Hip fractures are preventable: a proposal for osteoporosis screening and fall prevention in older people
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Hip fractures are preventable: a proposal for
osteoporosis screening and fall prevention in older people
Timothy CY Kwok, MD, FHKAM (Medicine)1,2,3; SW Law, MB, ChB, FHKAM (Orthopaedic Surgery)3,4; Edward MF Leung, MB, BS, FRCP3,5; Dicky TK Choy, MB, ChB, DCH2,3; Patti MS Lam, BSc, MSc (Health Services Management)2; Jason CS Leung, MSc2; SH Wong, MB, BS, FHKAM (Orthopaedic Surgery)6; TP Ip, MB, BS, FHKAM (Medicine)6,7; CL Cheung, BSc, PhD6,8
1 Department of Medicine and Therapeutics, Prince of Wales Hospital, The
Chinese University of Hong Kong, Hong Kong
2 Jockey Club Centre for Osteoporosis Care and Control, The Chinese University of Hong Kong, Hong Kong
3 Hong Kong Osteoporosis Foundation
4 Department of Orthopaedics and Traumatology, Alice Ho Miu Ling Nethersole Hospital, Hong Kong
5 Hong Kong Association of Gerontology
6 The Osteoporosis Society of Hong Kong
7 Department of Medicine, Tung Wah Hospital, Hong Kong
8 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Prof Timothy CY Kwok (tkwok@cuhk.edu.hk)
Abstract
Osteoporosis is highly prevalent but underdiagnosed
and undertreated in Hong Kong. Fragility fractures
associated with osteoporosis often result in loss
of independence and increased mortality for
home-dwelling patients, imposing a high socio-economic
burden on society. This issue requires
urgent attention given the rapid growth of the
elderly population in Hong Kong by approximately
4.3% each year. To address this situation, a group
of experts convened to discuss practical ways to
reduce the burden of fractures and formulated three
recommendations: first, all men (aged ≥70 years)
and women (aged ≥65 years) should receive universal
dual-energy X-ray absorptiometry assessment for
osteoporosis. Second, all men (aged ≥70 years)
and women (aged ≥65 years) with a fracture-risk
assessment-derived 10-year risk (hip fracture with
bone mineral density) ≥3% should receive ≥3 years of
anti-osteoporotic treatment. Third, comprehensive
structured assessment (including dual-energy X-ray
absorptiometry) should be conducted in older
patients with a history of falling. By implementing
these recommendations, we estimate that we could
prevent 5234 hip fractures in 10 years, an annual
incidence reduction of approximately 7%, and
save HK$425 million in direct medical costs plus
substantial indirect savings. Ample clinical and cost-effectiveness
data support these recommendations, and studies in Hong Kong and abroad could serve
as models on how to implement them. We are
confident that by applying these recommendations
rigorously and systematically, a significant reduction
in hip fractures in Hong Kong is achievable.
Introduction
The Hong Kong eldercare and healthcare system
faces a high burden from osteoporosis and hip
fragility fractures. The prevalence of osteoporosis
in people aged ≥50 years in Hong Kong has been
measured as high as 37% in some studies.1 A study
of 4000 community-dwelling older people in Hong
Kong found that 7% of men and 11% of women had
≥1 incident of major osteoporotic fracture over
9.9 and 8.8 years of follow-up, respectively.2 The
number of patients admitted for hip fracture surgery
increased from 3678 in 2000 to an estimated 6300
in 2020,3 a 71.3% increase over 20 years. Although
the risk of geriatric hip fracture in Hong Kong is
declining slightly, this is outweighed by the growing
elderly population.3 The life expectancy in Hong
Kong (81.9 years for men; 87.6 years for women) is among the highest in the world,4 5 and projections
suggest that by 2036, 31.1% of the population will be
aged ≥65 years.6 Without intervention, the ageing of
the population is predicted to result in a considerable
increase in the incidence of fragility fracture, with
estimates for hip fractures rising to more than 14 500
in 2040.3
Direct costs per hip fracture to Hong Kong
public hospitals have been estimated at HK$81 120
(2017 data by Su et al7). This equates to an annual
estimated cost of HK$511 million for the territory’s
Hospital Authority. Although no estimates of indirect
costs are available for Hong Kong, Taiwanese data
from 2016 suggest they are likely to be substantial.
Estimated annual indirect costs in Taiwan are
HK$13 728, HK$3744, or HK$9687 per patient if
discharged to a nursing home, foreign-paid home care, or domestic-paid home care, respectively.8 A
Hong Kong study found that 23% of patients with
hip fractures who had previously been living at home
were discharged to residential care homes for the
elderly.9
Follow-up care after hip fracture in Hong Kong
is suboptimal, with low usage of bone-enhancing
medication with poor follow-up rates.9 In a territory-wide
retrospective study, only 8.2% of patients were
prescribed anti-osteoporotic medication during the
year after hip fracture.10 Among patients with primary
hip fractures, 6% had fracture complications, and 4%
had secondary fracture(s) within 12 months.9 The
post-discharge mortality rate was also high: 17.3%
died within 1 year of hip surgery versus 1.6% of age-matched
controls.9
Local data on other major types of osteoporotic
fractures are scarce. In the Hong Kong Osteoporosis
Study (HKOS),11 the incidence of vertebral fracture
was 194 per 100 000 person-years in men and 508
per 100 000 person-years women aged ≥50 years.12
In 2013, in Hong Kong, the prevalence of vertebral
fracture was 17% in men and 30% in women aged 70
to 79 years.13 Many vertebral fractures do not require
immediate medical attention, making their impact
difficult to study,13 but international data suggest
they are associated with substantial morbidity,
possibly contributing to increased mortality.14
European and North American studies have
demonstrated that dual-energy X-ray absorptiometry
(DXA) screening followed by osteoporosis
treatment in older people can substantially reduce
hip fracture rates by 25% to 50% in ≤5 years and is
cost-effective.15 16 17 Therefore, many fractures and associated complications, including secondary
fractures and mortality, could be prevented by
routine osteoporosis screening in older people and
timely treatment initiation in at-risk individuals.
The rising burden of fragility hip fractures
demands a response. Based on existing studies from
Hong Kong and abroad, we believe that reducing
this burden is achievable. Here, we provide clear,
practical, evidence-based recommendations on how
to reduce hip fracture rates in Hong Kong with the
goal of reducing incidence by 6.8% annually, thereby
curbing rising incidence.
