© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Biological disease-modifying antirheumatic
drugs in juvenile idiopathic arthritis of
polyarticular course, enthesitis-related arthritis,
and psoriatic arthritis: a consensus statement
Assunta CH Ho, MB, ChB, FHKAM (Paediatrics)1; SN Wong, MB, BS, FHKAM (Paediatrics)2; Lettie CK Leung, MB, BS (Syd), FHKAM (Paediatrics)3; Winnie KY Chan, MB, BS, FHKAM (Paediatrics)4; Patrick CY Chong, MB, BS, FHKAM (Paediatrics)5; Niko KC Tse, MB, BS, FHKAM (Paediatrics)6; Roanna HM Yeung, MB, BS, FHKAM (Paediatrics)4; SY Kong, MB, ChB, FHKAM (Paediatrics)3; KP Lee, MB, ChB, FHKAM (Paediatrics)7
1 Department of Paediatrics, Prince of Wales Hospital, The Chinese
University of Hong Kong, Shatin, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Tuen Mun Hospital,
Tuen Mun, Hong Kong
3 Department of Paediatrics, Kwong Wah Hospital, Yaumatei, Hong Kong
4 Department of Paediatrics, Queen Elizabeth Hospital, Jordan, Hong
Kong
5 Department of Paediatrics and Adolescent Medicine, Queen Mary
Hospital, The University of Hong Kong, Pokfulam, Hong Kong
6 Department of Paediatrics and Adolescent Medicine, Princess Margaret
Hospital, Laichikok, Hong Kong
7 Department of Paediatrics and Adolescent Medicine, Pamela Youde
Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr Assunta CH Ho (assuntaho@cuhk.edu.hk)
Abstract
Objectives: Juvenile idiopathic arthritis (JIA) is
the most common type of inflammatory arthritis
in children. Treatment options have been expanded
since the introduction of biologics, which are highly
effective. The existing local JIA treatment guideline
was published more than a decade ago, when use
of biologics was not as common. In this article, we
review the latest evidence on using biologics in three
JIA subtypes: JIA of polyarticular course (pcJIA),
enthesitis-related arthritis (ERA), and psoriatic
arthritis (PsA). Based on the latest information,
an update on eligibility, response assessment,
termination, and safety information for using
biologics in these patients was performed.
Consensus process: The JIA Work Group, which
consisted of nine paediatricians experienced in
managing JIA, was convened in 2016. Publications
before July 2017 were screened. Eligible articles were
clinical trials, extension studies, systemic reviews,
and recommendations from international societies
and regulatory agencies about the use of biologics in
pcJIA, ERA, and PsA. Evidence extraction, appraisal,
and drafting of propositions were performed by
two reviewers. Extracted evidence and drafted
propositions were presented and discussed at the
first two meetings. Overwhelming consensus was
obtained at the final meeting in May 2018. Seven practice consensus statements were formulated.
Regular review should be performed to keep the
practice evidence-based and up-to-date.
Introduction
Biological disease-modifying antirheumatic drugs
(DMARDs) were introduced for the treatment of
juvenile idiopathic arthritis (JIA) in year 2000. They
are highly effective, especially for patients who are
refractory to conventional DMARDs (cDMARDs).
The current treatment algorithm for JIA in Hong
Kong was published more than a decade ago, when
the use of biologics was not as common.1 There has been a huge expansion in knowledge and approved
medications since then. An update of practice
based on the latest evidence is required, not only
for specialists but also paediatricians and family
physicians who may provide care to children with
JIA.
The Hong Kong Society for Paediatric Rheumatology commissioned the JIA Work Group
in 2016 to review this topic. The work group consists
of nine paediatricians experienced in managing JIA.
Our aims were: (1) to review the latest evidence
on biological DMARDs in polyarticular course JIA
(pcJIA, ie, those having arthritis affecting ≥5 joints
irrespective of subtype at onset), enthesitis-related
arthritis (ERA), and psoriatic arthritis (PsA); and (2)
to propose an updated practice consensus for using
biologics in these patients.
Methods
Articles about the use of biologics in pcJIA, ERA,
and PsA published in English before July 2017 were
identified by searching MEDLINE and PubMed.
The keywords used for searching included JIA, pcJIA, ERA, PsA, eoJIA (extended oligoarticular
arthritis), biologics, biological DMARDs, guidelines,
recommendation, practice review, registries, adverse
effects, malignancy, and infection. Publications
considered relevant were clinical trials (randomised
trials, reports on long-term extension phase of
clinical trials), open-label studies, results from
analysis of major registries, and recommendations
from international regulatory organisations (United
Kingdom: Clinical Commissioning Policy of National
Health Service, National Institute for Health and
Care Excellence; United States: American College of
Rheumatology [ACR]; Australia: the Pharmaceutical
Benefits Scheme, reimbursement programme for
biologics use). Article screening, grading of the
level of evidence, and propositions drafting were
performed by two reviewers. The extracted evidence
and draft propositions were presented to work group
members at the first meeting held in July 2017. They
were discussed and deliberated in a subsequent
meeting held in March 2018. At the final meeting in
May 2018, overwhelming consensus was reached on
seven practice consensus statements.
