Hong Kong consensus recommendations on the management of pancreatic ductal adenocarcinoma
Stephen L Chan, MD, FRCP1 #; CL Chiang, FRCR, FHKAM (Radiology)2 #; Kenneth SH Chok, MD, MS3 #; AS Lee, MB, ChB, FRCR4; Raymond SY Tang, MD5; Fiona MY Lim, FRCR, FHKAM (Radiology)6,7; KF Lee, FRCSEd, FHKAM (Surgery)8; Anna YP Tai, FRCR, FHKAM (Radiology)9; Sarah WM Lee, FRCR, FHKAM (Radiology)7; Regina CL Lo, MD, FRCPA7; Anthony WH Chan, FRCPA, FHKAM (Pathology)10; Francis PT Mok, FRCSEd, FRACS11; Endorsed by the Hong Kong Society of Hepatobiliary and Pancreatic Surgery and the Hong Kong Cancer Therapy Society
1 Department of Clinical Oncology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Clinical Oncology, Queen Mary Hospital, Hong Kong SAR, China
3 Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
4 Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
5 Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
6 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
7 Department of Pathology, School of Clinical Medicine, The University of Hong Kong, Hong Kong SAR, China
8 Department of Surgery, Prince of Wales Hospital, Hong Kong SAR, China
9 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China
10 Department of Anatomical and Cellular Pathology, Faculty of Medicine,
The Chinese University of Hong Kong, Hong Kong SAR, China
11 Department of Surgery and Combined Endoscopy Unit, Caritas Medical Centre, Hong Kong SAR, China
# Equal contribution
 Full paper in PDF
Full paper in PDF
Abstract
This project was undertaken to develop the
first set of consensus statements regarding the
management of pancreatic ductal adenocarcinoma
(PDAC) in Hong Kong, with the goal of providing
guidance to local clinicians. A multidisciplinary
panel of experts discussed issues surrounding
current PDAC management and reviewed evidence
gathered in the local context to propose treatment
recommendations. The experts used the Delphi
approach to finalise management recommendations.
Consensus was defined as ≥80% acceptance among
all expert panel members. Thirty-nine consensus
statements were established. These statements
cover all aspects of PDAC management, including
diagnosis, resectability criteria, treatment modalities
according to resectability, personalised management
based on molecular profiling, palliative care, and
supportive care. This project fulfils the need for
guidance regarding PDAC management in Hong
Kong. To assist clinicians with treatment decisions
based on varying levels of evidence and clinical
experience, treatment options are listed in several
consensus statements.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), a
malignant pancreatic epithelial tumour characterised
by glandular and ductal differentiation, constitutes
>90% of all pancreatic cancers and is usually
considered synonymous with the term ‘pancreatic
cancer’ itself.
1 Although the exact aetiology of PDAC
is unknown, many risk factors have been linked to
its development, including smoking, obesity, alcohol
intake, diabetes mellitus, chronic pancreatitis, and familial cancer syndromes.
2 3 4 Pancreatic ductal
adenocarcinoma is usually diagnosed in individuals
aged >70 years, with a male-to-female ratio of
1.4:1.0. Its incidence has been increasing worldwide,
particularly among individuals aged >50 years and
among women.
4 In 2020, PDAC had the 14th highest
incidence among cancers: approximately 495 773
people were diagnosed with PDAC, constituting
2.6% of new cancer cases.
5 Moreover, PDAC was
the eighth most common cause of cancer death in 2020, with 466 003 deaths (4.7% of all cancer deaths
worldwide).
5 In China, PDAC is one of the top 10
most common cancers in men and one of the top
10 most common causes of death among men and
women.
6
Pancreatic ductal adenocarcinoma, a highly
aggressive malignancy with a poor prognosis, has
one of the lowest 5-year survivals among cancers
(11%).
7 Surgical resection of localised disease
provides the best likelihood of a curative outcome,
but approximately 80% to 85% of cases are diagnosed
at an advanced, unresectable, or metastatic stage that
requires palliative management.
2 Although resection
of localised disease with adjuvant chemotherapy can
improve 5-year survival to approximately 30%, this
outcome depends upon complete removal of the
primary tumour and regional lymph nodes, a complex
procedure with a high rate of complications.
8
In Hong Kong, the incidence of PDAC has
been increasing since 2010; it had become the fifth
leading cause of cancer-related death by 2019.
9
Considering the challenges of late diagnosis, poor
clinical prognosis, and limited therapeutic options,
PDAC has emerged as a key local health concern.
Our group was established to develop the first
set of consensus recommendations regarding the
management of PDAC in Hong Kong. We initiated
this project to provide practical guidance to Hong
Kong healthcare practitioners based on the best
available evidence and expert opinions.
Methods
Based on a literature search in MEDLINE to identify
articles published in the past 10 years, consensus
development leads the first, second, and third
authors brainstormed and drafted preliminary
statements relevant to PDAC management that addressed diagnosis, imaging, and surveillance;
resectability criteria; stent management; stage-specific
treatment; personalised medicine; and
palliative care and supportive care. Subsequently,
they invited nine Hong Kong experts to complete
a 12-member consensus expert panel comprising
clinical oncologists, surgeons, a gastroenterologist,
and pathologists. All panel members were asked to
review the draft statements in the context of current
local practice and available evidence, then discuss
these issues during the consensus meeting.
A virtual consensus meeting was held on 12
February 2022 to refine and vote on the statements.
The consensus statements were developed through
the Delphi process: after discussion, the members
independently voted on each statement using a
5-point Likert scale (A: accept completely; B: accept
with minor reservations; C: accept with major
reservations; D: reject with reservations; E: reject
completely). A consensus was reached if at least 80%
of the panel members agreed with the statement (ie,
selected either ‘accept completely’ or ‘accept with
minor reservations’). If acceptance was <80%, the
panel members identified key concerns and proposed
revisions before a second vote. When applicable, the
level of evidence was evaluated using the Oxford
Centre for Evidence-Based Medicine 2011 Levels of
Evidence.
10
Consensus statements
Diagnosis
Statement 1: Early symptoms of pancreatic cancer result from a mass effect.
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Statement 2: In addition to progressive jaundice, patients may present with nonspecific symptoms
including abdominal pain, weight loss, and new-onset/recently worsening diabetes. A differential
diagnosis of PDAC should be considered in the
presence of the above symptoms.
A: 80%; B: 20%; C: 0%; D: 0%; E: 0%
Statement 3: The involvement of a multidisciplinary team is recommended for diagnosis and disease management.
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
The clinical presentation of PDAC varies
according to whether the tumour is in the pancreatic
head, neck, or tail, which would affect adjacent
structures. For example, jaundice can be related
to tumours in the head due to obstruction of the
common bile duct, whereas pain can be related
to effects on nearby vessels from tumours in the
pancreas.
11 12 However, many patients present with
nonspecific symptoms that may be attributed to
other diseases and cause further diagnostic delays
(
Table 1).
12 13 14 15 These symptoms should alert general practitioners and other healthcare professionals to
consider PDAC as a differential diagnosis. Clinicians
should attempt to distinguish stone-related
obstruction from malignancy-related obstruction.
In our clinical experience, stone-related obstruction
usually causes intermittent jaundice, whereas
malignancy-related obstruction causes progressive
jaundice. Notably, Chinese patients typically have
clay-coloured stool. They rarely present with the
steatorrhea that is common among Western patients
experiencing chronic pancreatitis from alcohol
consumption.
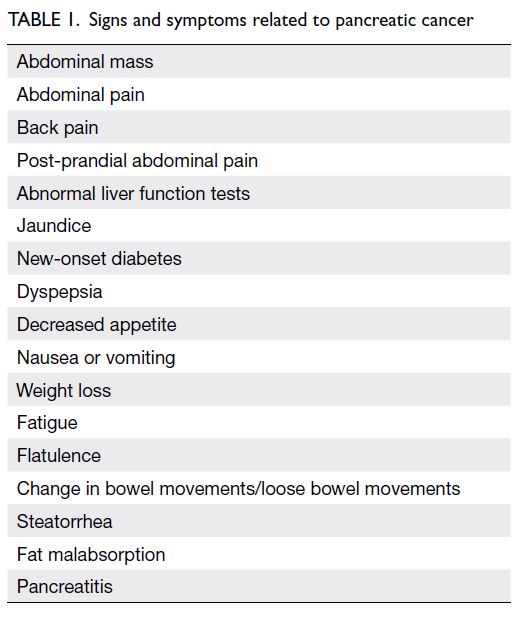 Table 1.
Table 1. Signs and symptoms related to pancreatic cancer
Further workup and management require a
multidisciplinary team encompassing a surgeon,
clinical oncologist, medical oncologist, radiologist,
and pathologist.
11 16 In Hong Kong, it is challenging
to involve a multidisciplinary team; nevertheless, we
recommend the multidisciplinary team approach
to address the evolving definition of resectability,
as well as the complexities of genetic profiling and
planning for various treatment modalities.
Statement 4: A thin-cut contrast-enhanced computed tomography scan of the entire abdomen should be performed for initial staging of the cancer. Positron emission tomography/computed tomography may be considered in selected cases. (Level 1)
A: 40%; B: 60%; C: 0%; D: 0%; E: 0%
In many centres, a baseline ultrasound is used to
initiate the investigation of gastrointestinal or biliary
complaints, such as jaundice. Subsequently, a high-quality
contrast-enhanced computed tomography
(CT) scan of the abdomen can detect a pancreatic mass and exclude other potential causes, such as
cancers of the gallbladder or bile ducts. Computed
tomography scanning is a well-validated method for
PDAC staging.
11 16 17 18 19 A thin-cut, pancreas-specific
CT scan can aid local staging by revealing adjacent
vessel infiltration and lymph node involvement.
17
Positron emission tomography (PET)/CT
can facilitate accurate staging, particularly in
cases with distant metastases. According to the
National Institute for Health and Care Excellence
of the United Kingdom, this approach may reduce
unnecessary surgeries by 20%.