Methods
Roundtable meetings of experts reviewing hip
fracture burden in Hong Kong were held in October
and December 2018 and June 2019. Tactics to relieve
the primary fracture burden were discussed, and
a consensus on a rigorous approach to screening,
prevention, and treatment was formed.
The calculated predictive performance and
simulated effects of our proposed strategies used a
similar population to that used by Su et al18 in the Mr
OS and Ms OS Hong Kong Cohort Study, employing
the same statistical model and simulation analysis
methods. Simulated analysis of the cohort was based
on DXA scans of the hip and spine performed on
men aged ≥70 years and women aged ≥65 years,
without pre-selection, followed by treatment if the
Fracture Risk Assessment Tool (FRAX; including
bone mineral density [BMD]) score was ≥3%.18
The number needed to DXA scan and number
needed to treat (NNT) were calculated using data
derived from the same simulated analysis as that
performed by Su et al.18 The present epidemiological
analysis assumes that only 50% of eligible patients
agree to undergo the DXA scan and engage in follow-up
steps.
Results
Better awareness of osteoporosis screening and
treatment among healthcare providers is urgently
needed, as is public financial support for evidence-based
tools that can reduce this problem. Discussions
resulted in the formulation of three evidence-based
recommendations.
Recommendation 1: Universal dual-energy
X-ray absorptiometry screening of the hip
and spine in all men aged ≥70 years and
women aged ≥65 years
Rationale for using age as screening criterion
We propose that the lower age limits for screening
of women and men be set to 65 and 70 years,
respectively, based on a 2017 study showing that the
average age of fragility fracture in Hong Kong was
82.1 years.9 From ages 65 to 69 years to 70 to 74 years, the annual incidence of hip fracture rose
exponentially from 156.0 to 364.4 per 100 000 person-years
in women and 102.6 to 212.2 per 100 000
person-years in men.19 The incidence continued to
double in every subsequent 5-year period.19 Our
proposed age thresholds align with those specified
in international guidelines and reflect the differing
ages at which the prevalence of reduced bone mass
increases in men and women.15 20
Rationale for universal screening
A prospective cohort study of almost 4000 men and
women aged ≥65 years in Hong Kong estimated the
effectiveness of hip fracture prevention strategies
using DXA.18 Based on the calculation method used
in this study, we recommend a strategy of universal
DXA measurement and treating patients with
FRAX (including BMD measurement) risk ≥3%. The
FRAX threshold of 3% was selected because our
statistical analysis suggests that this prevents the
greatest number of hip fractures while maintaining
acceptable NNT. Furthermore, this threshold aligns
with current local and international treatment
guidelines,13 and a United States study found that
this threshold was cost-effective.21
With our strategy, we found that one hip
fracture can be prevented for every 111 men (aged
≥70 years) DXA scanned, with 54 subsequently
treated; or for every 111 women (aged ≥65 years)
DXA scanned, with 73 subsequently treated (Table 1). Using the projected older population of 1.16
million,6 and assuming that only 50% of the target
population would be scanned, we could prevent
5234 hip fractures over the next 10 years.
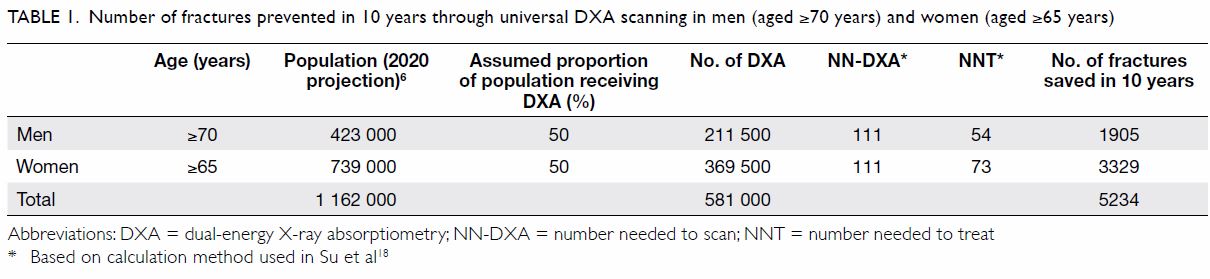
Table 1. Number of fractures prevented in 10 years through universal DXA scanning in men (aged ≥70 years) and women (aged ≥65 years)
To put the number of hip fractures prevented
into context, we considered the projected number
of hip fractures and the estimated growth rate. The
estimated number of hip fractures in 2020 according
to Man et al3 was 6300. If we apply the 4.3% estimated
annual growth of the older population to this figure,6
by 2029, we would expect 9167 hip fractures in Hong
Kong. Assuming the same 4.3% estimated annual
increase also applies to the number of fractures
prevented each year,6 we expect to prevent 431 hip
fractures in 2020, and 627 in 2029. Based on these
calculations, we estimate that by DXA screening and treating 581 000 men and women, the incidence
of hip fracture could be reduced by approximately
6.8% (Table 1). As the estimated growth of the older
population is 4.3% each year,6 an 6.8% annual hip
fracture reduction rate would outweigh the annual
increase in hip fracture cases among elderly patients
due to population growth.
