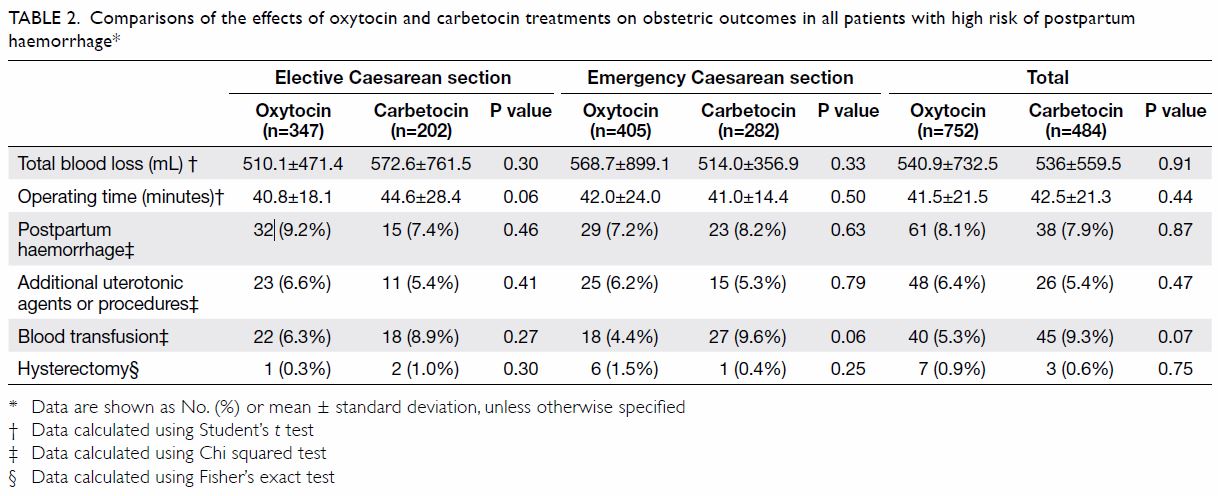
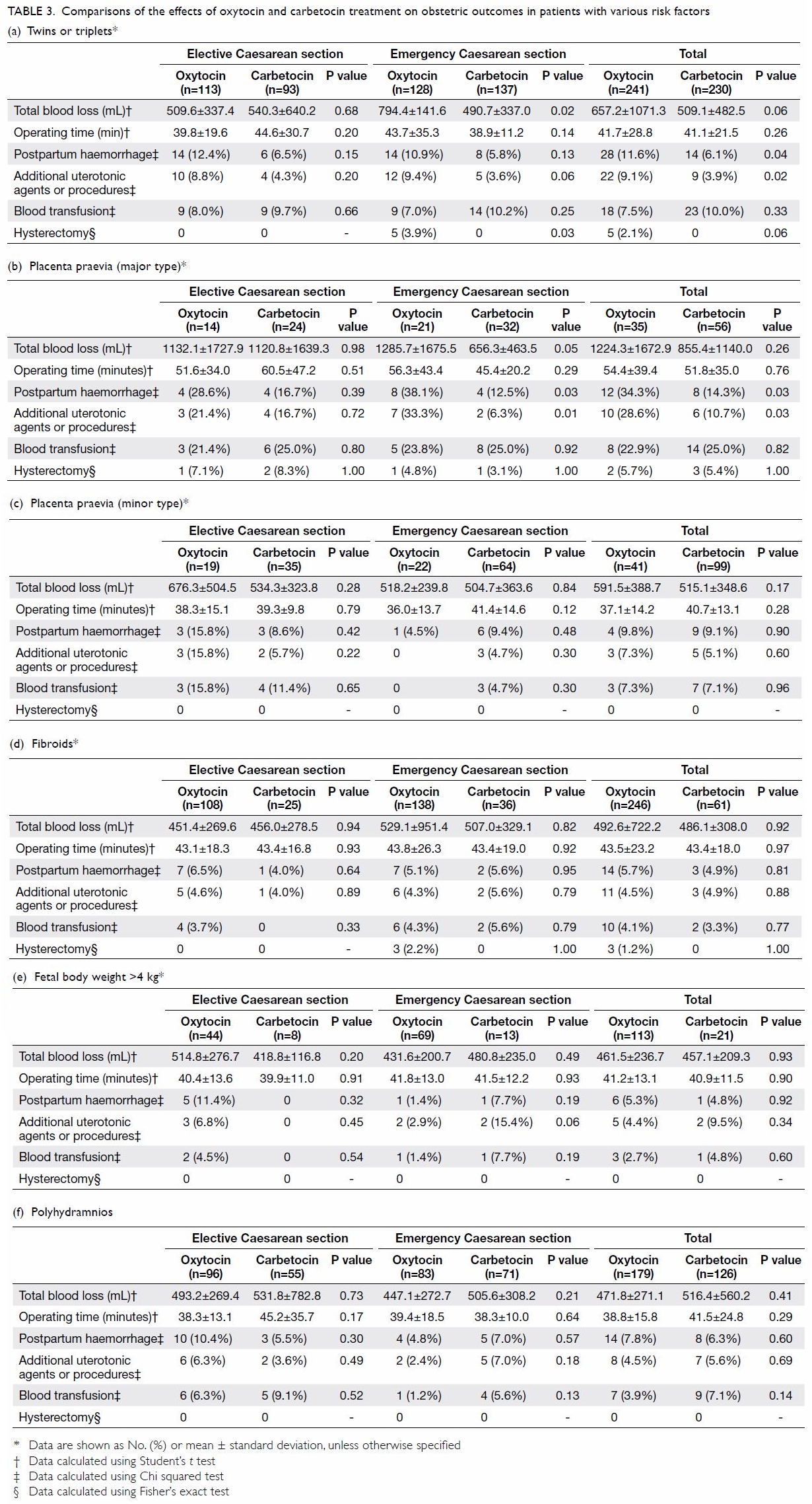
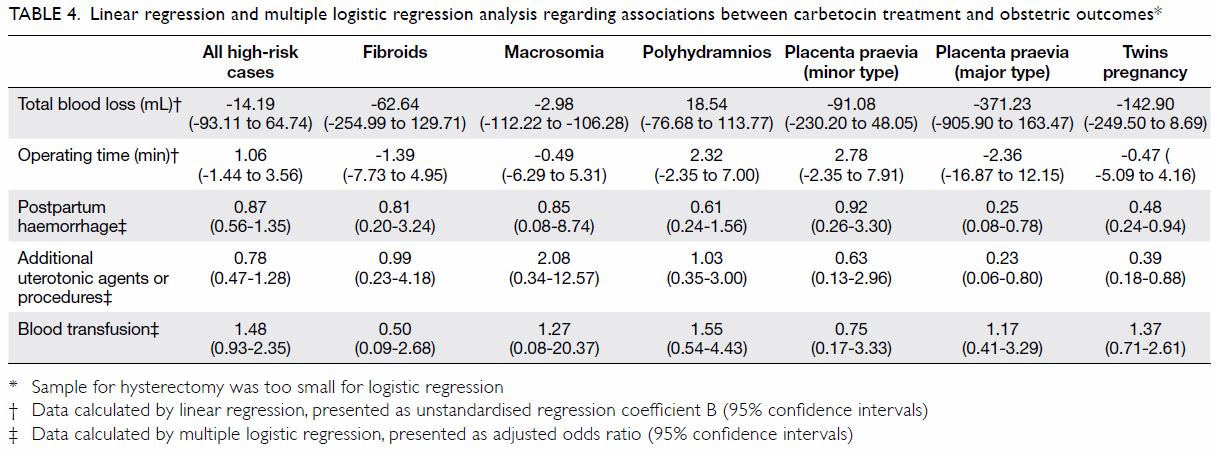
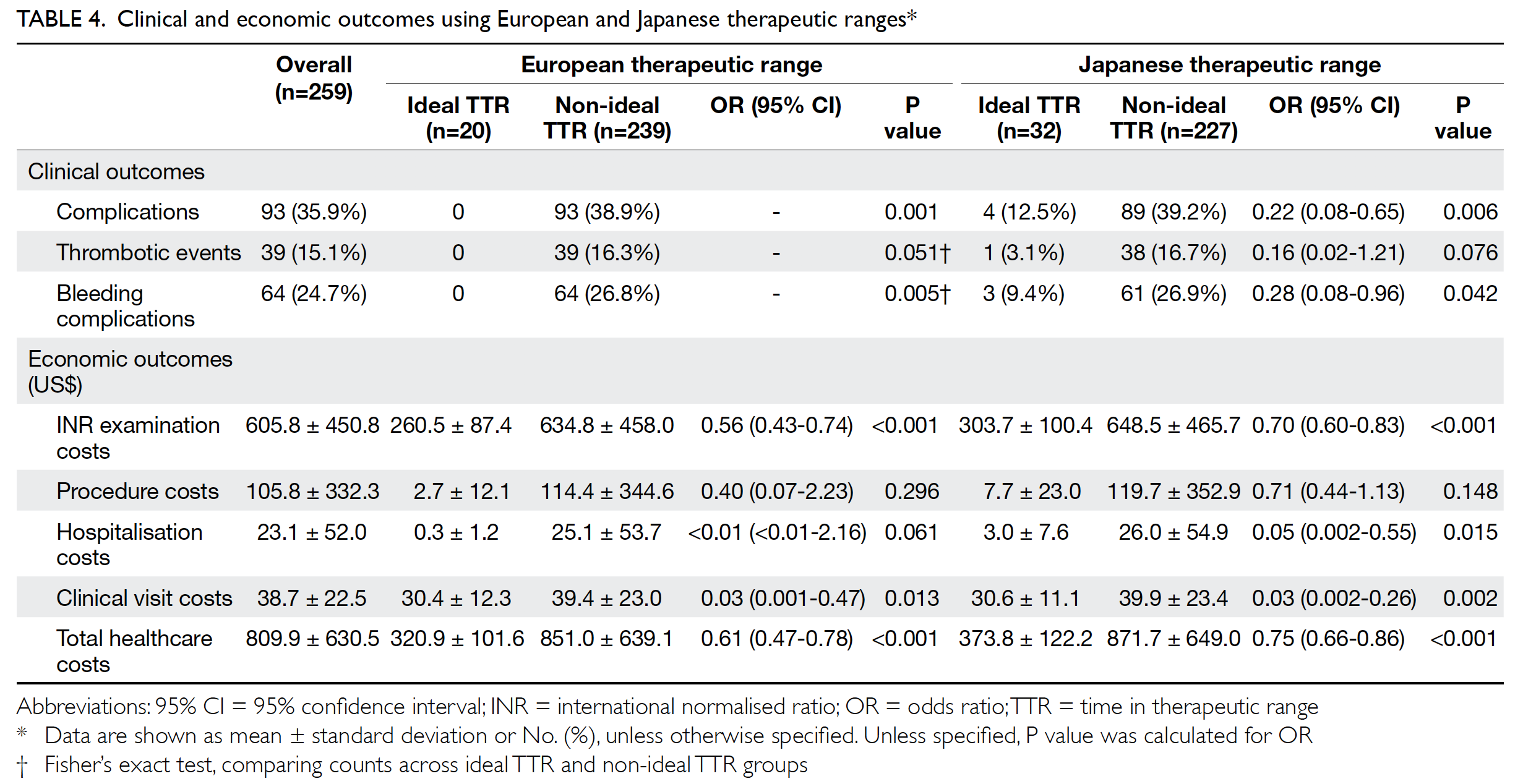
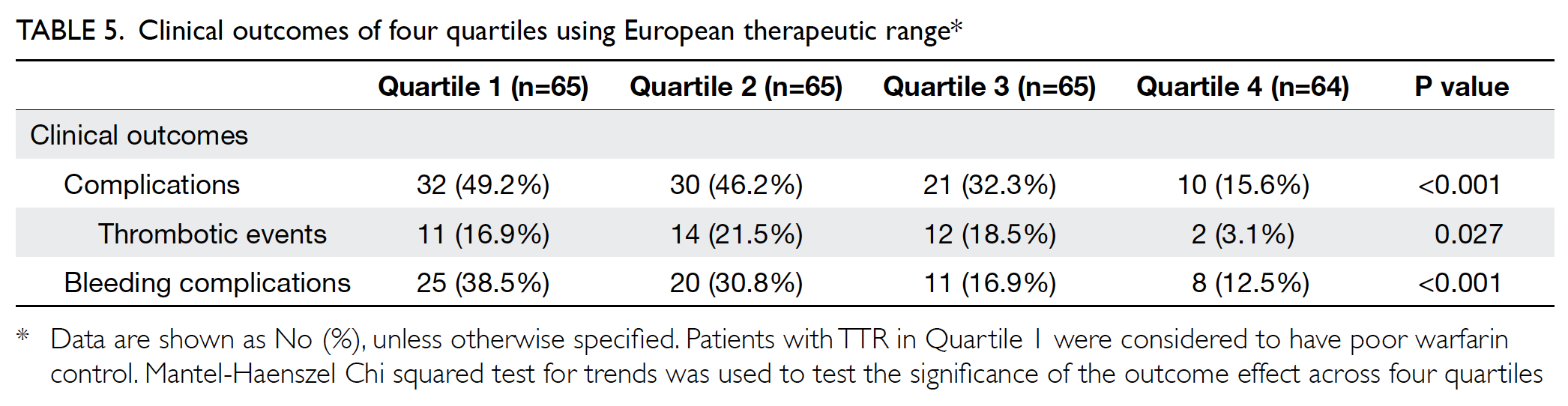
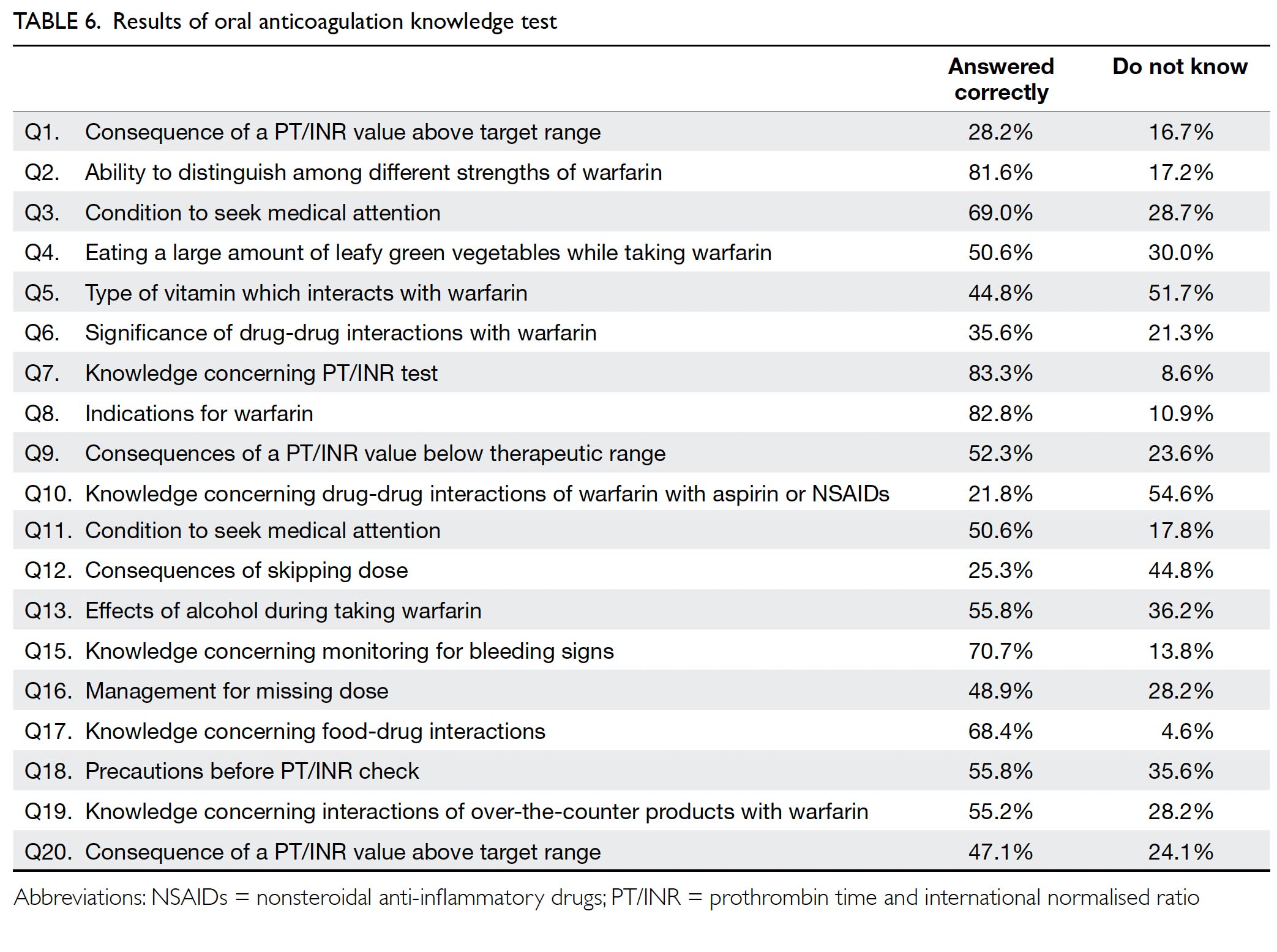
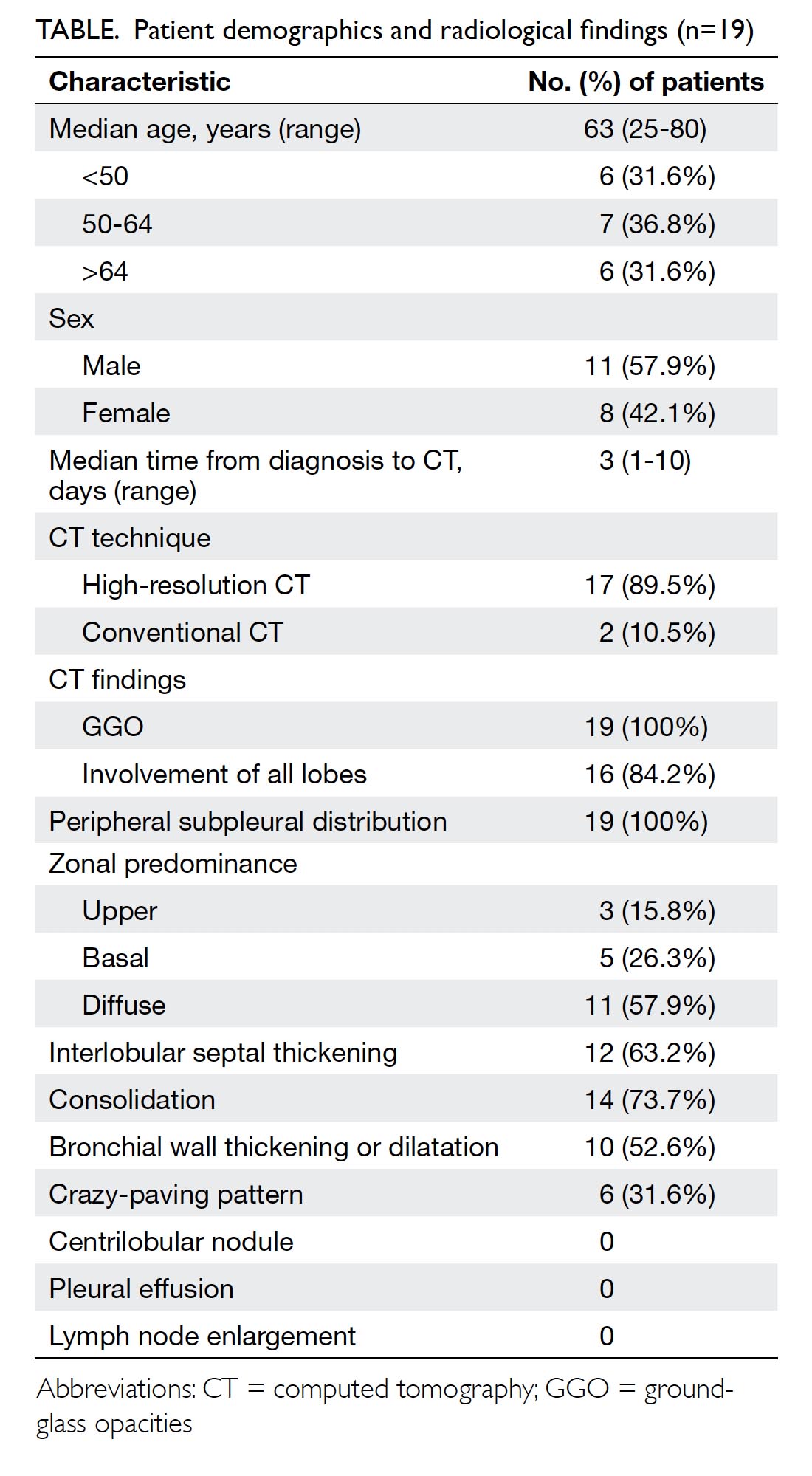
Treatment of patients with Mayer-Rokitansky-Küster-Hauser syndrome in a tertiary hospital
Hong Kong Med J 2020 Oct;26(5):397–403 | Epub 16 Oct 2020
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Treatment of patients with Mayer-Rokitansky-Küster-Hauser syndrome in a tertiary hospital