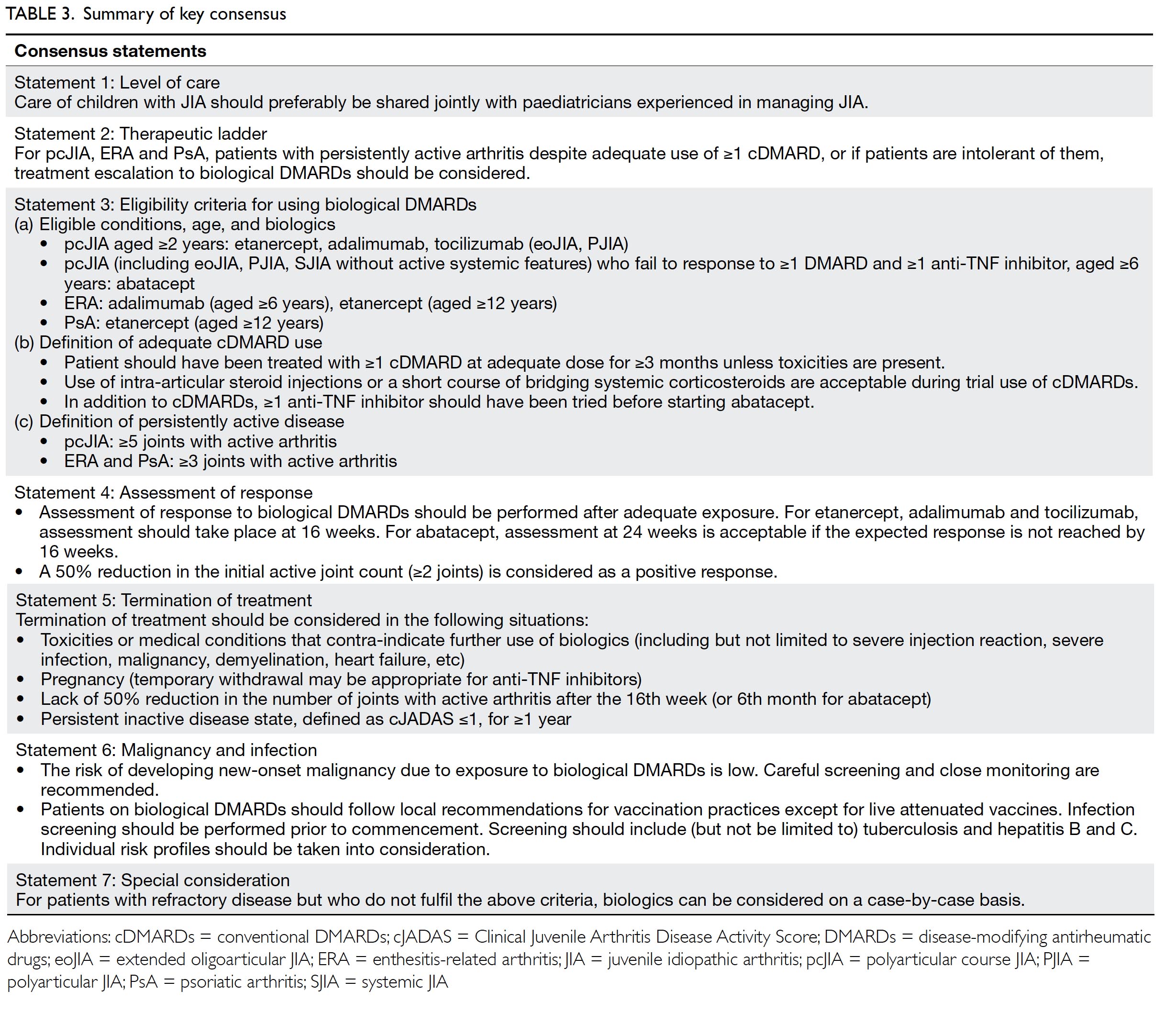
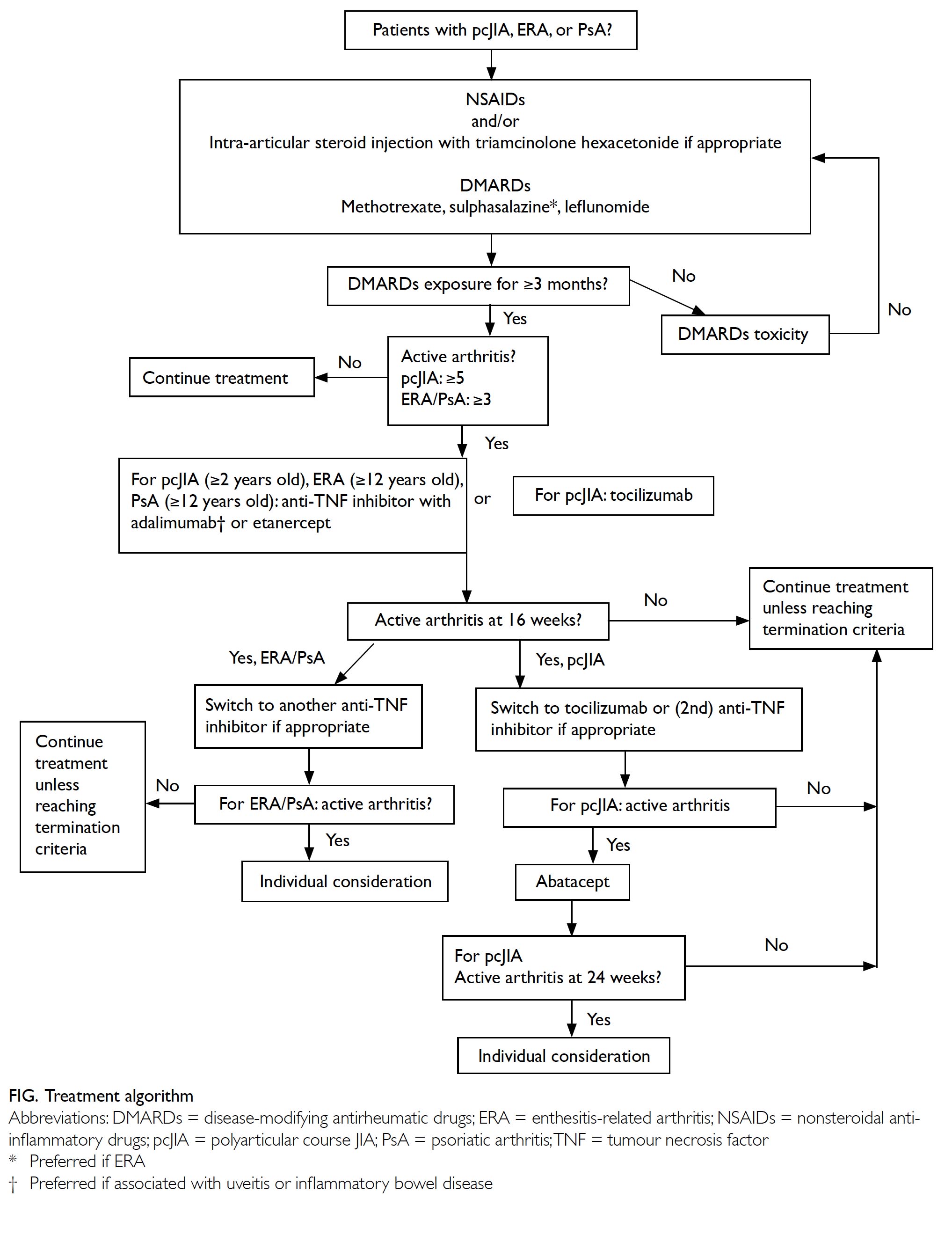
Statement 1: Level of care
Care of children with JIA should preferably be
shared jointly with paediatricians experienced in
managing JIA.
With modern treatment strategies, a high level
of disease control is possible. One study reported
that more than 70% of patients (except rheumatoid
factor positive polyarticular JIA) were able to reach
a clinically inactive disease state within 2 years.2 To
achieve the best outcome, care plans and treatment
targets should be carefully formulated together with
patients and paediatricians experienced in managing
JIA.
Statement 2: Therapeutic ladder
For pcJIA, ERA and PsA, patients with persistently
active arthritis despite adequate use of one or
more cDMARDs, or if patients are intolerant of
them, treatment escalation to biological DMARDs
should be considered.
Conventional DMARDs like methotrexate,
leflunomide, and sulphasalazine are effective in
treating pcJIA.3 4 5 6 The choice of cDMARDs usually
depends on JIA subtype and patient tolerance.
Nevertheless, some patients may not respond to
or may be intolerant of cDMARDs. Switching to
biologics should be considered in these cases.