Rationale for using dual-energy X-ray
absorptiometry
We selected DXA for screening because reduced
hip BMD in the femoral neck measured by DXA
is widely used in treatment guidelines to define
osteoporosis and set drug-treatment thresholds.22
Hip BMD is strongly associated with hip fracture
risk in both men and women23: its association with
fragility fracture is stronger than that of spine BMD
in Hong Kong Chinese people.24 25 Dual-energy X-ray
absorptiometry is cheaper than magnetic resonance
imaging (each DXA session cost approximately
HK$500 in Hong Kong in 2018)7; it is non-invasive,
and uses less irradiation than computed
tomography.26 Local guidelines do not recommend
the use of quantitative ultrasound for monitoring or
treatment of osteoporosis, and currently consider
the evidence for peripheral DXA to be insufficient.13
This recommendation is supported by health
economics studies. Screening strategies based on
DXA are more cost-effective in individuals aged
≥65 years than in younger people because hip fracture
is rare in the latter.15 American and European studies
have shown that screening strategies, including
DXA screening, are cost-effective for prevention of
hip fracture in elderly men and women.15 27 A recent
Hong Kong study that modelled screening strategies
found that DXA-based approaches, including
universal DXA with or without pre-screening,
were more cost-effective than no screening.7 In
women aged ≥65 years and men aged ≥70 years,
the incremental effectiveness gained by universal
DXA screening versus no screening incurred no
additional cost, which is well below the widely
acceptable willingness to pay (US$50 000 per quality-adjusted
life year).7 A modelling analysis using North
American data found that universal densitometry
and treatment with alendronate was highly cost-effective for women aged ≥65 years, and it was cost-saving
for ambulatory women aged ≥85 years.28 The
International Society for Clinical Densitometry
recommends DXA assessment for all women and
men aged ≥65 and ≥70 years, respectively.29 While
the United States Preventative Services Task Force
recommends screening in women aged ≥65 years
and in younger postmenopausal women with
elevated risk, it makes no recommendation for men
because of insufficient evidence.22 In Hong Kong,
however, the analysis of Su et al,18 which is based on
extensive local data, suggested that strategies using
DXA, including universal screening, can potentially
be cost-effective for fracture prevention in both
men and women at the age thresholds we propose.
Other countries such as Australia, Malaysia, Japan,
and Singapore also wholly or partially fund universal
DXA screening for older people.30 31 32 33
Recommendation 2: ≥3 years of treatment
with antiresorptive therapy for all men aged
≥70 years and women aged ≥65 years with
Fracture Risk Assessment Tool score ≥3%
Rationale for using Fracture Risk Assessment Tool
to guide treatment
Scanning alone would only be impactful if followed
up by a treatment plan. We recommend using FRAX
to guide treatment because it calculates the 10-year
fracture risk from a patient’s age, body mass index, and
risk-factor profile (fracture history, family history,
tobacco or alcohol use, use of oral glucocorticoids,
and presence of rheumatoid arthritis).34 It can be
used with or without BMD data; it is the most widely
adopted fracture risk assessment tool, and FRAX
has been extensively validated and adapted to local populations, including that of Hong Kong.34 35
The FRAX risk thresholds are widely used
to guide intervention in local and international
osteoporosis treatment guidelines.13 36 The 2019
European guidelines recommend country-specific
use of FRAX to assess risk in postmenopausal women,
including BMD in intermediate-risk individuals.34 An
example of a successful real-world fragility fracture-reducing
intervention incorporating FRAX has
been documented.37 A United Kingdom trial used a
questionnaire incorporating FRAX to promote DXA
screening in women aged 70 to 85 years, leading to a
28% hip fracture reduction over 5 years.37
Rationale for treatment duration of ≥3 years
We suggest 3 years as the duration of antiresorptive
treatment based on local studies of alendronate,
which showed increases in BMD of 5% to 6% at the
lumbar spine and 3% to 4% at the hip after 1 year
of treatment in postmenopausal women with
osteoporosis.38 39 Local guidelines recommend 5 years
of initial therapy with oral bisphosphonates but
acknowledge that no consensus exists regarding
the optimal treatment duration.13 We believe that
3 years of duration offers an appropriate balance
of efficacy and cost. Additional financial support
for longer-term therapy may become feasible
when our recommendations begin demonstrating
effectiveness in fragility fracture reduction, with
improved compliance, during the first 3 years.
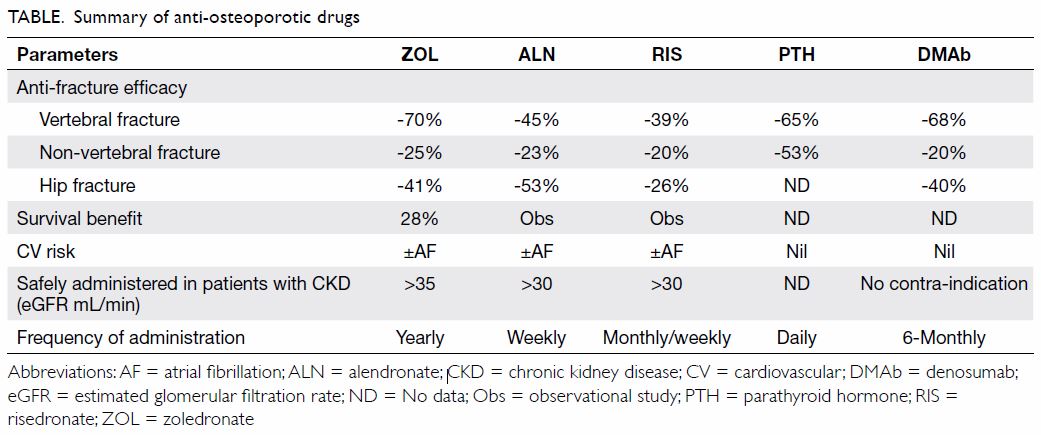
Rationale for treatment with antiresorptive drugs
Current osteoporosis treatments are appropriate for
addressing the hip fracture burden in Hong Kong
(Table 213). The efficacy and safety of locally approved antiresorptive therapies have been extensively
validated in numerous clinical trials in men and
postmenopausal women, including long-term
studies.40 41 42 Cost-effectiveness analyses support their
use for fracture prevention, with gains in quality-adjusted
life years for patients and incremental
cost-effectiveness ratios well within acceptability
thresholds (vs no treatment).7 21 A meta-analysis of
eight studies of four agents found that antiresorptive
therapy for osteoporosis led to significant mortality-rate
reductions.43 Recently, a study in Hong Kong
showed that real-world use of bisphosphonates
among hip fracture patients may protect against
cardiovascular diseases.10 Adverse event (AE)
imbalances suggesting cardioprotective effects were
also observed in a randomised trial of zoledronate
in 2000 women with osteopenia in New Zealand.44
Bisphosphonates are easily accessible in Hong Kong,
and many patients with osteoporosis could feasibly
be treated in primary care.13 Our analysis found that,
with universal DXA scanning of men aged ≥70 years
and women aged ≥65 years, a treatment threshold of
FRAX (with BMD) risk ≥3% for hip fracture yields
a NNT of 54 for men and 73 for women to prevent
one fracture.