Karen Ng, FHKAM (Obstetrics and Gynaecology), FHKCOG; Patricia NP Ip, MB, ChB; KW Yiu, FHKAM (Obstetrics and Gynaecology), FHKCOG; Jacqueline PW Chung, FHKAM (Obstetrics and Gynaecology), FHKCOG; Symphorosa SC Chan, MD, FHKCOG
Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Dr Karen Ng (ngkaren@cuhk.edu.hk)
Abstract
Introduction: Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome is an uncommon congenital
malformation characterised by agenesis or
hypoplasia of the vagina and uterus. Here, we
describe the treatment of patients with MRKH
syndrome in a tertiary hospital.
Methods: This retrospective study included patients
with MRKH syndrome attending the Paediatric and
Adolescent Gynaecology Clinic in a tertiary hospital.
Their clinical manifestations, examinations, and
methods for neovagina creation were recorded.
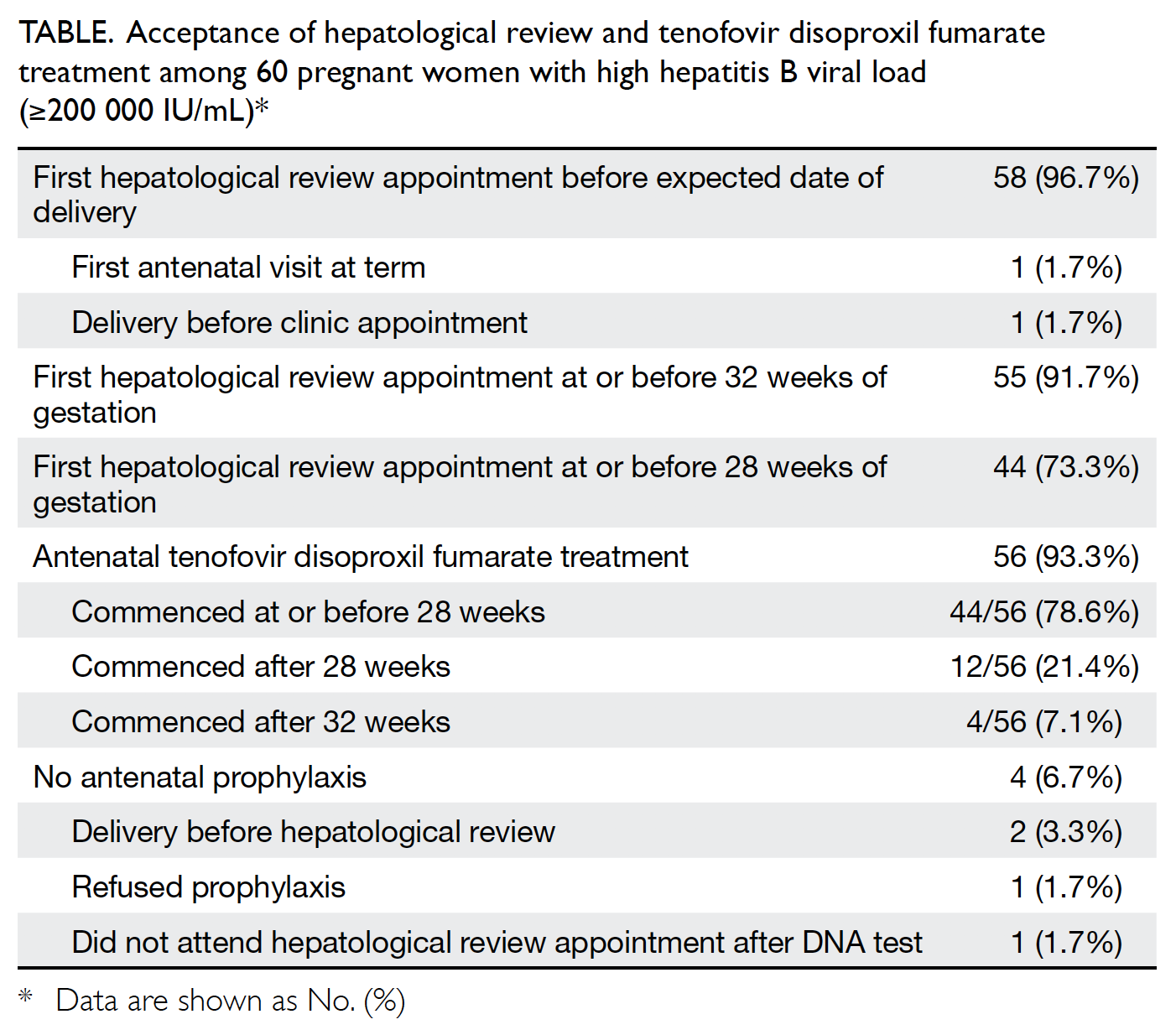
Among patients who underwent vaginal dilation
(VD), therapy duration, vaginal width and length
at baseline and after VD, complications, and sexual
activity and dyspareunia outcomes were evaluated.