Biological disease-modifying antirheumatic
drugs: evidence of effectiveness
Anti-tumour necrosis factor inhibitors
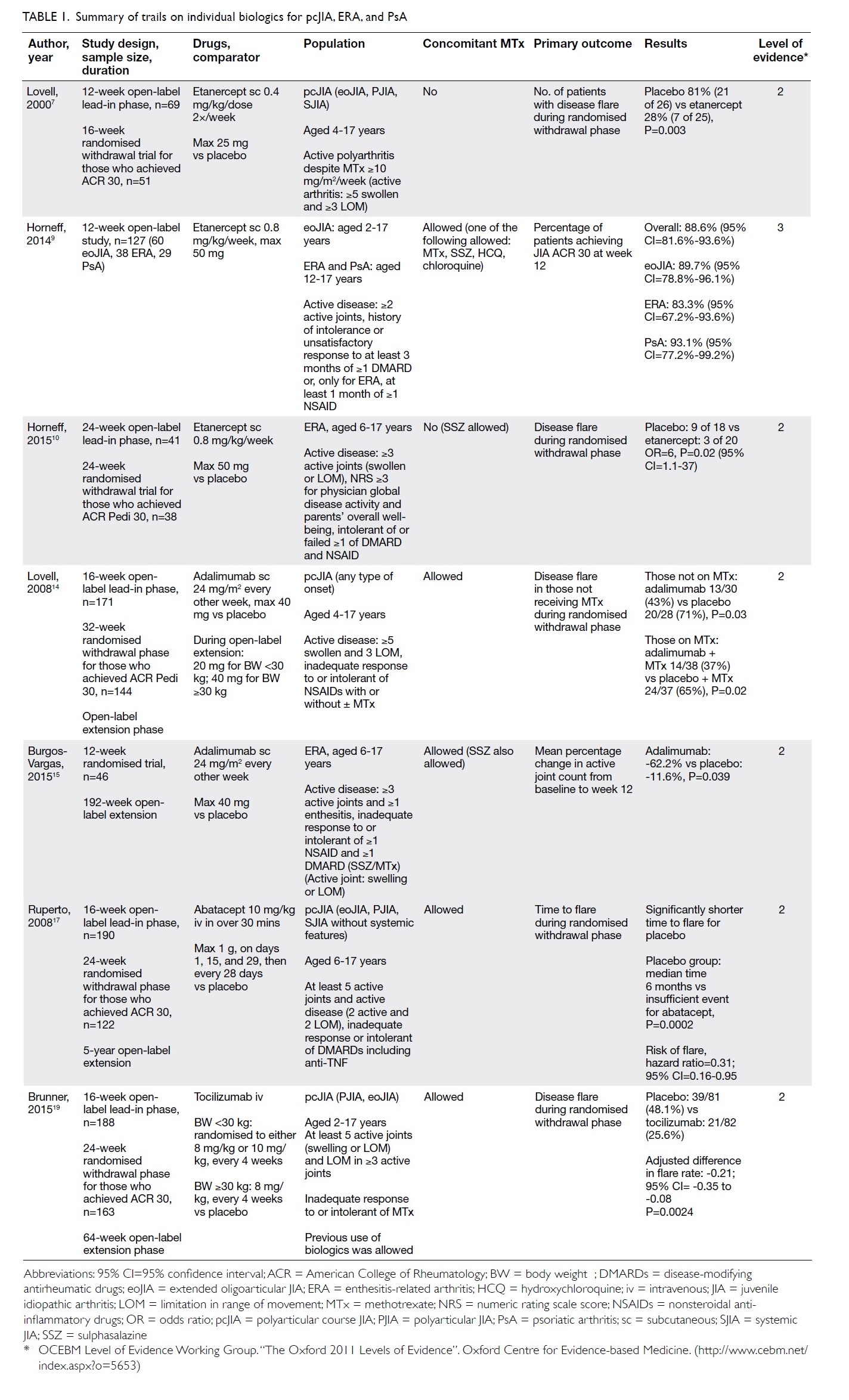
Etanercept
Etanercept is a chimeric fusion protein that acts as soluble TNF receptor. It is approved for use in
cases of: (1) pcJIA in children aged ≥2 years (Food
and Drug Administration [FDA] and European
Medicines Agency [EMA]) and (2) ERA and PsA in
children aged ≥12 years (EMA). The dose is 0.4 mg/kg
(max 25 mg) twice weekly or 0.8 mg/kg (max 50 mg)
once weekly subcutaneously.
Etanercept was the first anti-TNF inhibitor
approved for pcJIA. Its efficacy was demonstrated by
a randomised withdrawal trial7, in which 69 patients
with pcJIA aged 4 to 17 years who were refractory to
methotrexate were enrolled in the open-label lead-in
phase. Fifty one (74%) patients achieved an ACR 30
response at week 12. They were then randomised to
receive either etanercept or placebo for 16 weeks.
Etanercept was more effective in preventing arthritis
flares (28% vs 81%, P=0.003). The median time to
flare was significantly shorter in the placebo group
(28 days vs 116 days, P<0.001). Long-term data from
the extension phase confirmed the persistence of
efficacy.8
The use of etanercept in eoJIA, ERA, and PsA
was studied by Horneff et al.9 In all, 127 patients
(60 patients with eoJIA aged 2-17 years, 38 patients
with ERA aged 12-17 years, 29 patients with PsA
aged 12-17 years) were recruited in an open-label
multicentre study. All had persistently active disease
despite the use of nonsteroidal anti-inflammatory
drugs (NSAIDs; for ERA) and DMARDs (for eoJIA,
ERA, and PsA). At week 12, 88.6% (95% confidence
interval [CI]=81.6%-93.6%) had attained ACR 30
response. The proportion of response was similar
across all three subtypes.
The efficacy of etanercept in ERA was further
evaluated. In a phase III double-blinded study,
41 patients aged 6 to 17 years with active disease
despite taking at least one NSAID and one DMARD
were recruited. They received open-label etanercept
for 24 weeks. An ACR 30 response was achieved
in 38 (93%). These patients were then randomised
to receive etanercept or placebo in the subsequent
24-week withdrawal phase. The odds for having a
flare was significantly higher in placebo group (odds
ratio=6, 95% CI=1.1-37, P=0.02).10
The use of etanercept has now been extended
to 2 years old.11 The efficacy of administrating at 0.8
mg/kg weekly has been shown to be comparable to
twice weekly dosing.12
Etanercept can be used concomitantly with
methotrexate or as monotherapy.13
Adalimumab
Adalimumab is a fully humanised monoclonal anti-
TNF antibody. It is approved for use in cases of: (1)
pcJIA in children aged ≥2 years (FDA and EMA) and
(2) ERA in children aged ≥6 years (EMA). The dose is
once every other week at 10 mg for 10 to <15 kg, 20
mg for 15 to <30 kg, and 40 mg for ≥30 kg.