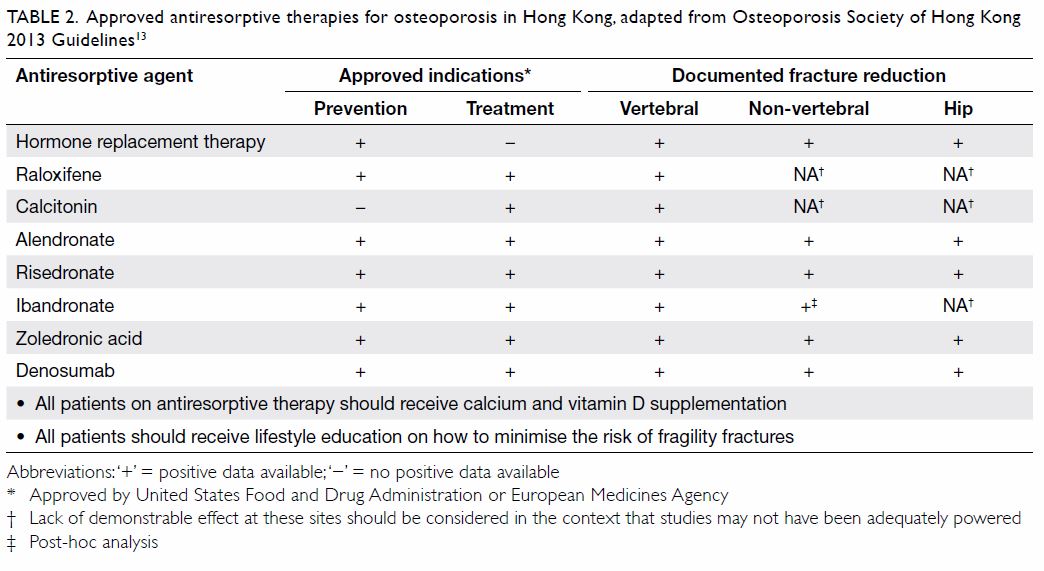
Table 2. Approved antiresorptive therapies for osteoporosis in Hong Kong, adapted from Osteoporosis Society of Hong Kong 2013 Guidelines13
In line with local treatment guidelines, we
recommend lifestyle measures, including a balanced
diet rich in calcium and vitamin D, regular exercise,
avoidance of smoking or excessive alcohol intake, and
adequate sunlight exposure, as a universal approach
to prevention and non-pharmacological management
of osteoporosis.13 Vitamin D supplementation should
be given alongside antiresorptive medications,
and calcium supplementation is recommended for
those unable to achieve adequate levels through
diet alone.13 This is particularly pertinent in Hong
Kong, where dietary vitamin D intake is generally
low and the prevalence of vitamin D insufficiency
or deficiency has been observed to be high.45 A
Cochrane systematic review found that exercise
has a small but significant effect on BMD in
postmenopausal women with osteoporosis.46 People
with osteoporosis and high hip fracture risk should
be treated with antiresorptive drugs combined with calcium or vitamin D and physical exercise.
Osteonecrosis of the jaw (ONJ) and atypical
femoral fracture (AFF) are well-known AEs of
antiresorptive therapies.13 47 However, the absolute
risk of ONJ with the administration of antiresorptive
therapy in patients with osteoporosis patients is low,
and the risk-benefit balance is highly favourable.48 49
Risk-minimising precautions against ONJ,
including close attention to good dental hygiene
during treatment and endodontic (rather than
surgical) corrective procedures, have already been
incorporated into local guidelines.13
Poor adherence is a challenge for long-term
use of oral bisphosphonates in osteoporosis.50 51 One
study showed persistence declining to <50% over
2 years.51 Factors associated with poor adherence
include advanced age, fear of AEs, and inadequate
education.52 Improving patient education,53
using antiresorptive therapies with longer dosing
intervals,54 and managing AEs appropriately may
help to improve adherence.55
Cost of Recommendations 1 and 2
For screening and follow-up treatment programmes
to be worthwhile, assessing the cost-benefit ratio is
important. The estimated costs of Recommendation
1 (universal DXA screening in men aged ≥70 years
and women aged ≥65 years) in 10 years are shown
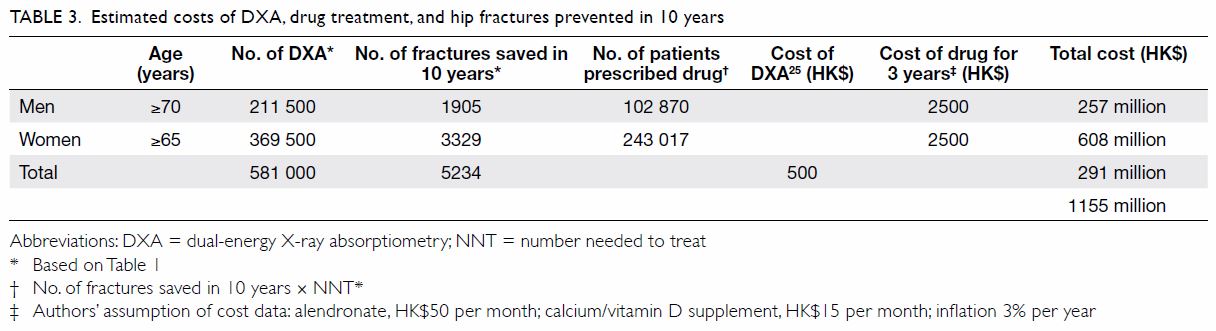
in Table 3.25
We assumed that 3 years of generic alendronate
plus calcium and vitamin D supplementation would
be given for treatment, costing approximately
HK$2500 (accounting for inflation; Table 325).
We chose alendronate because it is currently the
lowest-priced generic antiresorptive; patients
may self-fund other options should they prefer.
For instance, denosumab may be an option,
particularly for patients with high fracture risk,
poor adherence, contraindications, or intolerance
to bisphosphonates.13 49 The incidence of ONJ in
bisphosphonate-treated patients with osteoporosis
in Hong Kong appears to be low (73.53 per
100 000 person-years of oral treatment), and the reported cases were in patients with readily
modifiable risk factors.56 Estimates for the incidence
of AFF in Hong Kong are not available, but data
from abroad suggest that AFF is also very rare
(13.6 per 100 000 patient-years at 2.0-3.9 years of
bisphosphonate use).57 On this basis, we have not
included the costs associated with ONJ and AFF in
our calculations, as the associated cost is likely to be
low.