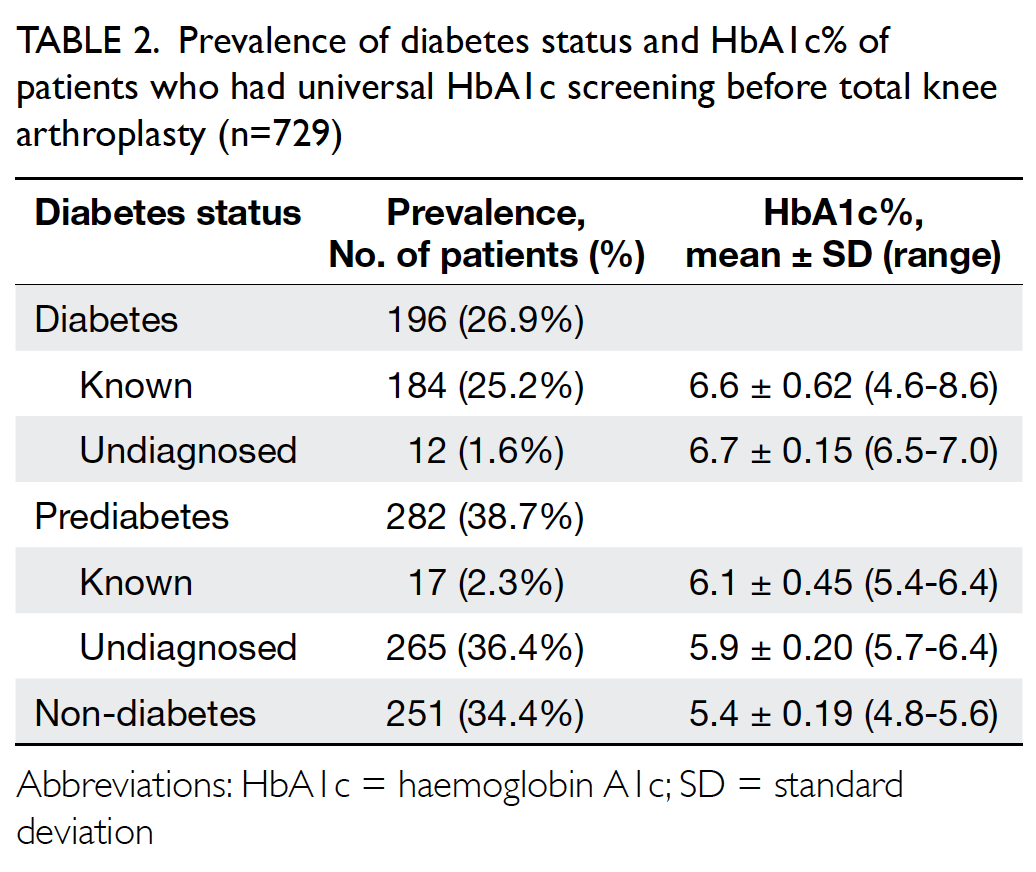
Results: Forty nine patients with MRKH syndrome
were identified. Their mean age at presentation was
17.9 years; 69.4% and 24.5% of patients presented for
primary amenorrhoea treatment and vaginoplasty,
respectively. Forty eight patients had normal renal
imaging findings and 46 XX karyotypes. Seventeen
(34.7%) patients underwent VD as first-line therapy;
three did not complete the therapy. Two had surgical
vaginoplasty, whereas five achieved adequate vaginal length by sexual intercourse alone; 25 had not yet
requested VD. The mean duration of VD was 16±10.2
(range, 4-35) weeks. The widths and lengths of the
vagina at baseline and after VD were 1.1±0.28 cm
and 1.3±0.7 cm, and 3.1±0.5 cm and 6.9±0.9 cm,
respectively. The overall success rate of VD was
92.3%. Vaginal spotting was the most common
complication (21%); only one patient reported
dyspareunia.
Conclusions: Mayer-Rokitansky-Küster-Hauser syndrome is an uncommon
condition that requires multidisciplinary specialist
care. Vaginal dilation is an effective first-line approach for
neovagina creation.
New knowledge added by this study
- Most patients were diagnosed with Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome after they presented with primary amenorrhoea. Most patients with MRKH syndrome exhibited normal renal imaging findings, but did not possess a uterus.
- Among patients with MRKH syndrome who completed vaginal dilation (VD) therapy, the success rate was 92.3%, based on reports of subjective sexual satisfaction.
- The most common complication during VD therapy was vaginal spotting (21% of patients), which resolved with conservative management or the use of vaginal oestrogen cream.
- For patients with MRKH syndrome who discontinued treatment prior to completion of VD therapy, a considerably shorter second course of treatment was sufficient to achieve satisfactory vaginal length.
- Resources should be allocated for provision of psychological services, including the option for group-based therapy, because these may be helpful for patients with MRKH syndrome and their caregivers.
- VD therapy should be recommended as first-line treatment for the creation of a neovagina in patients with MRKH syndrome, following careful consideration of local expertise, patient preferences, and patient ability to maintain compliance for the duration of therapy.
Introduction
Mayer-Rokitansky-Küster-Hauser (MRKH)
syndrome is a congenital malformation characterised
by failed development of the Müllerian duct, which
leads to vaginal agenesis, often accompanied by
uterine agenesis. It is estimated to occur in one in
4000 to 5000 births.1 Most patients present with primary amenorrhoea, but exhibit normal secondary
sexual characteristics. Mayer-Rokitansky-Küster-Hauser syndrome is reportedly associated with other
malformations including renal, skeletal, and auditory
manifestations.2 3 The creation of a functional
neovagina is a component of treatment performed
to aid women in achieving a normal sexual life. The timing for the creation of a neovagina depends on
the patient. However, treatment should be deferred
beyond late adolescence to allow each patient to
provided informed consent and participate in the
treatment process.4 Both surgical and non-surgical
methods have been described for the creation of
a neovagina. Regarding surgical options, various
grafts or moulds may be used; other techniques
include traction vaginoplasty.5 Surgical techniques
often achieve anatomical success, but result in
associated complications such as bladder injury,
neovaginal vault granulation, introital stenosis,
vaginal discharge, urinary tract infection, or graft
infection.4 6 7 After most surgical techniques, patients
often require postoperative utilisation of a mould or
dilator.5
Despite the availability of many surgical
methods, non-surgical methods with vaginal
dilation (VD) are advocated as first-line treatment
in many instances6 8; VD has been proven effective
in the creation of a neovagina.5 9 Notably, data are
available regarding surgical creation of a neovagina
in the Chinese population10; however, there is limited
information concerning the implementation of VD in
the Chinese population, despite the recommendation
of VD as first-line treatment for the creation of a
neovagina. Chinese adolescents or their caregivers
may prefer more conservative treatment options for
MRKH syndrome.11 12 In addition, pelvic connective
tissue has been proposed to differ between Chinese
women and Caucasian women.13
In this study, we investigated the treatment
of patients with MRKH syndrome, evaluated
the effectiveness of VD therapy, and identified
complications among patients with MRKH
syndrome who underwent VD. To the best of our
knowledge, this is the first report regarding VD
therapy in Chinese women with MRKH syndrome in
the English-language medical literature.
Methods
Patient population and standard treatment
A Paediatric and Adolescent Gynaecology Clinic has
been established in our tertiary university teaching
hospital since late 2002. All female patients with
MRKH syndrome underwent treatment in that clinic
by gynaecologists who specialised in paediatric and
adolescent gynaecology. Generally, diagnosis was
made on the basis of primary amenorrhoea, normal
secondary sexual characteristics, vaginal absence, or
vaginal dimple on perineal inspection. Ultrasound
assessment showed that most patients also did
not exhibit a uterus; when a uterus was present, it
was either functioning or rudimentary. Thorough
counselling concerning the diagnosis, including
implications for future sexual life and fertility,
was provided to the patients and their caregivers.
Ultrasound or magnetic resonance imaging for the
urinary system was performed to rule out urinary
tract anomalies. Patients returned for examination a
few weeks or months after the initial visit, then began
annual follow-up. During further follow-up, patients
received an explanation of vaginoplasty; they were
encouraged to discuss whether the procedure was
appropriate, following careful consideration.
Data collection
This retrospective observational study was performed
using information from a prospectively collected
database of all patients who had received treatment
for MRKH syndrome in our clinic. The patients’
medical notes were reviewed; demographic data,
presenting symptoms, previous imaging findings,
and history of sexual experience were recorded. The
method of vaginal creation, if any, was also recorded.
Regarding the outcome of VD therapy, patients
who had undergone VD as the primary method for
creation of a neovagina were included in the analysis.