16 20 However, for
initial staging, PET/CT generally does not offer
information beyond the results of a high-quality
CT scan of the abdomen.
16 20 21 Thus, a thin-cut
contrast-enhanced CT scan of the entire abdomen
is the imaging method of choice for initial staging.
Positron emission tomography/CT can be used
for preoperative staging in specific scenarios, such
as lesions with borderline resectability or cases
requiring lymph node staging.
16 The cost of PET/CT
should be discussed with patients and their families.
In Hong Kong, magnetic resonance imaging
may be utilised to investigate suspected lesions not
clearly defined by CT scanning, such as peritoneal
lesions. Although staging laparoscopy is rarely
performed, the laparoscopic approach (eg, during
the Whipple procedure) is common. Staging
laparoscopy can be selectively used to rule out
metastases and complement other imaging tools.
11 16
Statement 5: Tumour staging should follow the guidelines stipulated by the American Joint Committee on Cancer.
A: 90%; B: 10%; C: 0%; D: 0%; E: 0%
Statement 6: Pathology reports should contain all clinically significant essential parameters, including but not limited to tumour location, tumour size, histological type (according to the latest World Health Organization classification), histological grade, tumour extent, tumour response to neoadjuvant therapy (if any), lymphovascular invasion, perineural invasion, nodal status, and margin clearance status. Synoptic reports from the Royal College of Pathologists, Royal College of Pathologists of Australasia, and College of American Pathologists are recommended references.
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
Pancreatic ductal adenocarcinoma is staged
according to the most recent American Joint
Committee on Cancer tumour, node, and metastasis
classification,
22 a well-known and widely used
standard in the Hong Kong oncology community.
Clinicians can also categorise tumour resectability
into four levels, namely, resectable, borderline
resectable (BR), locally advanced (LA), and
metastatic.
2 3
Pathology data are necessary to fully assess the
extent of PDAC. In Hong Kong, most institutions
lack a standard pathology reporting protocol or
minimal dataset for pancreatic specimens. Moreover,
the Hong Kong College of Pathologists has not yet
developed a standard report format. In the absence
of such standards, we recommend that reports
include all clinically significant pathology data, such
as tumour location, tumour size, histological type
(according to the 2019 World Health Organization
classification), histological grade, tumour extent
(organ-confined or local invasion to adjacent
organs), tumour response to neoadjuvant therapy
(if any), lymphovascular invasion, perineural
invasion, nodal status, and margin clearance status
(
Table 2). The general structure of the report can
incorporate elements from datasets provided by
the Royal College of Pathologists, Royal College of
Pathologists of Australasia, and College of American
Pathologists.
23 24 25
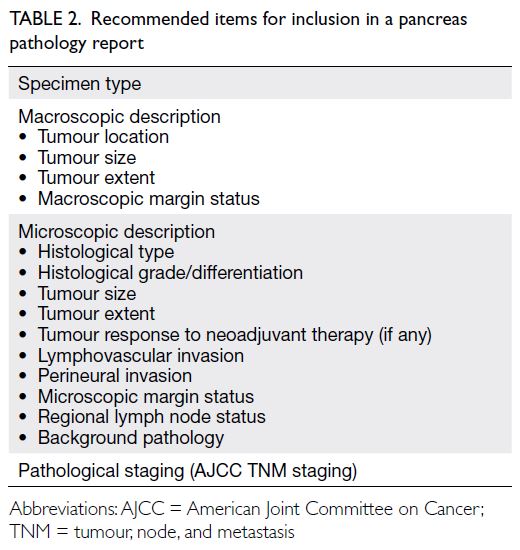 Table 2.
Table 2. Recommended items for inclusion in a pancreas
pathology report
Statement 7: For patients with suspected pancreatic head cancer without a definitive pancreatic mass observed on initial cross-sectional scan, endoscopic retrograde cholangiopancreatography and endoscopic ultrasound may be considered to detect small lesions in the pancreatic head or distal common bile duct. (Level 3)
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Statement 8: For patients with intraductal papillary mucinous neoplasms and ‘worrisome features’, as defined by the 2017 international consensus Fukuoka guidelines, endoscopic ultrasound may be considered for further workup.
A: 40%; B: 60%; C: 0%; D: 0%; E: 0%
Statement 9: Endoscopic ultrasound with fine-needle tissue acquisition is recommended when (a) there is a clinical need to exclude benign pathology, (b) tissue diagnosis is needed to guide treatment for locally advanced or metastatic disease, or (c) neoadjuvant treatment is planned.
A: 90%; B: 10%; C: 0%; D: 0%; E: 0%
Pancreatic head tumours usually present
with obstructive jaundice caused by bile duct
strictures. In these cases, endoscopic retrograde
cholangiopancreatography (ERCP) can be diagnostic
(through cytology from ERCP brushings and
biopsies) and therapeutic (through stent insertion
for biliary drainage). Endoscopic ultrasound (EUS)
with fine-needle aspiration (FNA) can also retrieve
tissue samples for the diagnosis of malignancy in
cases of obstructive jaundice, with high sensitivity
and specificity for detecting pancreatic masses and
malignant strictures.
26 27 28
Endoscopic ultrasound has a role in the
investigation of intraductal papillary mucinous
neoplasms (IPMNs). According to the Fukuoka
guidelines, EUS can be used to assess ‘worrisome
features’ and ‘high-risk stigmata’, with the latter
indicating a need for resection in surgically fit
patients.
29 In Hong Kong, surgery is usually advised
regardless of the EUS result because ‘worrisome
features’ indicate pre-malignancy, but the Fukuoka
guidelines suggest that EUS can facilitate further
characterisation of ambiguous areas that cannot be
resolved through cross-sectional CT scans, such as
tumour nodule and main duct features, as well as
cytological characteristics of the mass.
29
In Hong Kong, EUS is not commonly used
for routine staging. We concur with the National
Comprehensive Cancer Network (NCCN)
guidelines, which state that EUS with or without
fine needle tissue acquisition provides information
complementary to CT scans but is not recommended
for routine staging.
16 30 31 32 Endoscopic ultrasound
accuracy is largely operator-dependent and may
be affected by anatomical variations of the hepatic
arteries.
16 In the diagnosis of PDAC, EUS offers
specificity and sensitivity comparable to CT; it may
provide additional information for lesions with
inconclusive results or lesions <2 cm on initial CT.
18 21
Contrast-enhanced EUS, an evolving technique, can
distinguish characteristic traits of malignancy (eg,
hypoenhancement versus hyperenhancement) in
highly vascular neuroendocrine tumours.
33
Endoscopic ultrasound–FNA is an important
approach for obtaining tissue to establish a
cytologic diagnosis. We have listed indications that
require tissue diagnosis for treatment planning;
in such instances, EUS-FNA may be strongly
considered, especially for cases potentially requiring
chemotherapy or radiation therapy.
33 34 35 36 However, the
implementation of EUS-FNA may vary according to
each centre’s protocols and relevant expertise.
Surveillance
Statement 10: Serum carbohydrate antigen 19-9 is recommended for diagnosis of PDAC and for treatment response monitoring, but not for routine screening of PDAC. (Level 1)
A: 80%; B: 10%; C: 10%; D: 0%; E: 0%
For the diagnosis of PDAC in symptomatic
patients, serum carbohydrate antigen 19-9 (CA19-9)
exhibits a sensitivity of approximately 80% and a
specificity of 80% to 90%.
37 38 There is also robust
evidence suggesting that normal or decreased levels
can predict resectability and improved survival.
Carbohydrate antigen 19-9 levels <100 U/mL
suggest resectability, whereas levels ≥100 U/mL
suggest unresectability or metastatic disease. In the
preoperative period, normal levels (<37 U/mL) may
be prognostic of prolonged median survival (32-36
months) compared with elevated levels (≥37 U/mL;
12-15 months). Postoperative normalisation or
decrease from baseline by 20% to 50% is associated
with prolonged survival.
38 However, CA19-9 is not
an effective screening tool for PDAC, considering
its positive predictive value of <1% in symptomatic
patients.
16 38
Statement 11: For patients with unresectable disease, a biopsy is recommended to obtain histological proof of PDAC.
A: 20%; B: 60%; C: 20%; D: 0%; E: 0%
As discussed in the context of EUS-FNA, a
biopsy is needed to confirm a histological diagnosis
of PDAC before definitive therapy. This approach
is warranted when advanced or inoperable disease
is suspected and neoadjuvant or palliative therapy
is considered.
39 Considering that some suspicious
masses are not PDAC, histological proof is required
to guide treatment planning. Common differential
diagnoses include other malignant diseases, such
as neuroendocrine tumour and teratoma, or benign
conditions, such as autoimmune pancreatitis
and chronic pancreatitis. Patients with tumours
considered resectable based on imaging findings may
be directly referred for surgical treatment without a
routine biopsy.
40
Statement 12: There is no consensus on screening practices for PDAC.
A: 70%; B: 20%; C: 10%; D: 0%; E: 0%
In Hong Kong, patients’ families frequently
enquire about their PDAC risk and need for
screening. However, local clinicians lack a
standardised screening protocol for PDAC. Evidence
reviewed by the United States Preventive Services
Task Force suggests that screening is unnecessary
for asymptomatic individuals with a low risk of
PDAC.
41 According to the International Cancer of
the Pancreas Screening Consortium and the United
States Preventive Services Task Force, screening should be conducted in a research setting with a
multidisciplinary team for high-risk individuals—specifically, individuals with a history of familial
pancreatic cancer, individuals with inherited genetic
disorders linked to pancreatic cancer (eg, Peutz–Jeghers syndrome and hereditary pancreatitis), and
individuals with germline mutations such as
BRCA2
and
PALB—by age 50 or 10 years earlier than the
youngest relative was diagnosed with PDAC.
41 42
For these individuals, pancreatic imaging with CT,
magnetic resonance imaging, magnetic retrograde
cholangiopancreatography, and/or EUS is suggested
for annual pancreatic surveillance.