The total cost of Recommendations 1 and 2 is
HK$1155 million in 10 years (Table 325). In terms of
benefits, we estimate that our proposals will prevent
5234 hip fractures over 10 years; if the estimated
direct cost per hip fracture is HK$81 120 (2017),7 this
would save the healthcare system HK$425 million
in 10 years, with further savings from reduced
outpatient and indirect costs. Additionally, elderly
people and their families would avoid costs associated
with institutional care. Based on 6300 hip fractures
in Hong Kong in 2020,3 fracture reduction of 6.8%,
the proportion of all patients with hip fractures
discharged to institutional care (17%),9 and local
institutional care costs (HK$120 000 annually for
5 years), we estimate that the savings from reduced
institutional care needs would be HK$534 million
in 10 years. Therefore, the total cost savings would
be HK$959 million over 10 years; it should be
noted, though, that this estimation is conservative
and has not taken into account the year-on-year
increase in hip fracture rate or inflation. Although
the prevention of hip fractures alone may not reduce
the institutional care cost for all patients, it would
still likely be associated with substantial cost savings
for the majority of patients. A full cost-effectiveness
analysis of our proposal has not been performed, but
the above cost-savings calculation has not included
other fragility fractures, indirect healthcare cost
savings (eg, transportation, costs associated with
work productivity in family members), and inflation.
Therefore, we believe that our recommendations are
potentially cost-effective.
Recommendation 3: Comprehensive
structured assessment (including dual-energy
X-ray absorptiometry) of older people with a
history of falling
Rationale for assessing patients with a history of
falls
Because of ageing of muscles and declining balance
control, older adults are more likely to experience
falls than their younger counterparts.58 Fall history
is a risk factor for subsequent falls59 60 61 and an
independent predictor of fracture in older men and
women.62 Recently, a hip fracture prediction score
(HKOS score) was developed and validated for older
adults aged ≥80 years in Hong Kong, and fall history
is one of the risk factors included.63
Older people who have a history of falling
should therefore be offered a comprehensive fall risk
assessment. We also recommend DXA screening
in these high-risk individuals. Although a history
of falling is a good reason for fall risk and DXA
assessments, these assessments should not be
confined to those with a history of falling. Guidelines
from the American Geriatrics Society/British
Geriatrics Society recommend annual screening of
all community-dwelling older people aged ≥65 years
for falls and risk of falling.64
A meta-analysis of 159 trials in over 79 000
community-dwelling patients, including diverse
fall-risk reduction strategies, concluded that home
or group-based exercise programmes, including
Tai Chi and home modifications, can reduce fall
risk and fall-related fracture.65 In a study of elderly
Hong Kong patients with a history of falling, a single
risk assessment and home modification visit by an
occupational therapist resulted in a reduced fall
prevalence in the following 12 months compared
with controls (13.7% vs 20.4%, P=0.03).66
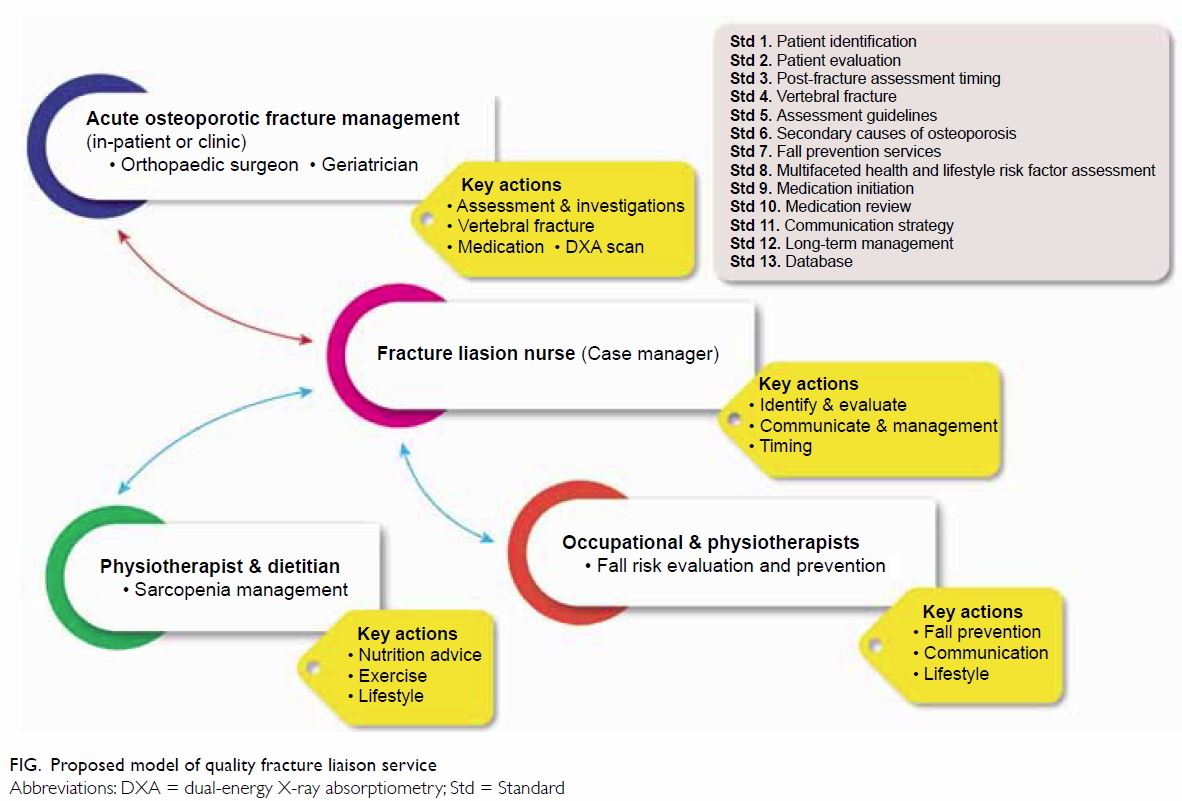
Suggestions for provision of care
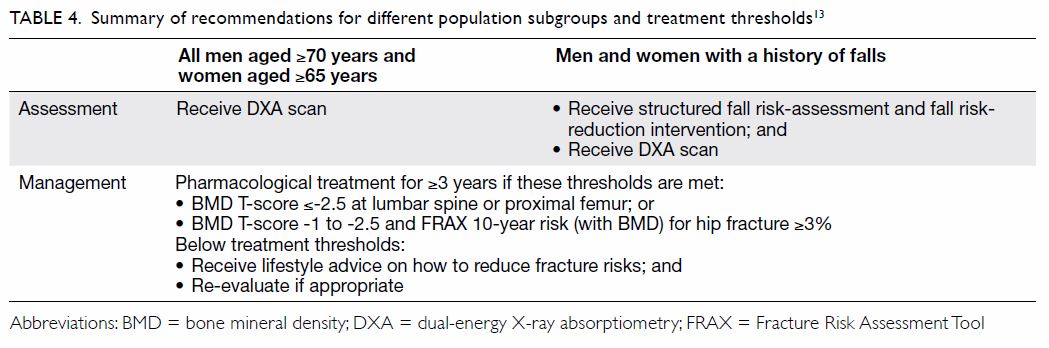
Table 413 summarises the key elements of our
recommendations, including treatment thresholds
specified by local guidelines. Osteoporosis and fall
risk assessment can be managed in the primary care
setting, especially in Hong Kong, where access to
DXA screening is excellent.