Adalimumab was shown to be effective in
treating pcJIA by Lovell et al.14 In total, 171 patients
aged 4 to 17 years with active arthritis despite
taking NSAIDs or methotrexate were enrolled in
the 16-week open-label phase. In all, 94% of the
adalimumab-methotrexate group and 74% of the
adalimumab monotherapy group demonstrated
an ACR 30 response. In the 32-week randomised
withdrawal phase, the flare rate was significantly
lower for those who remained on adalimumab. The
results were more pronounced in patients receiving
concomitant methotrexate (flare in placebo and
methotrexate vs adalimumab and methotrexate: 65%
vs 37%, P=0.02; flare in placebo vs adalimumab: 71%
vs 43%, P=0.03). Efficacy was maintained in the long-term
extension phase.
Burgos-Vargas et al15 studied the effectiveness
of adalimumab in 46 patients with ERA. These
patients, aged 6 to 17 years, had active arthritis and
enthesitis. They were randomised to receive either
adalimumab or placebo. At week 12, the adalimumab
group had a significantly greater percentage decrease
in the number of active joints (-62.2% vs -11.6%,
P=0.039).
The safety and effectiveness of adalimumab in
children aged 2 to 4 years weighing <15 kg were also
demonstrated.16
Adalimumab can be used together with
methotrexate or as monotherapy.
T cell co-stimulation inhibitor
Abatacept
Abatacept is a co-stimulation modulator that binds to the cluster of differentiation (CD) 80 or CD 86
ligands of the antigen-presenting cell and interferes
with its interactions with T cells. Abatacept is
indicated for pcJIA in children aged ≥6 years who
are unresponsive to cDMARDs and at least one anti-TNF (EMA, FDA). The dose is 10 mg/kg (max 1g)
infusion on day 1, 15, 29, and then every 28 days.
The efficacy of abatacept in pcJIA was assessed
in a randomised withdrawal trial by Ruperto et al.17
The enrolled patients were aged 6 to 17 years with
active disease despite DMARDs including anti-TNF
inhibitors. Systemic JIA patients without systemic
manifestations were also eligible. Patients with ERA
or PsA were not included. Among the 190 recruited
patients, 27% had previously been exposed to anti-TNF therapy. At the end of the 4-month open-label
phase, 123 (65%) demonstrated an ACR 30 response.
After one patient left the study, the remaining 122
patients were randomised to receive abatacept (60
patients) or placebo (62 patients) for 6 months. The
flare rate of the abatacept group was significantly
lower than that of the placebo group (20% vs 53%,
P=0.0003). The hazard ratio for having a flare in the
abatacept group was 0.31 (95% CI=0.16-0.95). The
median time to flare was 6 months for placebo and
not assessable for abatacept because of an inadequate
number of events (P=0.0002). Among patients who
did not achieve ACR 30 at the end of the 4-month
open-label phase, half achieved ACR 30 after longer
exposure. By day 589 of the long-term extension
phase, the proportions of patients achieving ACR
30, 50, 70, 90, and 100 were 90%, 88%, 75%, 57%, and
39%, respectively.18
Abatacept can be used concomitantly with
methotrexate or as monotherapy.
Anti-interleukin 6 inhibitor
Tocilizumab
Tocilizumab is a humanised anti–interleukin 6
monoclonal antibody. It is indicated for pcJIA in
child aged ≥2 years (FDA, EMA). The dose is once
every 4 weeks intravenous infusion, 10 mg/kg for
<30 kg, 8 mg/kg for ≥30 kg.
Tocilizumab is another non-anti-TNF option
for pcJIA. In a phase 3, three-part randomised
controlled study (CHERISH), 188 patients aged
2 to 17 years with active disease received open-label
tocilizumab for 16 weeks. In all, 166 (88%)
achieved an ACR 30 response. A total of 163 were
then randomised to receive tocilizumab (n=82) or
placebo (n=81) for 24 weeks. Flares of JIA occurred
significantly less often in the tocilizumab group
(25.6% vs 48.1%, difference in means adjusted
for stratification -0.21; 95% CI= -0.35 to -0.08;
P=0.0024). Numerically more patients on concurrent
methotrexate achieved ACR 70 and 90 responses.19
Tocilizumab can be used concomitantly with
methotrexate or as monotherapy.
Systemic reviews of biologics
The short-term efficacies of biologics have been
confirmed by systemic reviews.20 21 22 While there have
been no head-to-head trials between individual
biologics, analysis with indirect comparisons has not
shown obvious differences in efficacy. Longer-term
data (mainly for etanercept) and reports from major
registries also confirm biologics’ effectiveness and
safety.23 The results of clinical trials are summarised
in Table 1.
Statement 3: Eligibility criteria for
using biological disease-modifying
antirheumatic drugs
The work group achieved overwhelming consensus
on the following eligibility criteria.
