300-9. Crossref
14. Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 2011;106:13-22. Crossref
15. Shang J, Wang H, Zheng B, Rui M, Wang Y. Combined intravenous and topical tranexamic acid versus intravenous use alone in primary total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Int J Surg 2016;36:324-9. Crossref
16. Benson EE, McMillan DE, Ong B. The effects of active warming on patient temperature and pain after total knee arthroplasty. Am J Nurs 2012;112:26-33. Crossref
17. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108:71-7. Crossref
18. Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996;347:289-92. Crossref
19. Boutsiadis A, Reynolds RJ, Saffarini M, Panisset JC. Factors that influence blood loss and need for transfusion following total knee arthroplasty. Ann Transl Med 2017;5:418. Crossref
20. Ernest K. Hong Kong Red Cross issues urgent appeal for blood donors as supplies dwindle. South China Morning Post [newspaper on the internet]. 2017 May 4: Health & Environment. Available from: https://www.scmp.com/news/hong-kong/health-environment/article/2092982/hong-kong-red-cross-issues-urgent-appeal-blood. Accessed 10 Mar 2020.
21. City urged to donate blood amid severe shortage. The Standard [newspaper on the internet]. 2017 Aug 11: Local. Available from: https://www.thestandard.com.hk/breaking-news/section/3/94995/City-urged-to-donate-blood-amid-severe-shortage. Accessed 10 Mar 2020.
22. The Government of the Hong Kong Special Administrative Region. Urgent appeal for blood donation [press release]. 2018 Jan 9. Available from: https://www.info.gov.hk/gia/general/201801/09/P2018010900651.htm. Accessed 10 Mar 2020.
23. Coronavirus fears drain Hong Kong's blood banks. Radio Television Hong Kong [newspaper on the internet]. 2020 Feb 12. Available from: https://news.rthk.hk/rthk/en/component/k2/1508174-20200212.htm. Accessed 10 Mar 2020.
24. The Annual Report of Hong Kong Red Cross 2018/2019. Available from: https://www.redcross.org.hk/en/publications/annual_reports.html. Accessed 15 Jan 2020.
25. World Health Assembly. Resolution WHA63.12: Availability, safety and quality of blood products. Available from: https://apps.who.int/medicinedocs/documents/s19998en/s19998en.pdf. Accessed 10 Jan 2020.
26. Farmer SL, Towler SC, Leahy MF, Hofmann A. Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol 2013;27:43-58. Crossref
27. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016;56:2173-83. Crossref
28. Abdullah HR, Ang AL, Froessler B, et al. Getting patient blood management Pillar 1 right in the Asia-Pacific: a call for action. Singapore Med J 2019 May 2. Epub ahead of print. Crossref
29. D E H O, Hadi F, Stevens V. Health economic evaluation comparing iv iron ferric carboxymaltose, iron sucrose and blood transfusion for treatment of patients with iron deficiency anemia (Ida) in Singapore. Value Health 2014;17:A784. Crossref
30. Lee ES, Kim MJ, Park BR, et al. Avoiding unnecessary blood transfusions in women with profound anaemia. Aust N Z J Obstet Gynaecol 2015;55:262-7. Crossref
31. Yoon HM, Kim YW, Nam BH, et al. Intravenous ironsupplementation may be superior to observation in acute isovolemic anemia after gastrectomy for cancer. World J Gastroenterol 2014;20:1852-7. Crossref
32. Na HS, Shin SY, Hwang JY, Jeon YT, Kim CS, Do SH. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion 2011;51:118-24. Crossref
33. Lee QJ, Mak WP, Yeung ST, Wong YC, Wai YL. Blood management protocol for total knee arthroplasty to reduce blood wastage and unnecessary transfusion. J Orthop Surg (Hong Kong) 2015;23:66-70. Crossref
34. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013;95:2177-84. Crossref
35. Kim JL, Park JH, Han SB, Cho IY, Jang KM. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty 2017;32:320-5. Crossref
36. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty 2015;30:365-8. Crossref
37. Lindman IS, Carlsson LV. Extremely low transfusion rates: contemporary primary total hip and knee arthroplasties. J Arthroplasty 2018;33:51-4. Crossref