To promote DXA screening, Shepstone et al37
showed that a self-completed questionnaire
capturing FRAX risk factors was effective at alerting
older women to their fracture risk, resulting in a 28%
reduction in hip fracture incidence in 5 years. On
the basis of local prospective data, we demonstrated
that combining a short questionnaire for sarcopenia
(SARC-F) and the FRAX questionnaire could identify
>75% of older people who suffered an incident hip
fracture within 10 years.18 Furthermore, the well-calibrated
HKOS score may accurately identify the
oldest old (≥80 years) who are at high hip fracture
risk.63
Conclusion and call to action
The high primary hip fracture burden in Hong Kong
can be reduced with an evidence-based screening
programme. High-risk patients identified by our
proposed screening can be effectively managed using
well-established and affordable therapies within the
primary care field. Our proposals are supported by
extensive evidence: similar programmes have shown
efficacy, both in Hong Kong and abroad.
We propose that the Hong Kong government
provide public funding support for a universal DXA
screening programme for all men aged ≥70 years
and women aged ≥65 years. Patients with a history of falling must receive systematic fracture-risk
evaluations; patients with high fracture risk
identified through these measures should be treated
with antiresorptive agents and receive appropriate
multidisciplinary follow-up.
We recommend a multidisciplinary
effort including colleagues in general practice,
orthopaedics, geriatric medicine, etc, to improve
awareness of already available screening tools and
effective treatment options for osteoporosis. If
properly implemented and supported, we believe
that our goal of a 7% annual reduction in hip fracture
incidence in Hong Kong can be achieved, helping to
offset the annual increase in the elderly population.
Adoption of our proposals will shift government
spending from post-fracture management to
proactive osteoporosis management and fracture
prevention. If our proposals are adopted, the
estimated HK$1 billion that would be spent in
10 years on post-fracture management will instead
be spent on improved osteoporosis management
and fracture prevention for tens of thousands of
patients. Furthermore, preventing fractures would
also preserve the quality of life of many patients
and their families. Achieving better control of hip
fracture burden will be challenging, but it is well
within our reach.
Author contributions
All authors contributed to the literature review and
recommendations, critically revised the manuscript for
important intellectual content, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
This publication is funded by a supportive grant from Amgen Asia Holding Limited to the Hong Kong Osteoporosis
Foundation (HKOF) and the Osteoporosis Society of Hong
Kong (OSHK) Consensus Group on Prevention of Fractures in
Older People. All authors declare that they have no additional
conflicts of interest.
Acknowledgement
Editorial support for this manuscript was provided by Magdalene Chu and Dr Alister Smith of MIMS Hong Kong,
funded by the Hong Kong Osteoporosis Foundation.
Funding/support
This publication is funded by a supportive grant from Amgen Asia Holding Limited to the Hong Kong Osteoporosis
Foundation (HKOF) and the Osteoporosis Society of Hong
Kong (OSHK) Consensus Group on Prevention of Fractures
in Older People.
References
1. Yu F, Xia W. The epidemiology of osteoporosis, associated
fragility fractures, and management gap in China. Arch
Osteoporos 2019;14:32. Crossref
2. Su Y, Leung J, Hans D, Aubry-Rozier B, Kwok T. Added
clinical use of trabecular bone score to BMD for major
osteoporotic fracture prediction in older Chinese people:
the Mr. OS and Ms. OS cohort study in Hong Kong.
Osteoporos Int 2017;28:151-60. Crossref
3. Man LP, Ho AW, Wong SH. Excess mortality for operated
geriatric hip fracture in Hong Kong. Hong Kong Med J
2016;22:6-10. Crossref
4. The World Bank. World Development Indicators. Available
from: https://datacatalog.worldbank.org/dataset/world-development-indicators. Accessed 18 Mar 2019.
5. Department of Health, Hong Kong SAR Government. Life
expectancy. Available from: https://www.healthyhk.gov.hk/phisweb/en/healthy_facts/health_indicators/life_exp/. Accessed 3 Dec 2019.
6. Census and Statistics Department, Hong Kong SAR
Government. Hong Kong Population Projections 2017-2066. Available from: https://www.statistics.gov.hk/pub/B1120015072017XXXXB0100.pdf. Accessed 1 Dec 2019.