Eligible conditions, age, and biologics
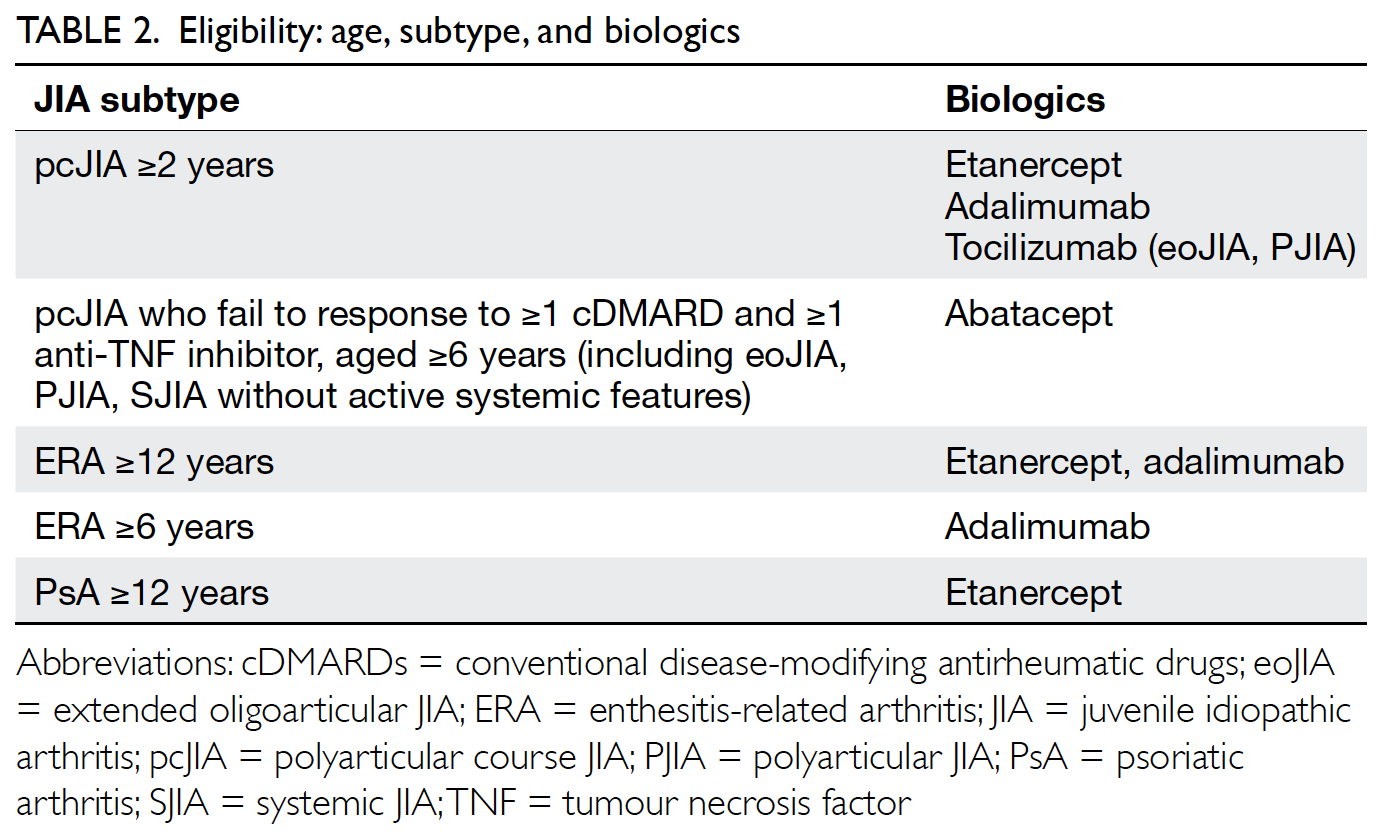
Table 2 lists the JIA subtypes and the corresponding biological DMARDs indicated. Anti-TNF inhibitors
are usually the first biologics to commence, except in
systemic JIA. The decision of which biologic to start
also depends on JIA subtype, the presence of extra-articular
manifestations (eg, uveitis, inflammatory
bowel disease), patient preferences, etc.24 25
Definition of adequate use of conventional
disease-modifying antirheumatic drugs
It usually takes 6 to 8 weeks before the effects
of cDMARDs can be seen. “Adequate cDMARDs
exposure” is often defined as at least 3 months.
However, if one develops significant intolerance or
toxicities during trial of cDMARDs, use of biologics
should be considered earlier.
Definition of persistently active disease
“Active arthritis” is defined as swelling, with
or without limitation in movement. Joints with
limitation in movement due to pain or tenderness
are also considered as “active”, especially those in
which swelling is difficult to assess.
When deciding the number of joints required
to define active disease, the work group adopted the
commonly used inclusion criteria of the clinical trials.
In view of the fact that the disease characteristics
(eg, number of joints involved, clinical course,
medication indicated) are somewhat different for ERA and PsA, the work group considered it more
appropriate to have seperate definitions for ERA and
PsA.
Statement 4: Assessment of
response
Patients usually experience improvement
within or around 3 months after commencement.
The exception is abatacept, which may take a bit
longer to achieve the desired therapeutic effect.20
The definition of positive response is
extrapolated from Pharmaceutical Benefits Scheme.