41 42
The American Gastroenterological Association
states that the advantages of PDAC screening for
high-risk individuals include the possibility of
detecting IPMNs, which may be precursor lesions
to PDAC.
43 There are no standard screening
protocols for IPMNs. However, the Fukuoka
guidelines suggest imaging for unresected, relatively
indolent lesions at intervals of 3 to 6 months
initially, then less frequently if the lesion size
remains small. Long-term surveillance for lesions
with ‘worrisome features’ or ‘high-risk stigmata’ may
require more frequent monitoring, at intervals of
3 to 9 months, to detect the potential development
of PDAC.
29
Although these international practices can be
considered, their applicability to the Hong Kong
setting is uncertain.
Management of localised disease
Statement 13: Resectability depends on the involvement of the venous and arterial vasculature, mainly the superior mesenteric artery, superior mesenteric vein, celiac trunk, and hepatic artery.
A: 20%; B: 60%; C: 20%; D: 0%; E: 0%
We established resectability criteria that are
consistent with the most recent NCCN guidelines.
16
The assessment of resection potential involves
determining the tumour’s extent into the following
critical structures: superior mesenteric vein
(SMV), portal vein (and its tributaries), superior
mesenteric artery (SMA), celiac trunk, hepatic
artery, and gastroduodenal artery.
44 ‘Resectable’
PDAC lacks tumour contact with critical vessels
and is characterised by the absence of metastasis.
The SMV and portal vein remain patent. Borderline
resectable PDAC is primarily characterised by
tumour abutment with (contact with <180° of vessel
wall circumference) the SMV, portal vein, SMA,
and/or celiac trunk, as well as abutment with or
limited enclosure of (contact with ≥180° of vessel
wall circumference) the common hepatic artery.
Locally advanced tumours are characterised by
major occlusion of the portal vein or SMV, as well
as enclosure of the SMA, celiac trunk, or proximal
hepatic artery.
44 45 46
Statement 14: Stent placement may be considered for cholangitis or severe jaundice, or if the waiting time for surgery exceeds 4 weeks.
A: 0%; B: 80%; C: 20%; D: 0%; E: 0%
Theoretically, preoperative biliary drainage
should relieve symptoms of hyperbilirubinaemia,
facilitate recovery from the metabolic derangements
caused by obstructive jaundice, and improve surgical
outcomes. However, as summarised by the NCCN,
retrospective and prospective studies have either
failed to show a decrease in postoperative mortality
or have shown increases in wound complications and
operating times among cases involving preoperative
drainage.
16 Furthermore, a randomised controlled
trial (RCT) showed a higher rate of complications
in the group undergoing routine preoperative biliary
drainage through ERCP with a plastic stent (74% in
the biliary drainage group vs 39% in the early surgery
group).
47 Considering the drainage preconditions in
that trial and the trends we have observed in clinical
practice, we recommend considering stent placement
for patients with active cholangitis or severe
jaundice, and in cases where the expected duration
of preoperative drainage exceeds 4 weeks. In our
experience, a bilirubin level of 250 μmol/L may be
an acceptable threshold for stent placement, but this
threshold should be evaluated in the context of the
patient’s overall clinical condition. The appropriate
technique for preoperative biliary stenting (ie,
percutaneous biliary drainage, endoscopic biliary
drainage, or ERCP) remains a subject of debate, as
does the need for preoperative stenting itself.
Statement 15: The optimal procedure for resection of tumours in the pancreatic head is pancreaticoduodenectomy (Whipple procedure). The optimal procedure for resection of tumours in the pancreatic body and tail is distal pancreatectomy.
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Surgical resection of the tumour is the best
option for patients with resectable PDAC. The
procedure of choice depends on tumour location
and its relationships with the bile duct and vessels.
Patients with tumours in the head and uncinate
process typically undergo pancreaticoduodenectomy
(ie, the Whipple procedure). Distal pancreatectomy
is usually performed as treatment for tumours of the
body or tail, but a margin-negative (R0) resection
should be targeted in such cases. If the tumour
invades the portal vein, en bloc resection and
reconstruction of the portal vein may achieve R0
resection.
16
The NCCN has noted the emerging role of
laparoscopic distal pancreatectomy, considering
reported decreases in blood loss and length of hospital
stay compared with open distal pancreatectomy.
16
Another important consideration regarding the
Whipple procedure is that outcomes are best when this surgical method is performed by surgeons who
complete >20 such procedures annually, usually
at high-volume centres.
2 16 Additionally, the best
outcomes are achieved when a multidisciplinary
team, with members whose experience ranges
from the operating room to the recovery room,
has extensive experience in perioperative care and
complication management.
Statement 16: Standard lymphadenectomy should involve the removal of ≥15 lymph nodes to allow adequate pathological staging of the disease.
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
This recommendation is based on the 2015
guidelines from the European Society for Medical
Oncology (ESMO).
11 The extent of lymphadenectomy
remains a subject of debate because there is
limited evidence of a benefit from extended
lymphadenectomy.
16 The International Study
Group of Pancreatic Surgery reviewed the available
evidence and identified lymph node stations that
should be included in a standard lymphadenectomy,
despite their acknowledgement that expert opinions
varied among group members.
48
Statement 17: Adjuvant therapy is recommended after surgical resection. Options include mFOLFIRINOX, gemcitabine plus capecitabine, gemcitabine monotherapy, or S-1. (Level 2)
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
Statement 18: After adjuvant treatment, patients are recommended to undergo monitoring every 3 to 6 months for 2 years and every 6 to 12 months thereafter.
A: 30%; B: 70%; C: 0%; D: 0%; E: 0%
Good outcomes from postoperative adjuvant
therapy have been demonstrated in RCTs. In
the CONKO-001 trial (Charité Onkologie–001)
[n=368], postoperative adjuvant chemotherapy
with gemcitabine alone significantly prolonged
overall survival (OS) compared with observation
(22.8 vs 20.2 months; hazard ratio [HR]=0.76,
95% confidence interval [CI]=0.61-0.95; P=0.010).
49
The ESPAC-4 study (European Study Group for
Pancreatic Cancer–4) [n=732] demonstrated that
the combination of gemcitabine and capecitabine
significantly prolonged postoperative OS compared
with gemcitabine monotherapy (28.0 vs 25.5
months; HR=0.82, 95% CI=0.68-0.98; P=0.032).
50
A mFOLFIRINOX (modified 5-fluorouracil with
leucovorin, irinotecan, and oxaliplatin) regimen
yielded significantly longer OS compared with
gemcitabine alone (54.4 vs 35.0 months; HR=0.64,
95% CI=0.48-0.86; P=0.003) in the PRODIGE 24-ACCORD 24/CCTG PA 6 study (n=493).
51 The
JASPAC 01 study (Japan Adjuvant Study Group
of Pancreatic Cancer) of 385 subjects in Japan showed significantly better OS with S-1, an oral
5-fluorouracil prodrug containing tegafur, gimeracil,
and oteracil potassium, compared with gemcitabine
alone (46.6 vs 25.5 months; HR=0.57, 95% CI=0.44-0.72; P<0.0001).
52
Although we do not recommend a standard
regimen, we have listed the available options
for Hong Kong clinicians who may need to plan
individualised therapy with limited resources.
Modified FOLFIRINOX may be considered for
patients with an Eastern Cooperative Oncology
Group performance status (PS) score of 0 to 1.
Those with a poor PS can receive gemcitabine plus
capecitabine or gemcitabine monotherapy.
16 S-1
may serve as an alternative to gemcitabine-based
therapies.
Locally, some R2 resections (with macroscopic
residual tumour) are followed by postoperative
radiotherapy (RT), although the administration
of RT in these cases is usually hindered by
challenges regarding localisation of the tumour and
administration of an adequate dose. In principle,
adjuvant RT may address suspected residual disease
or reduce local recurrence. However, the ESMO
guidelines cite results from the EORTC (European
Organisation for Research and Treatment of Cancer)
and ESPAC-1 trials, which showed no benefit and
suggested potential harm.
11 53 54 The ESMO panel
does not recommend postoperative adjuvant RT
except in clinical trials.
11
In our clinical experience, we have found it
challenging to ensure that patients continue follow-up
after curative treatment. Currently, there are no
evidence-based standards for the frequency and
timing of follow-up visits, use of CT scans and
other imaging methods, and assessment of tumour
biomarkers. Based on extensive discussion within
our group, we recommend follow-up monitoring
every 3 to 6 months for the first 2 years and every
6 months thereafter. This follow-up approach will
enable clinicians to diagnose recurrences, detect and
monitor complications, assess PS and quality of life,
and provide some education and counselling.
Management of localised disease
Statement 19: Neoadjuvant therapy is recommended for borderline resectable disease. (Level 1)
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
Statement 20: There is limited evidence to support the recommendation of specific neoadjuvant regimens. Generally, combination regimens are preferred. (Level 2/3)
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Statement 21: Stereotactic body radiation therapy is not recommended outside of a clinical trial.
A: 50%; B: 50%; C: 0%; D: 0%; E: 0%
Borderline resectable PDAC is characterised
by blood vessel infiltration that increases the risk of
R1 resection (with microscopic residual tumour)
and decreases the feasibility of upfront surgery.
11
Neoadjuvant therapy may improve the likelihood of
R0 resection, sterilise any potential metastasis, and
assess the biological aggressiveness of the tumour
to inform patient selection for surgery—if disease
progression or intolerability to neoadjuvant treatment
occurs, aggressive surgery may not be viable.
16
The feasibility of neoadjuvant therapy
in resectable and BR-PDAC was previously
substantiated by a meta-analysis that evaluated
various chemotherapy protocols, including
gemcitabine-based and 5-fluorouracil–based
combinations, with or without radiation.
34 Two
subsequent meta-analyses, based on the intention-to-treat approach, demonstrated that OS and
R0 resection rates favoured neoadjuvant therapy
(primarily gemcitabine-based, with or without
radiation) over upfront surgery.