7. Su Y, Lai FT, Yip BH, Leung JC, Kwok TC. Cost-effectiveness
of osteoporosis screening strategies for hip
fracture prevention in older Chinese people: a decision
tree modeling study in the Mr. OS and Ms. OS cohort in
Hong Kong. Osteoporos Int 2018;29:1793-805. Crossref
8. Chan DC, McCloskey EV, Chang CB, et al. Establishing
and evaluating FRAX® probability thresholds in Taiwan. J
Formos Med Assoc 2017;116:161-8. Crossref
9. Leung KS, Yuen WF, Ngai WK, et al. How well are we managing fragility hip fractures? A narrative report on
the review with the attempt to setup a Fragility Fracture
Registry in Hong Kong. Hong Kong Med J 2017;23:264-71. Crossref
10. Sing CW, Wong AY, Kiel DP, et al. Association of
alendronate and risk of cardiovascular events in patients
with hip fracture. J Bone Miner Res 2018;33:1422-34. Crossref
11. Cheung CL, Tan KC, Kung AW. Cohort Profile: The Hong
Kong Osteoporosis Study and the follow-up study. Int J
Epidemiol 2018;47:397-8f. Crossref
12. Bow CH, Cheung E, Cheung CL, et al. Ethnic difference of
clinical vertebral fracture risk. Osteoporos Int 2012;23:879-
85. Crossref
13. OSHK Task Group for Formulation of 2013 OSHK
Guideline for Clinical Management of Postmenopausal
Osteoporosis in Hong Kong, Ip TP, Cheung SK, et al. The
Osteoporosis Society of Hong Kong (OSHK): 2013 OSHK
guideline for clinical management of postmenopausal
osteoporosis in Hong Kong. Hong Kong Med J 2013;19
Suppl 2:1-40.
14. Hirsch JA, Beall DP, Chambers MR, et al. Management
of vertebral fragility fractures: a clinical care pathway
developed by a multispecialty panel using the RAND/UCLA Appropriateness Method. Spine J 2018;18:2152-61. Crossref
15. Dell R, Greene D. Is osteoporosis disease management cost
effective? Curr Osteoporos Rep 2010;8:49-55. Crossref
16. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis
disease management: the role of the orthopaedic surgeon.
J Bone Joint Surg Am 2008;90 Suppl 4:188-94. Crossref
17. Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood
GC. Osteoporosis disease management in a rural health
care population: hip fracture reduction and reduced costs
in postmenopausal women after 5 years. Osteoporos Int
2003;14:146-51. Crossref
18. Su Y, Woo JW, Kwok TC. The added value of SARC-F to
prescreening using FRAX for hip fracture prevention in
older community adults. J Am Med Dir Assoc 2019;20:83-9. Crossref
19. Tsang SW, Kung AW, Kanis JA, Johansson H, Oden A.
Ten-year fracture probability in Hong Kong Southern
Chinese according to age and BMD femoral neck T-scores.
Osteoporos Int 2009;20:1939-45. Crossref
20. Cheung CL, Ang SB, Chadha M, et al. An updated hip
fracture projection in Asia: the Asian Federation of
Osteoporosis Societies study. Osteoporos Sarcopenia
2018;4:16-21. Crossref
21. Tosteson AN, Melton LJ 3rd, Dawson-Hughes B, et al.
Cost-effective osteoporosis treatment thresholds: the
United States perspective. Osteoporos Int 2008;19:437-47. Crossref
22. US Preventive Services Task Force, Curry SJ, Krist AH,
et al. Screening for Osteoporosis to prevent fractures: US
preventive services task force recommendation statement.
JAMA 2018;319:2521-31. Crossref
23. Cummings SR, Cawthon PM, Ensrud KE, et al. BMD
and risk of hip and nonvertebral fractures in older men:
a prospective study and comparison with older women. J
Bone Miner Res 2006;21:1550-6. Crossref
24. Kung AW, Lee KK, Ho AY, Tang G, Luk KD. Ten-year risk of
osteoporotic fractures in postmenopausal Chinese women
according to clinical risk factors and BMD T-scores: a
prospective study. J Bone Miner Res 2007;22:1080-7. Crossref
25. Bow CH, Tsang SW, Loong CH, Soong CS, Yeung SC,
Kung AW. Bone mineral density enhances use of clinical
risk factors in predicting ten-year risk of osteoporotic fractures in Chinese men: the Hong Kong Osteoporosis
Study. Osteoporos Int 2011;22:2799-807. Crossref
26. Choi YJ. Dual-energy X-ray absorptiometry: beyond bone
mineral density determination. Endocrinol Metab (Seoul)
2016;31:25-30. Crossref
27. Nayak S, Greenspan SL. Cost-effectiveness of osteoporosis
screening strategies for men. J Bone Miner Res
2016;31:1189-99. Crossref
28. Schousboe JT, Ensrud KE, Nyman JA, Melton LJ 3rd, Kane
RL. Universal bone densitometry screening combined with
alendronate therapy for those diagnosed with osteoporosis
is highly cost-effective for elderly women. J Am Geriatr Soc
2005;53:1697-704. Crossref
29. International Society for Clinical Densitometry. 2019 ISCD
Official Positions–Adult. International Society for Clinical
Densitometry (ISCD). Available from: https://www.iscd.
org/official-positions/2019-iscd-official-positions-adult/.
Accessed 7 Aug 2019.
30. The Department of Health. Australian Government. Bone
densiometry. Available from: https://www1.health.gov.au/
internet/main/publishing.nsf/Content/diagnosticimagingbd.
htm. Accessed 7 Aug 2019.
31. International Osteoporosis Foundation. 2013 Asia-
Pacific Audit: Malaysia. Available from: https://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit-Malaysia_0_0.pdf. Accessed 23 Oct 2019.
32. International Osteoporosis Foundation. The Asian Audit.
Epidemiology, costs and burden of osteoporosis in Asia
2009. Available from: https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf. Accessed 23 Oct 2019.
33. International Osteoporosis Foundation. 2013 Asia-
Pacific Audit: Singapore. Available from: https://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit-Singapore_0_0.pdf. Accessed 23 Oct 2019.
34. Kanis JA, Cooper C, Rizzoli R, et al. European guidance
for the diagnosis and management of osteoporosis in
postmenopausal women. Osteoporos Int 2019;30:3-44. Crossref
35. The University of Sheffield, UK. FRAX Fracture Risk
Assessment Tool (Hong Kong). Available from: https://www.sheffield.ac.uk/FRAX/tool.aspx?country=20. Accessed 20 Mar 2019.