It is easy to apply and does not require inflammatory
markers like erythrocyte sedimentation rate and C-reactive
protein, as they are not always elevated.
Statement 5: Termination of
treatment
Termination of treatment should be considered in
the following situations:
The above termination criteria represent
the common “end points” for stopping biologics,
ie, the development of side-effects, conditions
contra-indicated for use of biologics, or continuous
remission of the index disease.
Pregnancy is no longer an absolute contraindication
in adult patients with rheumatoid arthritis
or ankylosing spondylitis who are receiving anti-TNF inhibitors. Temporary withdrawal, however,
is necessary for some agents. There have been no
recent updates from international organisations on
the use of biologics in pregnant patients with JIA.
This issue should be revisited during the next update.
Several definitions are used to describe
treatment response and disease remission in JIA.26 The Clinical Juvenile Arthritis Disease Activity Score
is a composite measurement that evaluates three
variables: active joint count, patient’s/parents’ global
well-being score, and physician’s global disease
activity score (both on visual analogue scales).
It has been shown to outperform other activity
measurements in predicting the need of escalation
to anti-TNF inhibitors.27 For both oligoarticular
arthritis and pcJIA, a score of ≤1 signifies inactive
disease.
Statement 6: Malignancy and
infection
In 2008, the FDA issued a black box warning
about the development of malignancy, particularly
lymphoproliferative types, in 48 children after
commencement of anti-TNF inhibitors between
2001 and 2008.28 In recent years, researchers
investigated the risk of malignancy by analysing
data generated from major registries and medical
reimbursement databases. These data suggested that
TNF inhibition alone is probably not causing the
apparent increase in incident malignancy. Instead,
those children’s background risk might have already
been elevated compared with that of the general
population. Nevertheless, much longer-term data
on larger patient samples are needed, as both cancer
and JIA are rare in children.29 30 31
Data on the associations between malignancy
and other non-anti-TNF biologics are still lacking.
Careful screening prior to commencement and
ongoing surveillance are necessary.
As for infection, biological DMARDs are
generally safe. Most infections associated with the
use of biologics are mild. Nevertheless, data from
registries and medical insurance databases did
show an increase in bacterial or serious infections
associated with TNF inhibition.32 33 34 Tuberculosis is a
genuine concern in our locality.35 36 Careful screening
and follow-up according to individual risk profiles
is strongly encouraged. The baseline infection
screening should include, but not be limited to, the
following:
While live attenuated vaccines should be
avoided during use of biologics, patients should
follow local guidelines for non-live vaccines.
Statement 7: Special consideration
The work group is aware that some patients
who do not fulfil the above criteria might still benefit
from biologics. These patients include those with
persistently active and disabling multiple enthesitis
or sacroiliitis despite NSAIDs and DMARDs or
oligoarticular JIA with severely active arthritis
and has exhausted treatment modalities including
intra-articular steroid injections and multiple
cDMARDs, etc. The use of biologics can be considered after careful assessment on a case-bycase basis.
Looking ahead: new indications
after the review period
The indications for use of biological DMARDs
have been expanding. For example, subcutaneous abatacept and tocilizumab are approved for pcJIA in
patients aged ≥2 years in the United States.37 38 39 The
ACR is also preparing a new update of the 2011 and
2013 guidelines. A review of the practice should be
performed regularly.
Conclusion
The use of biological DMARDs has greatly improved
the management of patients with JIA. Both patients and physicians could benefit from incorporating
the latest and best available evidence into disease
management. Periodic review should be performed
to keep clinical practice evidence-based and up-to-date.
Author contributions
All the authors contributed substantially to conception and design, acquisition and analysis of data, drafting and revising the article, and approval of the version to be published.
Conflicts of interest
All authors have no competing interest in the products
mentioned in this paper.