55 56 Recently, several
studies showed promising results for neoadjuvant
therapy, specifically in BR-PDAC. First, a phase II,
single-arm prospective trial (n=48) showed that
neoadjuvant FOLFIRINOX followed by proton
radiation (5 Gy in five fractions) with capecitabine
resulted in a high degree of R0 resection among
patients who underwent surgery (31/32).
57
Progression-free survival (PFS) and 2-year OS
among all patients were 14.7 months and 56%,
respectively; among patients who underwent
surgery, the respective values were 48.6 months and
72%.
57 Subsequently, Korean researchers conducted
the first multicentre phase II/III RCT of neoadjuvant
therapy for BR-PDAC (n=58), where intention-to-treat analysis showed that among patients
with BR-PDAC, gemcitabine-based neoadjuvant
chemoradiation followed by surgery yielded a
significantly higher 2-year survival than upfront
surgery followed by chemoradiation (40.7% vs
26.1%, HR=1.495, 95% CI=0.66-3.36; P=0.028).
58 The
R0 resection rate also was significantly higher with
neoadjuvant treatment (P=0.004).
58 More recently, in
the Dutch phase III PREOPANC trial (Perioperative
or Adjuvant mFOLFIRINOX for Resectable
Pancreatic Cancer) of patients with resectable and
BR-PDAC (n=248), intention-to-treat analysis
demonstrated improvements in distant metastasis-free
interval (P=0.32), locoregional failure-free
interval (P=0.0034), and R0 resection rate (P<0.001)
among patients who received gemcitabine-based
chemoradiation versus patients who underwent
upfront surgery.
59 The neoadjuvant group received
preoperative gemcitabine with radiation; both study
groups received postoperative adjuvant gemcitabine.
Final OS was significantly better with neoadjuvant
chemoradiation (15.7 vs 14.3 months, HR=0.73, 95%
CI=0.56-0.96; P=0.025). Five-year OS also favoured neoadjuvant treatment (20.5% vs 6.5%).
60
For tumours with a risk of incomplete resection,
preoperative radiation may be administered after
induction chemotherapy to increase the likelihood
of R0 resection. Compared with fractionated RT,
stereotactic body radiation therapy (SBRT) offers the
potential advantage of delivering higher radiation
doses while sparing adjacent tissues.
16 However,
the benefit of SBRT after induction chemotherapy
has not been established among patients with BR-PDAC.
Participants in the Alliance for Clinical
Oncology trial A021501 received, prior to surgery,
either eight cycles of mFOLFIRINOX or seven cycles
of mFOLFIRINOX followed by hypofractionated
image-guided radiation or SBRT. Patients without
disease progression after neoadjuvant treatment
underwent surgery and received adjuvant FOLFOX
(folinic acid, fluorouracil, and oxaliplatin).
61 The
results showed that the mFOLFIRINOX plus
SBRT group had worse median OS and worse
18-month OS compared with the group that
received mFOLFIRINOX alone; notably, only
19 of 56 chemoradiation patients underwent
resection.
61 Stereotactic body radiation therapy
with chemotherapy requires further research before
routine application in this setting.
Although the available literature does not
provide strong support for a specific regimen,
we recommend considering FOLFIRINOX or
gemcitabine-based regimens. Stereotactic body
radiation therapy with chemotherapy should
be administered within a clinical trial; other RT
techniques may be considered if neoadjuvant
chemoradiation is planned.
Statement 22: Surgical candidacy should be reassessed after neoadjuvant therapy, preferably through multidisciplinary team discussions.
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
After preoperative treatment, restaging is
recommended. The NCCN suggests repeating
CT and performing a staging laparoscopy (if
not previously conducted).
16 In our experience,
tumour assessment after neoadjuvant treatment
is challenging and requires the involvement of a
multidisciplinary team that will also contribute to
discussions of future treatment with the patient and
their family. Conventional imaging may not reliably
assess resectability. Regardless of radiographic
stability, clinical improvement and a decrease in
CA19-9 level, further evaluations are needed.
16
Before proceeding with resection, frozen section
analyses of tumours responsive to neoadjuvant
therapy should be performed to rule out metastasis
and examine critical structures.
Management of locally advanced disease
Statement 23: For locally advanced disease, systemic therapy is the primary treatment. Options include FOLFIRINOX, gemcitabine plus nab-paclitaxel, gemcitabine plus capecitabine, and gemcitabine monotherapy. (Level 1/2/3)
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
The extensive infiltration of critical vessels in
LA-PDAC precludes reconstruction and hinders
tumour resection. The primary treatment is systemic
chemotherapy. Similar to the statements regarding
resectable disease, we have listed the various
options for individualised management. Historically,
gemcitabine has been used for LA-PDAC, providing
a clinical benefit response of 23.8%, median OS
of 5.65 months, and 1-year survival of 18% in one
RCT focused on advanced PDAC.
62 A 6-month
treatment duration has been endorsed by the ESMO
guidelines.
11 Concerning 6-month OS, a meta-analysis
showed that gemcitabine plus capecitabine
reduced the mortality risk by 15% compared with
gemcitabine monotherapy (relative risk=0.85, 95%
CI=0.73-0.99; P=0.04).
63
FOLFIRINOX and gemcitabine plus nab-paclitaxel
regimens, initially established for
metastatic PDAC (mPDAC), have been applied
to LA-PDAC. A meta-analysis showed that the
median OS with FOLFIRINOX for LA-PDAC was
24.2 months, which was approximately twofold
longer than the OS of 6 to 13 months observed with
gemcitabine.
64 In one case series (n=485), despite
higher rates of RECIST (Response Evaluation
Criteria in Solid Tumors) partial response and
subsequent pancreatectomy among patients
receiving FOLFIRINOX compared to those receiving
gemcitabine plus nab-paclitaxel, both regimens (as
first-line chemotherapy for LA-PDAC) provided
similar OS (21 vs 20 months, HR=1.48, 95% CI=0.97-2.26; P=0.07).
65
Statement 24: Chemoradiation or stereotactic body radiation therapy can be considered for patients with no progression after chemotherapy.
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
After tumour stabilisation via post-induction
chemotherapy, concurrent chemoradiation is
usually considered for LA-PDAC to optimise
local control. Trials comparing chemoradiation
with chemotherapy alone have shown conflicting
results.
66 67 68 Notably, the contemporary phase III
LAP-07 study, which randomly assigned patients
with non-progressing LA-PDAC after 4 months of
gemcitabine plus erlotinib (n=269) to either receive
RT plus capecitabine or continue chemotherapy,
did not show a survival benefit from the addition of
RT (median OS from date of initial chemotherapy:
16.5 vs 15.2 months; P=0.83), despite a decrease in
locoregional progression (32% vs 46%; P=0.04).
69
Therefore, no standard chemotherapy regimen,
RT dose, or modality has been established. As previously discussed, the advantages of delivering
high RT doses while sparing critical tissues make
SBRT a promising option for LA-PDAC. Pooled
analyses of trials involving chemotherapy with
SBRT for LA-PDAC revealed a median OS of 17
months, a 1-year locoregional control rate of 72.3%,
and an overall severe adverse event incidence of
≤10%.
70 Another meta-analysis showed that SBRT
improved 2-year OS compared with conventionally
fractionated RT with concurrent chemotherapy
(26.9% vs 13.7%; P=0.004), although the rates of
late grade 3/4 toxicity were similar (9.0% vs 10.1%;
P=0.49).
71 Despite the limited evidence favouring a
specific protocol, the NCCN recommends systemic
therapy or induction chemotherapy for 4 to 6
months, followed by chemoradiation or SBRT.
16
Management of metastatic disease
Statement 25: The primary treatment for metastatic disease is palliative systemic therapy. (Level 2)
A: 90%; B: 10%; C: 0%; D: 0%; E: 0%
Statement 26: The treatment decision depends on performance status, bilirubin level, and the preferences of the clinician and patient. Combination therapy is generally recommended for patients with good performance status, bilirubin level <1.5 times the upper limit of normal, and intention to undergo aggressive treatment.
A: 90%; B: 10%; C: 0%; D: 0%; E: 0%
Statement 27: Combination treatment options include FOLFIRINOX, gemcitabine plus nab-paclitaxel, gemcitabine plus capecitabine, and gemcitabine plus S-1. (Level 2)
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Statement 28: Monotherapy options include S-1 alone and gemcitabine alone. (Level 2)
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
The benefit of systemic chemotherapy for
mPDAC has been confirmed in phase III RCTs.
62 72 73
Surgery does not improve OS and should not be
regarded as routine treatment.
74 75 With respect to
treatment planning, we noted that patients enrolled
in phase III RCTs for combination chemotherapy had
an Eastern Cooperative Oncology Group PS score
of 0 to 1 and a normal bilirubin level. The bilirubin
threshold of <1.5 times the upper limit of normal
was adapted from the American Society of Clinical
Oncology and ESMO guidelines.
11 76 In practice,
clinicians frequently accept a slightly higher level
for specific chemotherapy regimens. The intended
treatment strategy should be established based
on the balance of benefits and harms—aggressive
treatment with combination therapy may achieve
good tumour control, whereas less aggressive
options (eg, monotherapy) can maintain or improve quality of life for patients with clinical statuses that
preclude the use of combination therapy.
77
The results of the PRODIGE 4/ACCORD 11
trial (n=342) showed an improvement in median OS
among patients receiving FOLFIRINOX compared
with those receiving gemcitabine (11.1 vs 6.8 months,
HR=0.57, 95% CI=0.45-0.73; P<0.001). Additionally,
the median PFS and overall response rate were
significantly better.
72 However, FOLFIRINOX had an
inferior safety profile compared with gemcitabine.
72
The MPACT trial (n=861) demonstrated that the
combination of nab-paclitaxel and gemcitabine,
compared with gemcitabine alone, significantly the
improved median OS (8.5 vs 6.7 months, HR=0.72,
95% CI=0.62-0.83; P<0.001), median PFS, and
overall response rate.