The number of sessions, the starting and final vaginal
width and length, the interval until completion of
therapy, and any complications associated with
the therapy were reviewed. The outcomes of VD
in terms of sexual activity, dyspareunia, and sexual
satisfaction were reviewed.
Vaginal dilation therapy
Patients who requested VD therapy were
scheduled for individual treatment sessions with the gynaecologist. For the first session, patients
were admitted to the day ward; three intensive VD
sessions were performed on the first day. The dilator
(custom made by the Queen Charlotte and Chelsea
Hospital, United Kingdom; another dilator set, the
“Amielle Comfort vaginal dilators” manufactured
by Owen Mumford was also used) was placed at the
vaginal dimple and constant pressure was applied
using the dilator for 15 minutes. The first session
was performed by the gynaecologist, the second
session was performed by the patient under medical
supervision, and the third session was performed
independently by the patient. The patient was
discharged with instructions to perform two to three
VD sessions per day at home, 15 minutes per session.
Follow-up was arranged on an out-patient basis, at
intervals of 2 to 4 weeks. Patients were provided
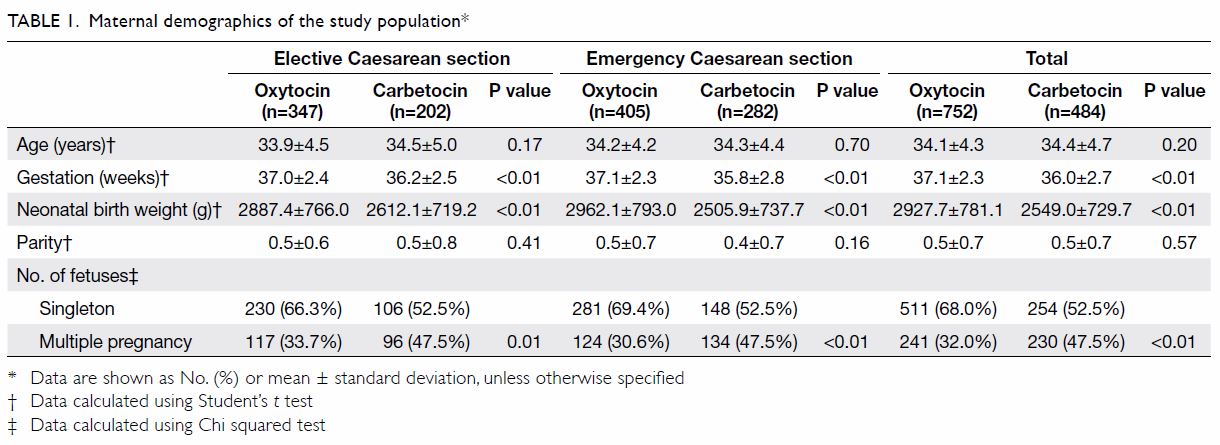
with appropriately sized dilators, typically larger
over time (Fig). Neovaginal width and length were
recorded at each follow-up visit. Any complications
and sexual function were also recorded. Vaginal
dilation therapy was discontinued when a patient
achieved satisfactory sexual intercourse.
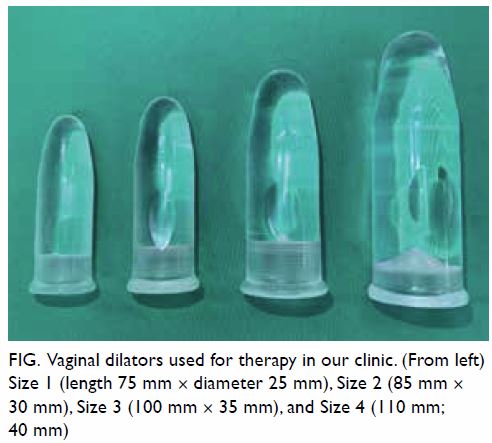
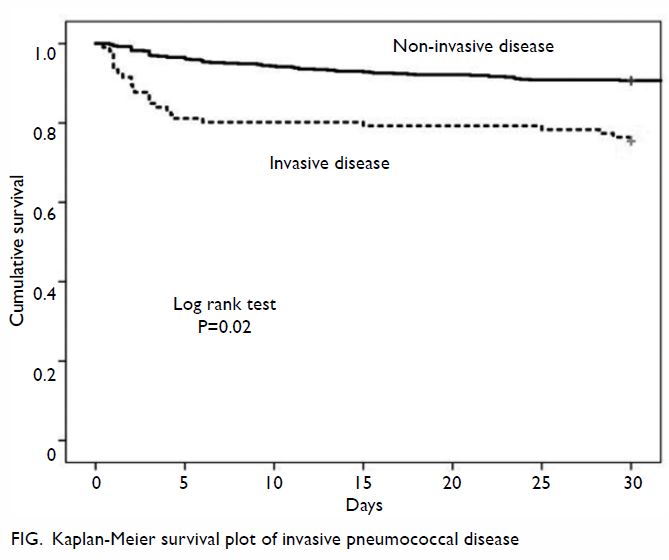
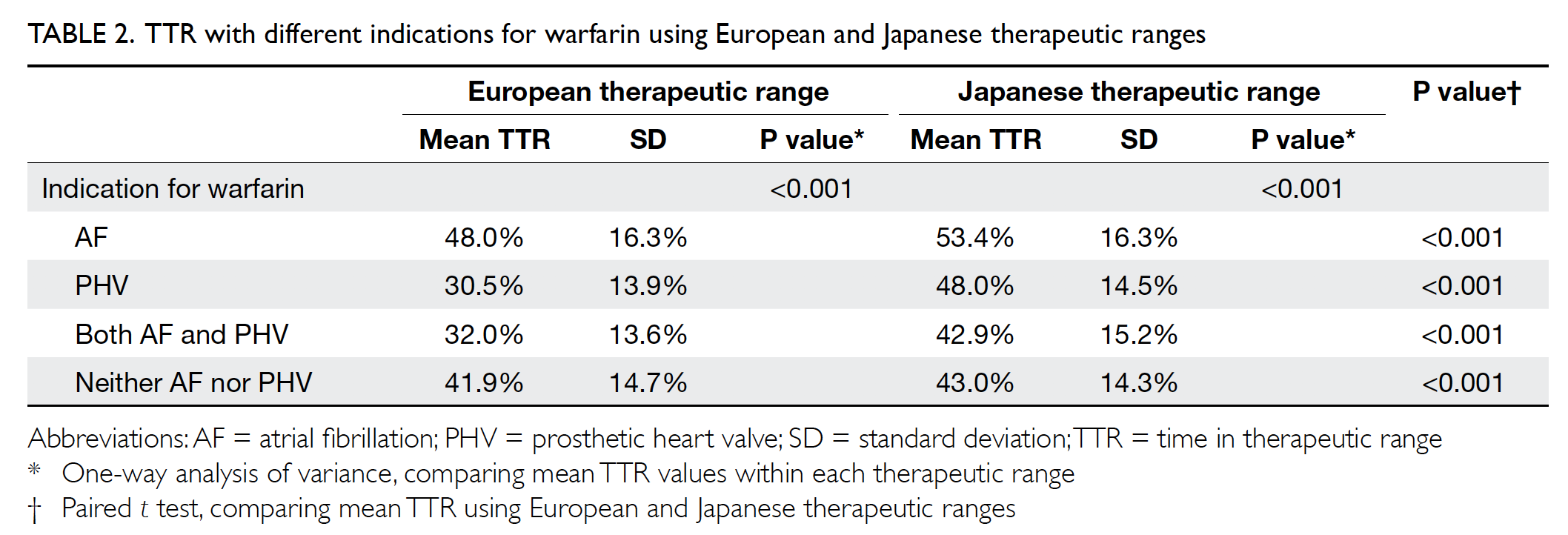
Fig. Vaginal dilators used for therapy in our clinic. (From left) Size 1 (length 75 mm × diameter 25 mm), Size 2 (85 mm × 30 mm), Size 3 (100 mm × 35 mm), and Size 4 (110 mm; 40 mm)
Data analysis
SPSS Statistics for Windows, version 21.0 (IBM
Corp, Armonk [NY], United States) was used to
analyse the data collected. Normally distributed data
are described as mean±standard deviation, whereas
non-normally distributed data are described as
median (range). Independent samples t tests were
used to compare results between two groups. P<0.05
was considered statistically significant.
Results
Patient characteristics and clinical treatment
In total, 49 patients with MRKH syndrome underwent
treatment in our clinic from 2002 to 2019. The mean patient age at presentation was 17.9±4.9 years (range,
11-36 years). Overall, 34 (69.4%) patients presented
with primary amenorrhoea and received diagnoses
of MRKH syndrome in our clinic. The mean age
among this group of patients was 16.7±2.4 years.