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
References
1. Lee TL, Lau YL, Chan W, et al. Juvenile idiopathic arthritis in Hong Kong and its current management. HK J Paediatr (new series) 2003;8:21-30. >
2. Guzman J, Oen K, Tucker LB, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854-60. Crossref
3. Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children's Study Group. N Engl J Med 1992;326:1043-9. Crossref
4. Woo P, Southwood TR, Prieur AM, et al. Randomised placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum 2000;43:1849-57. Crossref
5. Silverman E, Mouy R, Spiegel L, et al. Leflunomide or methotrexate for juvenile rheumatoid arthritis. N Engl J Med 2005;352:1655-66. Crossref
6. van Rossum MA, Fiselier TJ, Franssen MJ, et al. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomised, double-blind, placebo-controlled, multicenter study. Dutch Juvenile Chronic Arthritis Study Group. Arthritis Rheum 1998;41:808-16. Crossref
7. Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med 2000;342:763-9. Crossref
8. Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 2008;58:1496-504. Crossref
9. Horneff G, Burgos-Vargas R, Constantin T, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis- related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis 2014;73:1114-22. Crossref
10. Horneff G, Foeldvari I, Minden K, et al. Efficacy and safety of etanercept in patients with the enthesitis-related arthritis category of juvenile idiopathic arthritis. Arthritis Rheumatol 2015;67:2240-9. Crossref
11. Windschall D, Horneff G. Safety and efficacy of etanercept and adalimumab in children aged 2 to 4 years with juvenile idiopathic arthritis. Clin Rheumatol 2016;35:2925-31. Crossref
12. Horneff G, Ebert A, Fitter S, et al. Safety and efficacy of once weekly etanercept 0.8 mg/kg in a multicenter 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatology (Oxford) 2009;48:916-9. Crossref
13. Horneff G, De Bock F, Foeldvari I, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis 2009;68:519-25. Crossref
14. Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810-20. Crossref
15. Burgos-Vargas R, Tse SM, Horneff G, et al. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res (Hoboken) 2015;67:1503-12. Crossref
16. Kingsbury DJ, Bader-Meunier B, Patel G, Arora V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol 2014;33:1433-41. Crossref
17. Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet 2008;372:383-91. Crossref
18. Ruperto N, Lovell DJ, Quartier P, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1792-802. Crossref
19. Brunner HI, Ruperto N, Zuber Z, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomized, double-blind withdrawal trial. Ann Rheum Dis 2015;74:1110-7. Crossref
20. Shepherd J, Cooper K, Harris P, Picot J, Rose M. The clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis: a Crossref
21. Ungar WJ, Costa V, Burnett HF, Feldman BM, Laxer RM. The use of biologic response modifiers in polyarticular- course juvenile idiopathic arthritis: a systematic review. Semin Arthritis Rheum 2013;42:597-618. Crossref
22. Otten MH, Anink J, Spronk S, van Suijlekom-Smit LW. Efficacy of biological agents in juvenile idiopathic arthritis: a systematic review using indirect comparisons. Ann Rheum Dis 2013;72:1806-12. Crossref
23. Klotsche J, Niewerth M, Haas JP, et al. Long-term safety of etanercept and adalimumab compared to methotrexate in patients with juvenile idiopathic arthritis (JIA). Ann Rheum Dis 2016;75:855-61. Crossref
24. Anink J, Otten MH, Gorter SL, et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatology (Oxford) 2013;52:1674-9. Crossref
25. Kearsley-Fleet L, Davies R, Baildam E, et al. Factors associated with choice of biologics among children with juvenile idiopathic arthritis: results from two UK paediatric biologic registers. Rheumatology (Oxford) 2016;55:1556-65. Crossref
26. Smith EM, Foster HE, Beresford MW. The development and assessment of biological treatments for children. Br J Clin Pharmacol 2015;79:379-94. Crossref
27. Swart JF, van Dijkhuizen EH, Wulffraat NM, de Roock S. Clinical juvenile arthritis disease activity score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis 2018;77:336-42. Crossref
28. Diak P, Siegel J, La Grenada L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum 2010;62:2517-24. Crossref
29. Beukelman T, Haynes K, Curtis JR, et al. Rate of malignancy associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012;64:1263-71. Crossref
30. Ruperto N, Martini A. Juvenile idiopathic arthritis and malignancy. Rheumatology (Oxford) 2014;53:968-74. Crossref
31. Mannion ML, Beukelman T. Risk of malignancy associated with biologic agents in pediatric rheumatic disease. Curr Opin Rheumatol 2014;26:538-42. Crossref
32. Beukelman T, Xie F, Chen L, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012;64:2773- 80. Crossref
33. Davies R, Southwood TR, Kearsley-Fleet L, Lunt M, Hyrich KL; British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study. Medically significant infections are increased in patients with juvenile idiopathic arthritis treated with etanercept: results from the British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study. Arthritis Rheumatol 2015;67:2487-94. Crossref
34. Becker I, Horneff G. Risk of serious infection in juvenile idiopathic arthritis patients associated with tumor necrosis factor inhibitors and disease activity in the German biologic in pediatric rheumatology registry. Arthritis Care Res (Hoboken) 2017;69:552-60. Crossref
35. Mok CC, Tam LS, Chan TH, Lee GK, Li EK; Hong Kong Society of Rheumatology. Management of rheumatoid arthritis: consensus recommendations from the Hong Kong Society of Rheumatology. Clin Rheumatol 2011;30:303-12. Crossref
36. Wan R, Mok CC. The Hong Kong Society of Rheumatology Biologics Registry: Updated Report (May 2016). HK Bulletin Rheum Dis 2016;16:24-6. Crossref
37. Brunner HI, Tzaribachev N, Vega-Cornejo G, et al. Subcutaneous abatacept in patient with polyarticular- course juvenile idiopathic arthritis: results from a phase III open-label study. Arthritis Rheumatol 2018;70:1144-54. Crossref
38. Brunner H, Ruperto N, Martini A, et al. Identification of optimal subcutaneous doses of tocilizumab in children with polyarticular course juvenile idiopathic arthritis. Arthritis Rheumatol 2017;69(Suppl 4):Abstract 41.
39. De Benedetti F, Ruperto N, Lovell D, et al. THU0503 identification of optimal subcutaneous (SC) doses of tocilizumab in children with polyarticular-course juvenile idiopathic arthritis (PCJIA). Ann Rheum Dis 2017;76(Suppl 2):396. Crossref