73 Compared with gemcitabine,
the combination regimen had higher rates of
myelosuppression and peripheral neuropathy,
although these effects appeared to be reversible.
73
Clinicians in Hong Kong may prefer gemcitabine
plus capecitabine due to the convenience of the
oral formulation. Individual trial results for this
combination tended to indicate a survival benefit
but did not demonstrate statistical significance;
subsequent pooled analyses suggested a more robust
benefit.
78 79 80 81 A possible survival benefit was also
detected with gemcitabine plus S-1, which we have
included in the list of recommended combination
therapies (
Table 3).
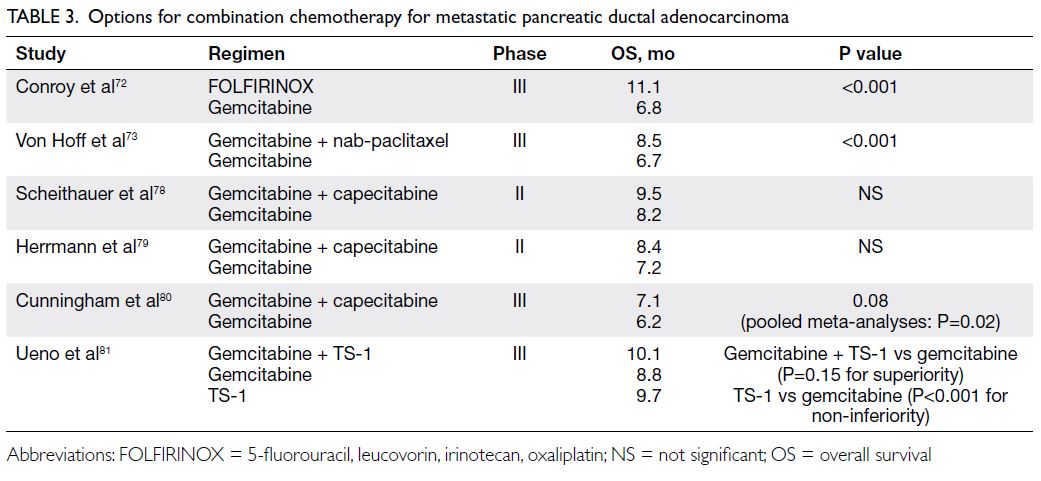 Table 3.
Table 3. Options for combination chemotherapy for metastatic pancreatic ductal adenocarcinoma
As previously stated, monotherapy options
are necessary for patients with poor PS or
elevated bilirubin levels that do not exhibit rapid
normalisation. Some clinicians and patients may
also prefer single-agent treatment. Gemcitabine
monotherapy for mPDAC is already established—an early phase III trial (n=126) revealed a clinical
benefit response in 23.8% of gemcitabine-treated
patients compared with 4.8% of 5-fluorouracil–treated patients (P=0.0022).
62 Additionally, OS
with gemcitabine in the MPACT and PRODIGE
trials was approximately 6 months.
72 73 In all trials,
gemcitabine was well-tolerated.
62 72 73 S-1 was
evaluated in a phase III trial (n=834); its use as
monotherapy led to a median OS of 9.7 months
with good tolerability.
81 S-1 also demonstrated non-inferiority
to gemcitabine (HR=0.96, 97.5% CI=0.78-1.18; P<0.001 for non-inferiority).
81 In Hong Kong,
capecitabine monotherapy is used for selected
patients. The efficacy and tolerability of capecitabine
are currently supported by phase II evidence.
82
Statement 29: The decision to undergo subsequent therapy after first-line treatment is highly individualised. Key factors to consider include the type and duration of first-line treatment, performance status, organ function, and treatment goals.
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
We recognise that some patients will undergo several lines of treatment, but there is currently
no consensus regarding the approach to next-line
therapy for mPDAC. According to a multivariate
analysis of patient variables from a cohort study of
second-line treatment, prognostic factors for OS
include liver metastases, PS, pain, jaundice, ascites,
duration of first-line treatment, and type of second-line
regimen.
83 These factors mirror our real-world
experience in establishing individualised regimens
for subsequent therapy. Evidence for next-line
treatment is based on cohort studies, phase II trials,
and phase III RCTs (
Table 4).
84 85 86 87 88 89 90 91 92 93 94 95 Only the regimen
of nanoliposomal irinotecan plus fluorouracil and
folinic acid has been evaluated in a multicentre phase
III trial demonstrating significant OS improvement;
thus, it is the first regimen with high-level evidence
supporting usage as second-line mPDAC treatment
for patients who progressed on first-line gemcitabine
treatment.
89
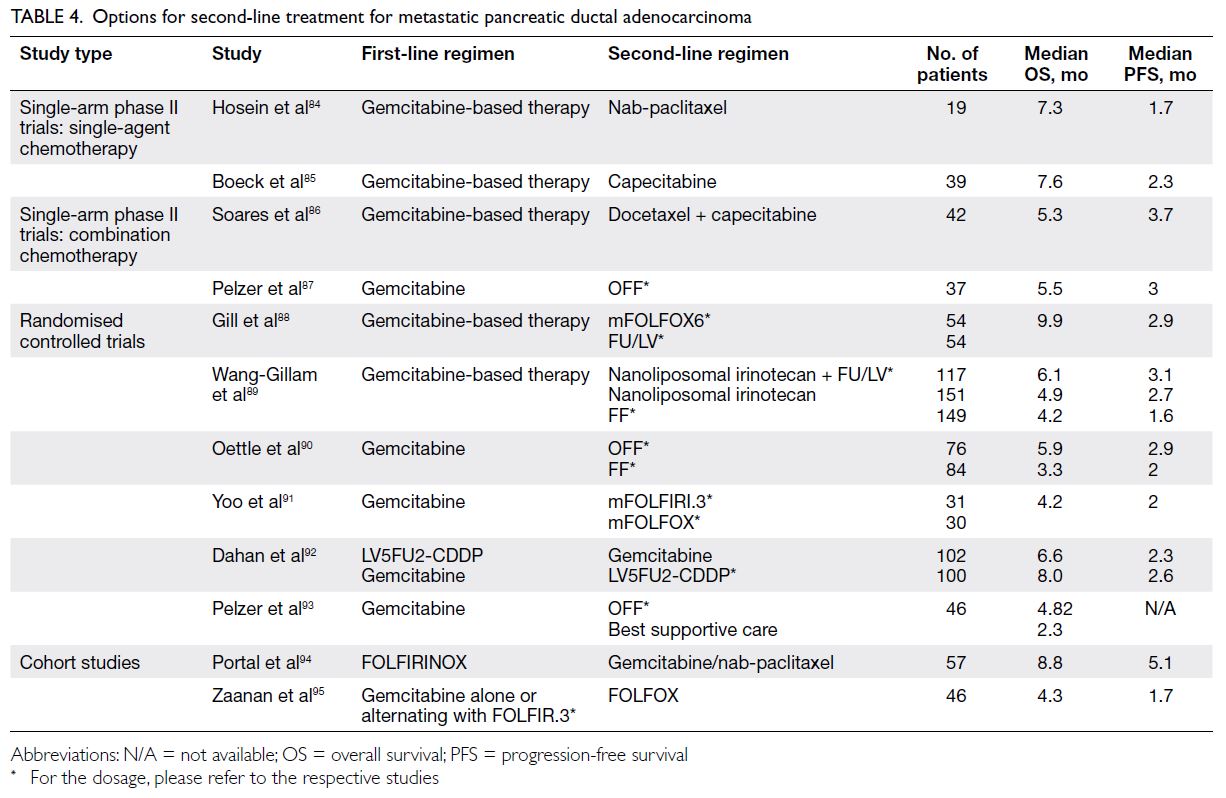 Table 4.
Table 4. Options for second-line treatment for metastatic pancreatic ductal adenocarcinoma
Personalised medicine
Statement 30: Germline testing of BRCA1/2 and somatic testing of microsatellite instability/mismatch repair can be considered for patients with unresectable disease, due to potential therapeutic implications.
A: 40%; B: 60%; C: 0%; D: 0%; E: 0%
Statement 31: Among patients who test positive for germline BRCA1 or BRCA2 mutations, olaparib may be considered for patients who have previously been treated with a platinum-based regimen and have not exhibited disease progression for at least 16 weeks. (Level 2)
A: 50%; B: 50%; C: 0%; D: 0%; E: 0%
Statement 32: For patients with tumours that harbour high microsatellite instability or genetic aberrations in DNA mismatch repair genes, immune checkpoint inhibitors may be considered.
A: 90%; B: 10%; C: 0%; D: 0%; E: 0%
Emerging evidence suggests that mPDAC
treatment can be tailored according to underlying
mutations, and we emphasise that individual
tumour profiling can be considered for selecting
patients who may benefit from such treatment.
The notion that PDAC with germline
BRCA1/2
mutations responds well to platinum-based
therapy is supported by retrospective analyses.
96 97
In contrast, the phase III POLO (Pancreas Cancer
Olaparib Ongoing) RCT demonstrated that targeted
therapy was effective for patients with a germline
BRCA mutation who had prior platinum-based
chemotherapy for mPDAC and whose disease
had not progressed for 16 weeks; these patients
experienced a clinical benefit with maintenance
olaparib, a poly (adenosine diphosphate-ribose)
polymerase inhibitor.
98 Among those 154 study
subjects, median PFS was significantly longer in
patients with maintenance olaparib than placebo
group (7.4 vs 3.8 months, HR=0.53, 95% CI=0.35-0.82; P=0.0038).
98 The preliminary OS in both
treatment groups was approximately 18 months.
98
Based on the inclusion criteria and results of the
POLO study, we recommend olaparib for patients
with
BRCA1/BRCA2-positive mPDAC that has not
progressed for 16 weeks.
Approximately 2% of pancreatic cancers
have mismatch repair (MMR) deficiency.