Another 12 (24.5%) patients were referred for further
treatment and/or VD therapy, following diagnosis in
another clinic. Two (4.1%) patients presented with
acute surgical complications, torsion of ovarian
cyst, and suspected appendicitis; the patients were
diagnosed with hematometra and hematosalpinx
at age 11 and 16 years, respectively, because of
incidental intra-operative pelvic findings. Finally,
one patient presented for labial minora hypertrophy
at age 12 years and was incidentally diagnosed with
vaginal agenesis during perineal examination. Forty
eight of the 49 patients had a 46 XX karyotype; one
patient had a 47 XXX karyotype. Two patients had
a unilateral functioning uterus, while three patients
had a non-functioning rudimentary uterine horn.
Overall, 45 patients underwent imaging, either
ultrasound or magnetic resonance imaging, to assess
the renal system; some patients also underwent
intravenous urography. Of these 45 patients, 44
had normal findings; one patient had a dilated
pelvicalyceal system and ureter with suspected low
insertion into the bladder, but no signs of obstruction
were found. The remaining four patients were either
waiting for the imaging appointment or did not have
imaging information in their medical notes.
Overall, 17 (34.7%) patients underwent VD
therapy as first-line therapy for the creation of a
neovagina. Two (4.1%) patients had surgeries as
first-line treatment: one patient underwent Creatsas
vaginoplasty in our hospital and one underwent
vaginoplasty at a hospital in China. Both of these
two patients required VD therapy, at 12 years and
1 year, respectively, after their original surgeries
because of vaginal stenosis. Furthermore, five
(10.2%) patients achieved creation of a neovagina
by sexual intercourse alone. Finally, 25 (51%)
patients had not yet requested vaginoplasty; these
patients were significantly younger than patients
who had undergone vaginoplasty (22.8±5.2 years vs
30±7.0 years, P<0.005) [Table].
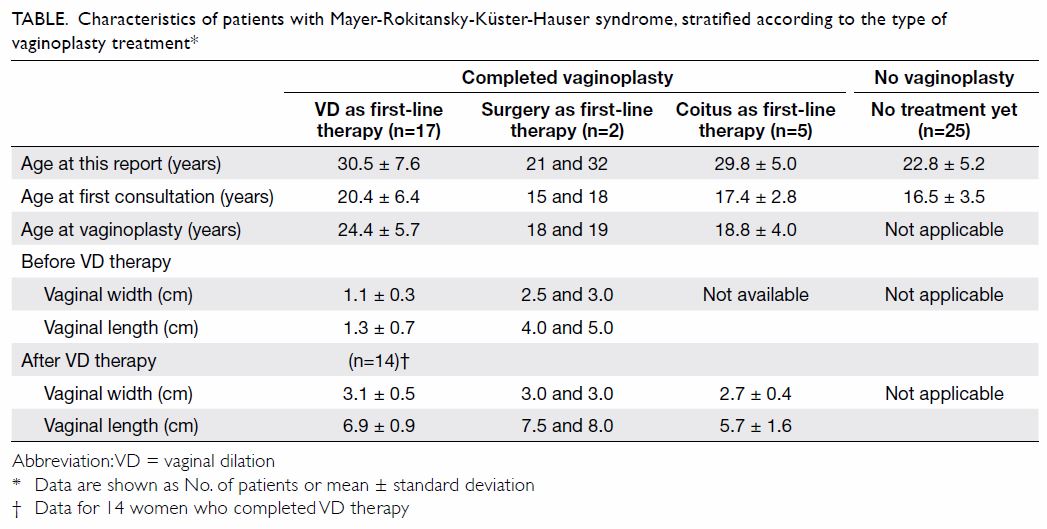
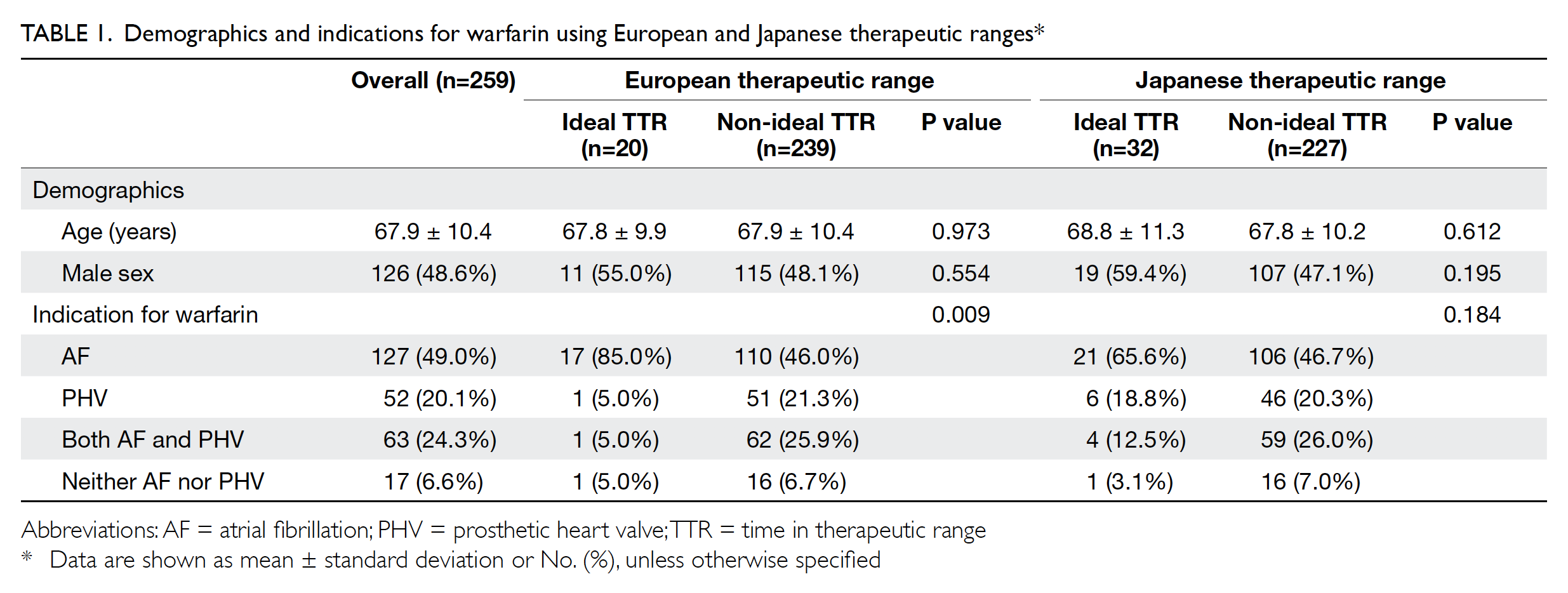
Table. Characteristics of patients with Mayer-Rokitansky-Küster-Hauser syndrome, stratified according to the type of vaginoplasty treatment
Vaginal dilation outcomes and follow-up
Of the 17 patients who underwent VD therapy as
first-line treatment, four (23.5%) reported that they
had previously attempted unsuccessful coitus and
therefore requested VD therapy. For these four
patients, the mean age at initiation of VD therapy
was 24.4±5.7 years (range, 18-36 years). The mean
width and length of the vagina at baseline were
1.1±0.28 cm (range, 1-2 cm) and 1.3±0.7 cm (range,
0.5-3 cm), respectively. There were no differences in
starting width and length between the four patients
who had previously attempted coitus and the remaining 13 who had not. Three patients did not
complete VD therapy because their relationships
had ended and they chose to discontinue the therapy.
Among the patients who completed VD therapy, the
final width and length of the vagina were 3.1±0.5 cm
(range, 2-4 cm) and 6.9±0.9 cm (range, 6-9 cm),
respectively. The mean interval until the completion
of VD therapy was 16±10.2 (range, 4-35) weeks.
Notably, there was no difference in duration of VD
therapy between patients who had and patients
who had not previously attempted coitus before
commencement of therapy (P=0.83).
Of the 14 patients who completed VD therapy,
one was lost to follow-up; 13 were included in further
analysis. All 13 of these patients were sexually active
after VD therapy, and 12 reported subjective sexual
satisfaction. Therefore, the success rate of VD
therapy was 92.3% in our cohort. The remaining
patient reported mild superficial dyspareunia and
that the vaginal length of 6 cm was inadequate for
achievement of sexual satisfaction; thus, surgical
vaginoplasty is planned.
Complications and subsequent therapy
The most common complication during VD was
vaginal spotting, which occurred in four (21%) of
the 19 patients who had undergone VD therapy. In
all patients, spotting subsided with conservative
management or the use of vaginal oestrogen cream.
One patient had one episode of urinary tract
infection, which resolved following treatment with
oral antibiotics.
Among the three patients in the VD group who
did not complete therapy when their relationships
ended, all began a second course of VD therapy
following new requests for treatment. The second course required a considerably shorter duration
(4-8 weeks) to achieve satisfactory vaginal length for
sexual intercourse.