36. Kanis JA, Harvey NC, Cooper C, et al. A systematic review
of intervention thresholds based on FRAX: a report
prepared for the National Osteoporosis Guideline Group
and the International Osteoporosis Foundation. Arch
Osteoporos 2016;11:25. Crossref
37. Shepstone L, Lenaghan E, Cooper C, et al. Screening in the
community to reduce fractures in older women (SCOOP):
a randomised controlled trial. Lancet 2018;391:741-7. Crossref
38. Kung AW, Yeung SS, Chu LW. The efficacy and tolerability
of alendronate in postmenopausal osteoporotic Chinese
women: a randomized placebo-controlled study. Calcif
Tissue Int 2000;67:286-90. Crossref
39. Lau EM, Woo J, Chan YH, Griffith J. Alendronate prevents
bone loss in Chinese women with osteoporosis. Bone
2000;27:677-80. Crossref
40. Tu KN, Lie JD, Wan CK, et al. Osteoporosis: a review of
treatment options. P T 2018;43:92-104.
41. Zhou J, Ma X, Wang T, Zhai S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network
meta-analyses. Osteoporos Int 2016;27:3289-300. Crossref
42. Lyu H, Jundi B, Xu C, et al. Comparison of denosumab and
bisphosphonates in osteoporosis patients: a meta-analysis
of randomized controlled trials. J Clin Endocrinol Metab
2019;104:1753-65. Crossref
43. Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of
osteoporosis treatment on mortality: a meta-analysis. J
Clin Endocrinol Metab 2010;95:1174-81. Crossref
44. Reid IR, Horne AM, Mihov B, et al. Fracture prevention
with zoledronate in older women with osteopenia. N Engl
J Med 2018;379:2407-16. Crossref
45. Leung RY, Cheung BM, Nguyen US, Kung AW, Tan KC,
Cheung CL. Optimal vitamin D status and its relationship
with bone and mineral metabolism in Hong Kong Chinese.
Bone 2017;97:293-8. Crossref
46. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing
and treating osteoporosis in postmenopausal women.
Cochrane Database Syst Rev 2011;(7):CD000333. Crossref
47. Amgen. Prolia (denosumab prescribing information).
Highlights of prescribing information. Prolia (denosumab
prescribing information). Available from: https://www.
pi.amgen.com/~/media/amgen/repositorysites/pi-amgencom/
prolia/prolia_pi.pdf. Accessed 25 Apr 2019.
48. Khan AA, Morrison A, Hanley DA, et al. Diagnosis and
management of osteonecrosis of the jaw: a systematic
review and international consensus. J Bone Miner Res
2015;30:3-23. Crossref
49. Bone HG, Wagman RB, Brandi ML, et al. 10 Years of
denosumab treatment in postmenopausal women with
osteoporosis: results from the phase 3 randomised
FREEDOM trial and open-label extension. Lancet Diabetes
Endocrinol 2017;5:513-23. Crossref
50. Abrahamsen B. Are long-term bisphosphonate users
a reality? Dose years for current bisphosphonate users
assessed using the Danish National Prescription Database.
Osteoporos Int 2013;24:369-72. Crossref
51. Burden AM, Paterson JM, Solomon DH, Mamdani M,
Juurlink DN, Cadarette SM. Bisphosphonate prescribing,
persistence and cumulative exposure in Ontario, Canada.
Osteoporos Int 2012;23:1075-82. Crossref
52. Diab DL, Watts NB. Postmenopausal osteoporosis. Curr
Opin Endocrinol Diabetes Obes 2013;20:501-9. Crossref
53. Sewerynek E, Horst-Sikorska H, Stępień-Kłos W, et al.
The role of counselling and other factors in compliance of
postmenopausal osteoporotic patients to alendronate 70
therapy. Arch Med Sci 2013;9:288-96. Crossref
54. Cooper A, Drake J, Brankin E, PERSIST Investigators.
Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results
from the PERSIST study. Int J Clin Pract 2006;60:896-905. Crossref
55. Inderjeeth CA, Inderjeeth AJ, Raymond WD. Medication
selection and patient compliance in the clinical management
of osteoporosis. Aust Fam Physician 2016;45:814-7.
56. Kwok T, Choy TK, Kwok WL. Prevalence of
bisphosphonate-related osteonecrosis of the jaw in Hong
Kong. Hong Kong Med J 2016;22 Suppl 2:S46-7.
57. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical
nontraumatic diaphyseal fractures of the femur. J Bone
Miner Res 2012;27:2544-50. Crossref
58. Guerado E, Sandalio RM, Caracuel Z, Caso E.
Understanding the pathogenesis of hip fracture in the
elderly, osteoporotic theory is not reflected in the outcome
of prevention programmes. World J Orthop 2016;7:218-28. Crossref
59. Scott V, Votova K, Scanlan A, Close J. Multifactorial and
functional mobility assessment tools for fall risk among
older adults in community, home-support, long-term and
acute care settings. Age Ageing 2007;36:130-9. Crossref
60. Wu TY, Chie WC, Yang RS, Kuo KL, Wong WK, Liaw CK.
Risk factors for single and recurrent falls: a prospective
study of falls in community dwelling seniors without
cognitive impairment. Prev Med 2013;57:511-7. Crossref
61. Woo J, Leung J, Wong S, Kwok T, Lee J, Lynn H. Development of a simple scoring tool in the primary care
setting for prediction of recurrent falls in men and women
aged 65 years and over living in the community. J Clin Nurs
2009;18:1038-48. Crossref
62. Harvey NC, Odén A, Orwoll E, et al. Falls predict fractures independently of FRAX probability: a meta-analysis of the
osteoporotic fractures in men (MrOS) study. J Bone Miner
Res 2018;33:510-6. Crossref
63. Lam MT, Sing CW, Li GH, Kung AW, Tan KC, Cheung CL. Development and validation of a risk score to predict the
first hip fracture in the oldest old: a retrospective cohort
study. J Gerontol A Biol Sci Med Sci 2020;75:980-6. Crossref
64. Panel on Prevention of Falls in Older Persons, American Geriatrics Society, British Geriatrics Society. Summary
of the Updated American Geriatrics Society/British
Geriatrics Society clinical practice guideline for prevention
of falls in older persons. J Am Geriatr Soc 2011;59:148-57. Crossref
65. Gillespie LD, Robertson MC, Gillespie WJ, et al.
Interventions for preventing falls in older people
living in the community. Cochrane Database Syst Rev
2012;(9):CD007146. Crossref
66. Chu MM, Fong KN, Lit AC, et al. An occupational therapy
fall reduction home visit program for community-dwelling
older adults in Hong Kong after an emergency department
visit for a fall. J Am Geriatr Soc 2017;65:364-72. Crossref