99 Patients
with advanced MMR-deficient cancers respond
to programmed cell death protein 1 blockade.
The efficacy of the anti–programmed cell death
protein 1 antibody pembrolizumab was evaluated
in patients with MMR-deficient tumour types.
Among 86 patients, eight had pancreatic tumours.
Overall, 53.5% (46/86) of the patients exhibited objective radiographic responses, whereas 76.7%
(66/86) demonstrated disease control.
99 These
results indicate that immune checkpoint inhibition
should be considered for high microsatellite
instability mPDAC. The potential benefit of this
approach has been acknowledged by international
guidelines.
16 76 100
Germline testing of
BRCA1/2 and somatic
testing of microsatellite instability/MMR are
conducted separately. In contrast to countries with
extensive reimbursement, routine testing with
comprehensive gene panels is not routinely feasible
for all patients due to the limited resources in Hong
Kong. Hong Kong clinicians, especially in private
clinics, may utilise next-generation sequencing
services to obtain a comprehensive genetic mutation
profile. In next-generation sequencing, a broad
mutational analysis panel can identify potentially
actionable alterations, including
BRCA1/2
mutations. However, one study showed that only
1.3% of patients (3/225) received targeted therapy
for PDAC based on next-generation sequencing
results.
101 This observation is similar to our clinical
experience, suggesting that next-generation
sequencing has limited therapeutic utility for PDAC.
Statement 33: Genetic counselling is recommended for patients who test positive for a germline mutation.
A: 50%; B: 50%; C: 0%; D: 0%; E: 0%
Germline testing for
BRCA mutations in
PDAC is expected to increase in Hong Kong. We
recommend genetic counselling for patients who
plan to undergo tests for pathogenic variants. The
NCCN also recommends germline testing and
subsequent referral for genetic counselling at the
time of PDAC diagnosis, especially for patients with
suspected familial risk based on a family history
of
BRCA-linked tumours.
16 No detailed guidance
regarding genetic counselling for PDAC is currently
available. Nonetheless, guidelines regarding
BRCA-associated
tumours, particularly breast and ovarian
tumours, recommend the provision of genetic
counselling services for patients with germline
pathogenic mutations.
102 103 104 105
Palliative and supportive care
Statement 34: Assessments of physical and psychological symptoms should be performed for all patients with PDAC. Palliative management should be considered when clinically indicated.
A: 70%; B: 30%; C: 0%; D: 0%; E: 0%
Statement 35: Biliary drainage should be considered for patients with obstructive jaundice. Options include endoscopic or percutaneous drainage and surgical bypass.
A: 70%; B: 20%; C: 10%; D: 0%; E: 0%
Statement 36: Options for the management of gastric outlet obstruction include surgical bypass and endoscopic stenting.
A: 100%; B: 0%; C: 0%; D: 0%; E: 0%
Statement 37: Aggressive pain control is mandatory and frequently requires the involvement of a pain specialist
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
Statement 38: In addition to pharmacological interventions, a coeliac axis block can be considered to optimise pain control.
A: 60%; B: 40%; C: 0%; D: 0%; E: 0%
Statement 39: Palliative radiation can be considered to relieve severe tumour-associated pain and/or bleeding from the primary tumour site.
A: 40%; B: 60%; C: 0%; D: 0%; E: 0%
We acknowledge that palliative care and
supportive care for PDAC are therapeutic aspects
often overlooked by clinicians. Key guidelines
have emphasised the need to coordinate palliative
and supportive care with therapeutic care, thereby
optimising quality of life and potentially improving
survival. These guidelines have highlighted
interventions to address symptoms such as pain,
biliary obstruction, gastric outlet obstruction, and
bleeding.
11 16
Symptomatic biliary obstruction occurs in up
to 75% of patients with pancreatic head tumours.
Obstructive jaundice can lead to generalised
wasting; untreated biliary obstruction can result in
cholangitis and liver dysfunction, with the potential
for early mortality.
106 Primary treatments consist
of endoscopic or percutaneous drainage. Surgical
bypass should only be utilised as a palliative option
in cases where the planned Whipple procedure
revealed an unresectable tumour.
Tumour invasion into the duodenum leads to
gastric outlet obstruction. The choice of treatment
depends on PS and predicted length of survival
16—in an otherwise young and healthy patient with an
unresectable tumour, surgical bypass is the best
palliative option with respect to quality of life.
Endoscopic enteral stenting may be preferred for
frail patients.
Pain is experienced by almost all patients
with advanced PDAC and requires aggressive
management. Experts in pain management, such
as pain specialists or oncologists with extensive
experience in pain medicine, should often be included in the care team. A coeliac axis block,
which interrupts visceral pain innervation from
the pancreas and nearby structures through
injections of corticosteroids and anaesthetics,
may be considered for severe pain refractory to
analgesics or narcotics.
16 107 A coeliac axis block is
usually performed under fluoroscopic or CT-based
guidance, but EUS-based guidance provides better
visualisation of the coeliac plexus.
108
Palliative RT can be used to control pain
caused by the tumour or sites of metastasis. Patients
with non-mPDAC and poor PS or co-morbidities
that preclude definitive therapy may be offered
palliative RT. Additionally, RT is an option for the
management of tumour-induced gastrointestinal
bleeding.
11 16
Conclusion
Despite its relatively low incidence among cancers
worldwide and in Hong Kong, PDAC represents a
major health burden because of its aggressive nature
and the complexities involved in its diagnosis and
management. To familiarise Hong Kong clinicians
with all aspects of PDAC care and provide practical
guidance, our consensus group developed this initial
set of recommendations for clinical management
of PDAC. We discussed the current state of PDAC
management, reviewed the best available evidence
and international guidelines, and crafted statements
that address real-world situations encountered
by clinicians. We recognise that many aspects of
PDAC treatment lack high-level evidence; moreover,
clinical experiences, patient preferences, and
resources availability vary across Hong Kong. Thus,
several of our statements suggest options, rather
than endorsing a specific technique or regimen,
to facilitate individualised management based on
available evidence and clinical judgement.
Author contributions
Concept or design: SL Chan, CL Chiang, KSH Chok.
Acquisition of data: SL Chan, CL Chiang, KSH Chok.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual
content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
SL Chan has served as an advisor for AstraZeneca, MSD, Eisai, and Ipsen, and has received research funding from Bayer, Eisai, Ipsen, Sirtex, and MSD. CL Chiang has served as
an advisor for AstraZeneca, MSD, and Eisai, and has received
research funding from Merck KGaA, AstraZeneca, and Taiho.
Other authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Dr Jose Miguel (Awi) Curameng, Dr Mita Pabari, and Dr Pia Villanueva of MIMS (Hong Kong) Limited for support with meeting logistics, coordination, and medical
writing.
Funding/support
The consensus meeting and manuscript development were funded by AstraZeneca. The funder had no role in study design, data collection/analysis/interpretation, or manuscript
preparation.
References
1. Grant TJ, Hua K, Singh A. Molecular pathogenesis of pancreatic cancer. Prog Mol Biol Transl Sci 2016;144:241-75.
Crossref2. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA 2021;326:851-62.
Crossref3. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic
cancer. Lancet 2020;395:2008-20.
Crossref4. Huang J, Lok V, Ngai CH, et al. Worldwide burden
of, risk factors for, and trends in pancreatic cancer.
Gastroenterology 2021;160:744-54.
Crossref5. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics
2020: GLOBOCAN estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer J
Clin 2021;71:209-49.
Crossref6. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing
profiles of cancer burden worldwide and in China: a
secondary analysis of the Global Cancer Statistics 2020.
Chin Med J (Engl) 2021;134:783-91.
Crossref7. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics,
2022. CA Cancer J Clin 2022;72:7-33.
Crossref8. Strobel O, Neoptolemos J, Jäger D, Büchler MW.
Optimizing the outcomes of pancreatic cancer surgery.
Nat Rev Clin Oncol 2019;16:11-26.
Crossref9. Hospital Authority, Hong Kong SAR Government.
Overview of Hong Kong Cancer Statistics of 2019.
Available from:
https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202019.pdf. Accessed 3 May 2022.
10. Nuffield Department of Primary Care Health Sciences.
Centre for Evidence-Based Medicine. OCEBM levels of
evidence. Available from:
https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.Accessed 30 April 2022.
11. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v56-68.
Crossref12. Schmidt-Hansen M, Berendse S, Hamilton W. Symptoms
of pancreatic cancer in primary care: a systematic review.
Pancreas 2016;45:814-8.
Crossref13. Walter FM, Mills K, Mendonça SC, et al. Symptoms
and patient factors associated with diagnostic intervals
for pancreatic cancer (SYMPTOM pancreatic study): a
prospective cohort study. Lancet Gastroenterol Hepatol
2016;1:298-306.
Crossref14. Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic
cancer: symptoms at presentation and their relation to
tumour site and stage. Clin Transl Oncol 2005;7:189-97.
Crossref15. Wong HC, Lam KY, Chong CC, Chan AW, Chan SL. Impact of weight loss during chemotherapy in Chinese patients with unresectable pancreatic cancer. Nutr Cancer
2019;71:954-70.
Crossref16. National Comprehensive Cancer Network. NCCN
guidelines: pancreatic adenocarcinoma. V1.2022.
Available from:
https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. Accessed 27 Mar 2024.
17. Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic
ductal adenocarcinoma radiology reporting template:
consensus statement of the Society of Abdominal
Radiology and the American Pancreatic Association.
Radiology 2014;270:248-60.
Crossref18. Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am 2010;90:235-49.
Crossref19. Bipat S, Phoa SS, van Delden OM, et al. Ultrasonography,
computed tomography and magnetic resonance imaging
for diagnosis and determining resectability of pancreatic
adenocarcinoma: a meta-analysis. J Comput Assist
Tomogr 2005;29:438-45.
Crossref20. National Institute for Health and Care Excellence.