Findings after neovagina creation by coitus
alone
Among the five patients who achieved creation of a
neovagina by coitus alone, their age at initial sexual
intercourse ranged from 13 to 24 years. Their mean
vaginal width and length were 2.7 cm and 5.7 cm,
respectively. One patient had a rectovaginal fistula;
she reported faecal incontinence from the vagina
and the passage of semen from the anus after sexual
intercourse. Examination revealed a 5- × 2-mm
rectovaginal fistula in the posterior vaginal wall,
3 cm above the introitus. Vaginal repair of the fistula
was performed; the patient reported no further
faecal incontinence nor abnormal passage of semen.
Findings in patients with functioning
endometrium
Regarding the two patients with a functioning
endometrium, one had a hysterectomy at age 18 years,
shortly after she had undergone Creatsas vaginoplasty.
The other patient had a unicornuate uterus that was
initially suppressed with gonadotropin-releasing
hormone analogue with supplemental oestrogen
therapy until age 21 years; she then selected uterine-preserving
surgery. After VD therapy, the patient
underwent uterovaginal anastomosis by laparotomy
and the perineal route. Two months after surgery,
she experienced an episode of cervical stenosis. The
hematometra was drained by manual dilation using
Hegar dilators at the bedside. Subsequently, she
has experienced regular monthly menstruation for
8 months postoperatively.
Discussion
In our cohort, the most common presenting
symptom was primary amenorrhoea. The patients
presented at mean age 16.7±2.4 years, which is
the appropriate time for consultation for primary
amenorrhoea. This indicated that the young women
and their caregivers did not seek to delay treatment
for this gynaecological problem. Moreover, the
healthcare providers referred the young women at
the appropriate age.
The diagnosis of MRKH syndrome is mainly
based on clinical assessment. Ultrasound can be a
useful modality to confirm the absence of a uterus
because of its relatively low cost, non-invasive nature,
and widespread availability in many gynaecology
units. However, the effectiveness of ultrasound
is operator-dependent and image quality can be
affected by each patient’s body build. Although three-dimensional
ultrasound is useful for assessment of
Müllerian anomalies,14 15 it may not be necessary to
detect the absence of a uterus. Magnetic resonance
imaging is another useful modality, with good soft
tissue resolution; it allows good visualisation of any
rudimentary horns, possible endometrium, and
ovaries. Magnetic resonance imaging reportedly
exhibited 100% sensitivity when diagnostic
laparoscopy was performed in women with uterine
anomalies14 16; therefore, diagnostic laparoscopy is
rarely required or recommended for the diagnosis
of MRKH syndrome. Even in patients with acute
hematometra of the obstructing uterus, conservative
treatment might be appropriate if the diagnosis of
MRKH syndrome is made prior to surgery. In our
cohort, a patient with acute hematometra presented
to the surgical unit and received a provisional
diagnosis of acute appendicitis. In women with
congenital androgen insensitivity syndrome, the
absence of a uterus can be a similar finding5; however,
clinical examination of women with congenital
androgen insensitivity syndrome has shown that
these patients do not have axillary hair or pubic
hair, and a simple karyotype analysis can be used to
differentiate between the two possible diagnoses.
Psychological concerns can be an important
factor in patients with MRKH syndrome. These
patients can develop a negative self-image because
they perceive themselves to be different from their
peers; they can also develop low self-esteem.17 18 19
Upon diagnosis and treatment, the patients and
their caregivers should be offered guidance and
psychological support.20 Resources should be
allocated for provision of psychological services,
which is an essential component of multidisciplinary
care. Although our service has been available for
many years, more services of this type are needed in
the future.
The creation of a neovagina is an important
aspect of treatment for patients with MRKH syndrome. Various neovagina creation techniques
have been described over the years. A non-surgical
method involving the utilisation of handheld vaginal
dilators was first described by Frank in 1938.21
Ingram22 later modified the technique with the use
of a bicycle stool. The respective success rates for
Frank’s and Ingram’s methods are reportedly 66% to
95%5 6 9 23 24 25 and 92%.22 Despite the success of these
non-surgical techniques, various surgical techniques
have also been developed for vaginoplasty. These
include the McIndoe technique, usage of various
grafts (eg, amnion, skin, or bowel), the Davydov
technique, and the Vecchietti technique. However,
these surgical techniques may result in some
complications. Following intestinal vaginoplasty,
introital stenosis and vaginal discharge can occur in
up to 9% and 7% of patients, respectively.5 7 26 27 28 There
have also been case reports regarding the onset of
adenocarcinoma in bowel grafts29 30 and the onset
of hair growth or squamous cell carcinoma in skin
grafts.31 Following the use of the Davydov technique
(ie, downward stretching of the pelvic peritoneum
to create a vagina), neovaginal vault granulation
can occur in up to 8% of patients.5 7 Intra-operative
bladder injury can occur in 1% to 2% of patients who
undergo the Vecchietti procedure, which comprises
progressive upward stretching of the perineal skin
by means of threads that exit from the anterior
abdominal wall.5 7 32 Strictures and contractures are
also a concern in patients who undergo any surgical
technique; therefore, a mould or dilation is often
required during the early postoperative period.
A relatively longer hospital stay of 2 to 9 days is a
notable concern following surgical vaginoplasty.5 7
Given its lower risk for potential complications
and high success rate, VD has become the first-line
technique for the creation of a neovagina in Australia,
the United Kingdom, the United States, and parts of
Russia.7 Previous studies have also demonstrated
that VD is more cost-effective, compared with first-line
surgical treatment.5 33
Edmonds et al9 reported an average of 5 months
to achieve satisfactory vaginal length, which they
defined as 6 cm, or when the patient is able to
achieve satisfactory sexual coitus; our results were
comparable (ie, 4 months). The mean vaginal length
of 6.9 cm achieved in our cohort was also comparable
with the findings in previous studies.7 9 23 25 Generally,
VD results in a shorter average vaginal length,
compared with surgical vaginoplasty.5 7 Notably,
6.6 cm has been proposed as the ideal vaginal length
for satisfactory sexual activity.7 24 Of the patients who
completed VD therapy in our cohort, 92.3% reported
satisfactory coitus. This success rate was comparable
to the 94.9% reported by Edmonds et al,9 who
published the largest study regarding VD thus far.
Sexual coitus has been shown to successfully
create a neovagina9 34 35; this outcome was achieved by a few patients in our cohort. Importantly,
previous coital attempts in our patients did not
affect the starting width and length of the vagina, or
the interval required to complete the dilation. This is
presumably because the patients abandoned further
coital attempts after unsuccessful coitus.
A common cause for the failure of VD is a lack
of motivation.36 The success rate was high among
patients in our cohort. Roberts et al37 reported that
women younger than 18 years of age at the initiation
of therapy had a significantly higher failure rate. The
mean age of patients in our group was 24 years; most
patients (79%) were in a relationship before initiation
of therapy (data not shown), which might have
enhanced their motivation to complete the therapy.
Accordingly, we only commence VD therapy when
patients are well prepared for this therapy, such that
they fully understand the importance of compliance.
Following the creation of a neovagina, regular
coitus or dilation is required to maintain it. Three
patients in our cohort required a second course
of VD because they experienced shrinkage of the
neovagina after discontinuation of self-dilation and
cessation of regular coitus. However, a short duration
of therapy was required to re-establish a satisfactory
neovagina. Notably, vaginal spotting occurred in
four patients during VD therapy. In all patients,
spotting resolved with conservative management
or with local application of oestrogen cream to the
neovagina.
This study demonstrated the characteristics
and treatment of Chinese patients with an
uncommon condition, MRKH syndrome, as well as
the outcome of VD therapy in those patients. The
study may have been limited by the small number of
patients, which is attributable to the rarity of MRKH
syndrome. In addition, standardised validated
questionnaires were not employed to assess sexual
function among the patients in this study. Future
research using validated questionnaires should be
performed to assess the psychological aspects of
these patients before, during, and after VD therapy.
Conclusion
Mayer-Rokitansky-Küster-Hauser syndrome is
an uncommon gynaecological condition. Careful
treatment by healthcare providers familiar with
this condition may aid patients in achieving
suitable outcomes. Vaginal dilation therapy is an
effective first-line treatment for the creation of a
neovagina. The results achieved in our cohort of
Chinese women are comparable with the findings
in previously published studies in other nations.
However, long-term data collection, including the
use of validated questionnaires, will provide more
objective information regarding sexual function in
patients with MRKH syndrome.
Author contributions
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Concept or design: K Ng, SSC Chan.
Acquisition of data: K Ng, SSC Chan, KW Yiu.
Analysis or interpretation of data: K Ng, SSC Chan.
Drafting of the manuscript: K Ng, SSC Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: K Ng, SSC Chan, KW Yiu.
Analysis or interpretation of data: K Ng, SSC Chan.