Pancreatic cancer. Quality standard [QS177] Published:
20 December 2018. Quality statement 2: staging using
FDG-PET/CT. Available from:
https://www.nice.org.uk/guidance/qs177/chapter/quality-statement-2-staging-using-fdg-petct. Accessed 3 May 2022.
21. Toft J, Hadden WJ, Laurence JM, et al. Imaging modalities in
the diagnosis of pancreatic adenocarcinoma: a systematic
review and meta-analysis of sensitivity, specificity and
diagnostic accuracy. Eur J Radiol 2017;92:17-23.
Crossref22. Kakar S, Pawlik TM, Allen PJ. Exocrine Pancreas. In: Amin
MB, Edge SB, Greene FL, editors. AJCC Cancer Staging
Manual. 8th ed. New York, NY: Springer; 2017: 337-47.
23. The Royal College of Pathologists. Dataset for the
histopathological reporting of carcinomas of the pancreas,
ampulla of Vater and common bile duct. October
2019. Available from:
https://www.rcpath.org/uploads/assets/34910231-c106-4629-a2de9e9ae6f87ac1/G091-Dataset-for-histopathological-reporting-of-carcinomas-of-the-pancreas-ampulla-of-Vater-and-common-bile-duct.pdf. Accessed 8 May 2022.
24. Royal College of Pathologists of Australasia. Cancer
of the exocrine pancreas, ampulla of Vater and distal
common bile duct: structured reporting protocol. 2nd
edition 2020. Available from:
https://www.rcpa.edu.au/getattachment/0e0524b6-32cb-491c-959f-4f60355d0509/Protocol-pancreatic-cancer.aspx. Accessed 8 May 2022.
25. College of American Pathologists. Protocol for the
examination of specimens from patients with carcinoma
of the pancreas. November 2021. Available from:
https://documents.cap.org/protocols/Panc.Exo_4.2.0.2.REL_CAPCP.pdf. Accessed 8 May 2022.
26. Chung HG, Chang JI, Lee KH, Park JK, Lee KT, Lee JK.
Comparison of EUS and ERCP-guided tissue sampling in
suspected biliary stricture. PLoS One 2021;16:e0258887.
Crossref27. Malak M, Masuda D, Ogura T, et al. Yield of endoscopic
ultrasound-guided fine needle aspiration and endoscopic
retrograde cholangiopancreatography for solid pancreatic
neoplasms. Scand J Gastroenterol 2016;51:360-7.
Crossref28. Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS
for tissue diagnosis of biliary strictures? A prospective
comparative study. Gastrointest Endosc 2004;60:390-6.
Crossref29. Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas.
Pancreatology 2017;17:738-53.
Crossref30. Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L.
Endoscopic ultrasound-guided fine needle aspiration and
multidetector spiral CT in the diagnosis of pancreatic
cancer. Am J Gastroenterol 2004;99:844-50.
Crossref31. Dewitt J, Devereaux BM, Lehman GA, Sherman S,
Imperiale TF. Comparison of endoscopic ultrasound and
computed tomography for the preoperative evaluation of
pancreatic cancer: a systematic review. Clin Gastroenterol
Hepatol 2006;4:717-25; quiz 664.
Crossref32. Nawaz H, Fan CY, Kloke J, et al. Performance
characteristics of endoscopic ultrasound in the staging of
pancreatic cancer: a meta-analysis. JOP 2013;14:484-97.
Crossref33. Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K,
Yamashita Y. Impact of endoscopic ultrasonography on
diagnosis of pancreatic cancer. J Gastroenterol 2019;54:19-32.
Crossref34. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H,
Kleeff J. Preoperative/neoadjuvant therapy in pancreatic
cancer: a systematic review and meta-analysis of response
and resection percentages. PLoS Med 2010;7:e1000267.
Crossref35. Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy
to distinguish autoimmune pancreatitis from pancreatic
cancer. Clin Gastroenterol Hepatol 2009;7:1097-103.
Crossref36. ASGE Standards of Practice Committee; Eloubeidi MA,
Decker GA, et al. The role of endoscopy in the evaluation
and management of patients with solid pancreatic
neoplasia. Gastrointest Endosc 2016;83:17-28.
Crossref37. Goonetilleke KS, Siriwardena AK. Systematic review of
carbohydrate antigen (CA19-9) as a biochemical marker
in the diagnosis of pancreatic cancer. Eur J Surg Oncol
2007;33:266-70.
Crossref38. Ballehaninna UK, Chamberlain RS. The clinical utility
of serum CA19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: an evidence-based
appraisal. J Gastrointest Oncol 2012;3:105-19.
Crossref39. Hartwig W, Schneider L, Diener MK, Bergmann F,
Büchler MW, Werner J. Preoperative tissue diagnosis for
tumours of the pancreas. Br J Surg 2009;96:5-20.
Crossref40. Függer R, Gangl O, Fröschl U. Clinical approach to
the patient with a solid pancreatic mass. Wien Med
Wochenschr 2014;164:73-9.
Crossref41. United States Preventive Services Task Force; Owens DK,
Davidson KW, et al. Screening for pancreatic cancer:
US Preventive Services Task Force Reaffirmation
Recommendation Statement. JAMA 2019;322:438-44.
Crossref42. Goggins M, Overbeek KA, Brand R, et al. Management of
patients with increased risk for familial pancreatic cancer:
updated recommendations from the International Cancer
of the Pancreas Screening (CAPS) Consortium. Gut
2020;69:7-17.
Crossref43. Aslanian HR, Lee JH, Canto MI. AGA clinical
practice update on pancreas cancer screening in high-risk
individuals: expert review. Gastroenterology
2020;159:358-62.
Crossref44. Callery MP, Chang KJ, Fishman EK, Talamonti MS,
William Traverso L, Linehan DC. Pretreatment
assessment of resectable and borderline resectable
pancreatic cancer: expert consensus statement. Ann Surg
Oncol 2009;16:1727-33.
Crossref45. Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg
Oncol 2013;20:2787-95.
Crossref46. Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic
adenocarcinoma, version 2.2012: featured updates to the
NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13.
Crossref47. van der Gaag NA, Rauws EA, van Eijck CH, et al.
Preoperative biliary drainage for cancer of the head of
pancreas. N Engl J Med 2010;362:129-37.
Crossref48. Tol JA, Gouma DJ, Bassi C, et al. Definition of a
standard lymphadenectomy in surgery for pancreatic
ductal adenocarcinoma: a consensus statement by the
International Study Group on Pancreatic Surgery (ISGPS).
Surgery 2014;156:591-600.
Crossref49. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant
chemotherapy with gemcitabine and long-term outcomes
among patients with resected pancreatic cancer: the
CONKO-001 randomized trial. JAMA 2013;310:1473-81.
Crossref50. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison
of adjuvant gemcitabine and capecitabine with
gemcitabine monotherapy in patients with resected
pancreatic cancer (ESPAC-4): a multicentre, open-label,
randomised, phase 3 trial. Lancet 2017;389:1011-24.
Crossref51. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or
gemcitabine as adjuvant therapy for pancreatic cancer. N
Engl J Med 2018;379:2395-406.
Crossref52. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant
chemotherapy of S-1 versus gemcitabine for resected
pancreatic cancer: a phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57.
Crossref53. Smeenk HG, van Eijck CH, Hop WC, et al. Long-term
survival and metastatic pattern of pancreatic and
periampullary cancer after adjuvant chemoradiation or
observation: long-term results of EORTC trial 40891. Ann
Surg 2007;246:734-40.
Crossref54. Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant
chemoradiotherapy and chemotherapy in resectable
pancreatic cancer: a randomised controlled trial. Lancet
2001;358:1576-85.
Crossref55. Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis
comparing upfront surgery with neoadjuvant treatment
in patients with resectable or borderline resectable
pancreatic cancer. Br J Surg 2018;105:946-58.
Crossref56. Cloyd JM, Heh V, Pawlik TM, et al. Neoadjuvant therapy
for resectable and borderline resectable pancreatic cancer:
a meta-analysis of randomized controlled trials. J Clin
Med 2020;9:1129.
Crossref57. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant
therapy with FOLFIRINOX followed by individualized
chemoradiotherapy for borderline resectable pancreatic
adenocarcinoma: a phase 2 clinical trial. JAMA Oncol
2018;4:963-9.
Crossref58. Jang JY, Han Y, Lee H, et al. Oncological benefits of
neoadjuvant chemoradiation with gemcitabine versus
upfront surgery in patients with borderline resectable
pancreatic cancer: a prospective, randomized, open-label,
multicenter phase 2/3 trial. Ann Surg 2018;268:215-22.
Crossref59. Versteijne E, Suker M, Groothuis K, et al. Preoperative
chemoradiotherapy versus immediate surgery for
resectable and borderline resectable pancreatic cancer:
results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020;38:1763-73.
Crossref60. Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant
chemoradiotherapy versus upfront surgery for resectable
and borderline resectable pancreatic cancer: long-term
results of the Dutch randomized PREOPANC trial. J Clin
Oncol 2022;40:1220-30.
Crossref61. Katz MH, Shi Q, Meyers JP, et al. Alliance A021501:
preoperative mFOLFIRINOX or mFOLFIRINOX plus
hypofractionated radiation therapy (RT) for borderline
resectable (BR) adenocarcinoma of the pancreas. J Clin
Oncol 2021;39(3_Suppl):377.
Crossref62. Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements
in survival and clinical benefit with gemcitabine as first-line
therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol 1997;15:2403-13.
Crossref63. Xie DR, Yang Q, Chen DL, et al. Gemcitabine-based
cytotoxic doublets chemotherapy for advanced pancreatic
cancer: updated subgroup meta-analyses of overall
survival. Jpn J Clin Oncol 2010;40:432-41.
Crossref64. Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX
for locally advanced pancreatic cancer: a systematic
review and patient-level meta-analysis. Lancet Oncol
2016;17:801-10.
Crossref65. Perri G, Prakash L, Qiao W, et al. Response and survival
associated with first-line FOLFIRINOX vs gemcitabine
and nab-paclitaxel chemotherapy for localized pancreatic
ductal adenocarcinoma. JAMA Surg 2020;155:832-9.
Crossref66. Ambe C, Fulp W, Springett G, Hoffe S, Mahipal A. A meta-analysis
of randomized clinical trials of chemoradiation
therapy in locally advanced pancreatic cancer. J
Gastrointest Cancer 2015;46:284-90.
Crossref67. Chang JS, Chiu YF, Yu JC, Chen LT, Ch’ang HJ. The role
of consolidation chemoradiotherapy in locally advanced
pancreatic cancer receiving chemotherapy: an updated
systematic review and meta-analysis. Cancer Res Treat
2018;50:562-74.
Crossref68. Wang C, Liu X, Wang X, Wang Y, Cha N. Effects of
chemoradiotherapy and chemotherapy on survival of
patients with locally advanced pancreatic cancer: a
meta-analysis of randomized controlled trials. Medicine
(Baltimore) 2018;97:e12260.
Crossref69. Hammel P, Huguet F, van Laethem JL, et al. Effect
of chemoradiotherapy vs chemotherapy on survival
in patients with locally advanced pancreatic cancer
controlled after 4 months of gemcitabine with or without
erlotinib: the LAP07 randomized clinical trial. JAMA
2016;315:1844-53.
Crossref70. Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M,
Barni S. Stereotactic body radiation therapy for locally
advanced pancreatic cancer: a systematic review and
pooled analysis of 19 trials. Int J Radiat Oncol Biol Phys
2017;97:313-22.
Crossref71. Tchelebi LT, Lehrer EJ, Trifiletti DM, et al. Conventionally
fractionated radiation therapy versus stereotactic body
radiation therapy for locally advanced pancreatic cancer
(CRiSP): an international systematic review and meta-analysis.
Cancer 2020;126:2120-31.
Crossref72. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX
versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med 2011;364:1817-25.
Crossref73. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival
in pancreatic cancer with nab-paclitaxel plus gemcitabine.
N Engl J Med 2013;369:1691-703.
Crossref74. Gu J, Xu Z, Ma Y, et al. Surgical resection of metastatic
pancreatic cancer: is it worth it?—a 15-year experience at
a single Chinese center. J Gastrointest Oncol 2020;11:319-28.
Crossref75. Lillemoe KD, Cameron JL, Yeo CJ, et al. Pancreaticoduodenectomy. Does it have a role in the
palliation of pancreatic cancer? Ann Surg 1996;223:718-25.
Crossref76. Sohal DP, Kennedy EB, Khorana A, et al. Metastatic
pancreatic cancer: ASCO clinical practice guideline
update. J Clin Oncol 2018;36:2545-56.
Crossref77. Chan SL, Chan ST, Chan EH, He ZX. Systemic treatment
for inoperable pancreatic adenocarcinoma: review and
update. Chin J Cancer 2014;33:267-76.
Crossref78. Scheithauer W, Schüll B, Ulrich-Pur H, et al. Biweekly
high-dose gemcitabine alone or in combination with
capecitabine in patients with metastatic pancreatic
adenocarcinoma: a randomized phase II trial. Ann Oncol
2003;14:97-104.
Crossref79. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine
plus capecitabine compared with gemcitabine alone in
advanced pancreatic cancer: a randomized, multicenter,
phase III trial of the Swiss Group for Clinical Cancer
Research and the Central European Cooperative Oncology
Group. J Clin Oncol 2007;25:2212-7.
Crossref80. Cunningham D, Chau I, Stocken DD, et al. Phase
III randomized comparison of gemcitabine versus
gemcitabine plus capecitabine in patients with advanced
pancreatic cancer. J Clin Oncol 2009;27:5513-8.
Crossref81. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III
study of gemcitabine plus S-1, S-1 alone, or gemcitabine
alone in patients with locally advanced and metastatic
pancreatic cancer in Japan and Taiwan: GEST study. J Clin
Oncol 2013;31:1640-8.
Crossref82. Cartwright TH, Cohn A, Varkey JA, et al. Phase II study of
oral capecitabine in patients with advanced or metastatic
pancreatic cancer. J Clin Oncol 2002;20:160-4.
Crossref83. Vienot A, Beinse G, Louvet C, et al. Overall survival
prediction and usefulness of second-line chemotherapy in
advanced pancreatic adenocarcinoma. J Natl Cancer Inst
2017;109(10).
Crossref84. Hosein PJ, de Lima Lopes G Jr, Pastorini VH, et al. A phase
II trial of nab-paclitaxel as second-line therapy in patients
with advanced pancreatic cancer. Am J Clin Oncol
2013;36:151-6.
Crossref85. Boeck S, Wilkowski R, Bruns CJ, et al. Oral capecitabine in
gemcitabine-pretreated patients with advanced pancreatic
cancer. Oncology 2007;73:221-7.
Crossref86. Soares HP, Bayraktar S, Blaya M, et al. A phase II study
of capecitabine plus docetaxel in gemcitabine-pretreated
metastatic pancreatic cancer patients: CapTere. Cancer
Chemother Pharmacol 2014;73:839-45.
Crossref87. Pelzer U, Stieler J, Roll L, et al. Second-line therapy in
refractory pancreatic cancer. Results of a phase II study.
Onkologie 2009;32:99-102.
Crossref88. Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized
phase III study of fluorouracil/leucovorin with or
without oxaliplatin for second-line advanced pancreatic
cancer in patients who have received gemcitabine-based
chemotherapy. J Clin Oncol 2016;34:3914-20.
Crossref89. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal
irinotecan with fluorouracil and folinic acid in metastatic
pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57.
Crossref90. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin,
folinic acid, and fluorouracil versus folinic acid and
fluorouracil alone for gemcitabine-refractory pancreatic
cancer: outcomes from the CONKO-003 trial. J Clin
Oncol 2014;32:2423-9.
Crossref91. Yoo C, Hwang JY, Kim JE, et al. A randomised phase
II study of modified FOLFIRI.3 vs modified FOLFOX
as second-line therapy in patients with gemcitabine-refractory
advanced pancreatic cancer. Br J Cancer
2009;101:1658-63.
Crossref92. Dahan L, Bonnetain F, Ychou M, et al. Combination
5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP)
followed by gemcitabine or the reverse sequence in
metastatic pancreatic cancer: final results of a randomised
strategic phase III trial (FFCD 0301). Gut 2010;59:1527-34.
Crossref93. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care
(BSC) versus oxaliplatin, folinic acid and 5-fluorouracil
(OFF) plus BSC in patients for second-line advanced
pancreatic cancer: a phase III-study from the German
CONKO-study group. Eur J Cancer 2011;47:1676-81.
Crossref94. Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus
gemcitabine for metastatic pancreatic adenocarcinoma
after FOLFIRINOX failure: an AGEO prospective
multicentre cohort. Br J Cancer 2015;113:989-95.
Crossref95. Zaanan A, Trouilloud I, Markoutsaki T, et al. FOLFOX
as second-line chemotherapy in patients with pretreated
metastatic pancreatic cancer from the FIRGEM study.
BMC Cancer 2014;14:441.
Crossref96. Golan T, Kanji ZS, Epelbaum R, et al. Overall survival
and clinical characteristics of pancreatic cancer in
BRCA
mutation carriers. Br J Cancer 2014;111:1132-8.
Crossref97. Pishvaian MJ, Blais EM, Brody JR, et al. Outcomes in
patients with pancreatic adenocarcinoma with genetic
mutations in DNA damage response pathways: results
from the Know Your Tumor Program. JCO Precis Oncol
2019;3:1-10.
Crossref98. Golan T, Hammel P, Reni M, et al. Maintenance olaparib
for germline
BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317-27.
Crossref99. Le DT, Durham JN, Smith KN, et al. Mismatch repair
deficiency predicts response of solid tumors to PD-1
blockade. Science 2017;357:409-13.
Crossref100. Sohal DP, Kennedy EB, Cinar P, et al. Metastatic
pancreatic cancer: ASCO guideline update. J Clin Oncol
2020;38:3217-30.
Crossref101. Lowery MA, Jordan EJ, Basturk O, et al. Real-time genomic
profiling of pancreatic ductal adenocarcinoma: potential
actionability and correlation with clinical phenotype. Clin
Cancer Res 2017;23:6094-100.
Crossref102. Hoogerbrugge N, Jongmans MC. Finding all BRCA
pathogenic mutation carriers: best practice models. Eur J
Hum Genet 2016;24 Suppl 1:S19-26.
Crossref103. National Collaborating Centre for Cancer. Familial
Breast Cancer: Classification and Care of People at Risk
of Familial Breast Cancer and Management of Breast
Cancer and Related Risks in People with a Family History
of Breast Cancer. Cardiff (UK): National Collaborating
Centre for Cancer (UK); 2013.
104. Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk
assessment: breast, ovarian, and pancreatic, version
2.2021, NCCN Clinical Practice Guidelines in Oncology. J
Natl Compr Canc Netw 2021;19:77-102.
Crossref105. Pujol P, Barberis M, Beer P, et al. Clinical practice
guidelines for
BRCA1 and
BRCA2 genetic testing. Eur J
Cancer 2021;146:30-47.
Crossref106. House MG, Choti MA. Palliative therapy for pancreatic/biliary cancer. Surg Clin North Am 2005;85:359-71.
Crossref107. Wong GY, Schroeder DR, Carns PE, et al. Effect of
neurolytic celiac plexus block on pain relief, quality
of life, and survival in patients with unresectable
pancreatic cancer: a randomized controlled trial. JAMA
2004;291:1092-9.
Crossref108. Santosh D, Lakhtakia S, Gupta R, et al. Clinical trial:
a randomized trial comparing fluoroscopy guided
percutaneous technique vs. endoscopic ultrasound
guided technique of coeliac plexus block for treatment
of pain in chronic pancreatitis. Aliment Pharmacol Ther
2009;29:979-84.
Crossref