Drafting of the manuscript: K Ng, SSC Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Conflicts of interest
As an editor of the journal, JPW Chung was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref CRE.2020.023). The requirement for
written informed consent was waived.
References
1. Creighton SM. Long-term sequelae of genital surgery.
In: Balen AH, Creighton SM, Davies MC, MacDougall
J, Stanhope R, editors. Paediatric and Adolescent
Gynaecology: A Multidisciplinary Approach. Cambridge:
Cambridge University Press; 2004. Crossref
2. Morcel K, Camborieux L, Programme de Recherches sur
les Aplasies Müllériennes, Guerrier D. Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome. Orphanet J Rare Dis
2007;2:13. Crossref
3. Oppelt P, Renner SP, Kellermann A, et al. Clinical
aspects of Mayer-Rokitansky-Kuester-Hauser syndrome:
recommendations for clinical diagnosis and staging. Hum
Reprod 2006;21:792-7. Crossref
4. Laufer MR. Congenital absence of the vagina: in search
of the perfect solution. When, and by what technique,
should a vagina be created? Curr Opin Obstet Gynecol
2002;14:441-4. Crossref
5. Callens N, De Cuypere G, De Sutter P, et al. An update
on surgical and non-surgical treatments for vaginal
hypoplasia. Hum Reprod Update 2014;20:775-801. Crossref
6. Ismail-Pratt IS, Bikoo M, Liao LM, Conway GS, Creighton
SM. Normalization of the vagina by dilator treatment
alone in complete androgen insensitivity syndrome and
Mayer-Rokitansky-Kuster-Hauser syndrome. Hum Reprod
2007;22:2020-4. Crossref
7. McQuillan SK, Grover SR. Dilation and surgical
management in vaginal agenesis: a systematic review. Int
Urogynecol J 2014;25:299-311. Crossref
8. Committee on Adolescent Health Care. Committee opinion
no. 562: Müllerian agenesis: diagnosis, management, and
treatment. Obstet Gynecol 2013;121:1134-7. Crossref
9. Edmonds DK, Rose GL, Lipton MG, Quek J. Mayer-Rokitansky-Kuster-Hauser syndrome: a review of 245
consecutive cases managed by a multidisciplinary approach
with vaginal dilators. Fertil Steril 2012;97:686-90. Crossref
10. Qin C, Luo G, Du M, et al. The clinical application of laparoscope-assisted peritoneal vaginoplasty for the
treatment of congenital absence of vagina. Int J Gynaecol
Obstet 2016;133:320-4. Crossref
11. Chan SS, Yiu KW, Yuen PM, Sahota DS, Chung TK.
Menstrual problems and health-seeking behaviour in
Hong Kong Chinese girls. Hong Kong Med J 2009;15:18-
23.
12. Yiu KW, Chan SS, Chung TK. Mothers’ attitude to the use
of a combined oral contraceptive pill by their daughters for
menstrual disorders or contraception. Hong Kong Med J
2017;23:150-7. Crossref
13. Zacharin RF. A Chinese anatomy—The pelvic supporting
tissues of Chinese and Occidental female compared and
contrasted. Aust Nz J Obstet Gyn 1977;17:1-11. Crossref
14. Deutch TD, Abuhamad AZ. The role of 3-dimensional
ultrasonography and magnetic resonance imaging in the
diagnosis of Mullerian duct anomalies: a review of the
literature. J Ultrasound Med 2008;27:413-23. Crossref
15. Ahmadi F, Haghighi H. Detection of congenital Mullerian
anomalies by real-time 3D sonography. J Reprod Infertil
2012;13:65-6.
16. Pellerito JS, McCarthy SM, Doyle MB, Glickman MG,
DeCherney AH. Diagnosis of uterine anomalies: relative
accuracy of MR imaging, endovaginal sonography, and
hysterosalpingography. Radiology 1992;183:795-800. Crossref
17. Heller-Boersma JG, Edmonds DK, Schmidt UH. A
cognitive behavioural model and therapy for utero-vaginal
agenesis (Mayer-Rokitansky-Kuster-Hauser syndrome:
MRKH). Behav Cogn Psychother 2009;37:449-67. Crossref
18. Heller-Boersma JG, Schmidt UH, Edmonds DK.
Psychological distress in women with uterovaginal agenesis
(Mayer-Rokitansky-Kuster-Hauser Syndrome, MRKH).
Psychosomatics 2009;50:277-81. Crossref
19. Patterson CJ, Crawford R, Jahoda A. Exploring the
psychological impact of Mayer-Rokitansky-Kuster-
Hauser syndrome on young women: an interpretative
phenomenological analysis. J Health Psychol 2016;21:1228-
40. Crossref
20. Wagner A, Brucker SY, Ueding E, et al. Treatment
management during the adolescent transition period of
girls and young women with Mayer-Rokitansky-Kuster-
Hauser syndrome (MRKHS): a systematic literature review.
Orphanet J Rare Dis 2016;11:152. Crossref
21. Frank R. The formation of an artificial vagina without
operation. Am J Obstet Gynecol 1938;35:1053-5. Crossref
22. Ingram JM. The bicycle seat stool in the treatment of
vaginal agenesis and stenosis: a preliminary report. Am J
Obstet Gynecol 1981;140:867-73. Crossref
23. Bach F, Glanville JM, Balen AH. An observational study of
women with Mullerian agenesis and their need for vaginal
dilator therapy. Fertil Steril 2011;96:483-6. Crossref
24. Callens N, De Cuypere G, Wolffenbuttel KP, et al. Long-term
psychosexual and anatomical outcome after vaginal dilation or vaginoplasty: a comparative study. J Sex Med
2012;9:1842-51. Crossref
25. Ketheeswaran A, Morrisey J, Abbott J, Bennett M, Dudley J,
Deans R. Intensive vaginal dilation using adjuvant
treatments in women with Mayer-Rokitansky-Kuster-Hauser syndrome: retrospective cohort study. Aust N Z J
Obstet Gynaecol 2018;58:108-13. Crossref
26. Kölle A, Taran FA, Rall K, Schöller D, Wallwiener D,
Brucker SY. Neovagina creation methods and their
potential impact on subsequent uterus transplantation: a
review. BJOG 2019;126:1328-35. Crossref
27. Cai B, Zhang JR, Xi XW, Yan Q, Wan XP. Laparoscopically
assisted sigmoid colon vaginoplasty in women with Mayer-Rokitansky-Kuster-Hauser syndrome: feasibility and
short-term results. BJOG 2007;114:1486-92. Crossref
28. Darai E, Toullalan O, Besse O, Potiron L, Delga P. Anatomic
and functional results of laparoscopic-perineal neovagina
construction by sigmoid colpoplasty in women with
Rokitansky’s syndrome. Hum Reprod 2003;18:2454-9. Crossref
29. Hiroi H, Yasugi T, Matsumoto K, et al. Mucinous
adenocarcinoma arising in a neovagina using the sigmoid
colon thirty years after operation: a case report. J Surg
Oncol 2001;77:61-4. Crossref
30. Kita Y, Mori S, Baba K, et al. Mucinous adenocarcinoma
emerging in sigmoid colon neovagina 40 years after its
creation: a case report. World J Surg Oncol 2015;13:213. Crossref
31. Idrees MT, Deligdisch L, Altchek A. Squamous papilloma
with hyperpigmentation in the skin graft of the neovagina
in Rokitansky syndrome: literature review of benign and
malignant lesions of the neovagina. J Pediatr Adolesc
Gynecol 2009;22:e148-55. Crossref
32. Brucker SY, Gegusch M, Zubke W, Rall K, Gauwerky JF,
Wallwiener D. Neovagina creation in vaginal agenesis:
development of a new laparoscopic Vecchietti-based
procedure and optimized instruments in a prospective
comparative interventional study in 101 patients. Fertil
Steril 2008;90:1940-52. Crossref
33. Routh JC, Laufer MR, Cannon GM Jr, Diamond DA,
Gargollo PC. Management strategies for Mayer-Rokitansky-Kuster-Hauser related vaginal agenesis: a cost-effectiveness
analysis. J Urol 2010;184:2116-21. Crossref
34. D’Alberton A, Santi F. Formation of a neovagina by coitus.
Obstet Gynecol 1972;40:763-4. Crossref
35. Moen MH. Creation of a vagina by repeated coital
dilatation in four teenagers with vaginal agenesis. Acta
Obstet Gynecol Scand 2000;79:149-50. Crossref
36. Liao LM, Doyle J, Crouch NS, Creighton SM. Dilation
as treatment for vaginal agenesis and hypoplasia: a pilot
exploration of benefits and barriers as perceived by
patients. J Obstet Gynaecol 2006;26:144-8. Crossref
37. Roberts CP, Haber MJ, Rock JA. Vaginal creation for
Mullerian agenesis. Am J Obstet Gynecol 2001;185:1349-52. Crossref