Antibiotic management of acute pharyngitis in primary care
Hong
Kong Med J 2019 Feb;25(1):58–63 | Epub 31 Jan 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Antibiotic management of acute pharyngitis in primary
care
The Advisory Group on Antibiotic Stewardship
Programme in Primary Care
Angus MW Chan, MB, ChB (Glasg), FHKAM (Family
Medicine)1; Winnie WY Au, MB, BS2; David VK Chao,
FRCGP, FHKAM (Family Medicine)3; K Choi, MB, BS, FHKAM (Family
Medicine)4; KW Choi, MB, ChB, FHKAM (Medicine)5;
Sarah MY Choi, MB, ChB, FHKAM (Community Medicine)6; Y Chow,
MB, BS, FHKAM (Psychiatry)7; Cecilia YM Fan, MB, BS, FHKAM
(Family Medicine)8; PL Ho, MD, FACP9; Eric MT Hui,
FHKCFP, FHKAM (Family Medicine)10; KH Kwong, MB, BS, MFM (Clin)
(Monash)11; Benjamin YS Kwong, BSc Pharm, MPharmS12;
TP Lam, MD, FHKAM (Family Medicine)13; Edman TK Lam, MB, ChB,
FHKAM (Pathology)14; KW Lau, BSc Pharm, MPharmS14;
Leo Lui, MB, BS, FHKAM (Pathology)14; Ken HL Ng, MB, BS, FHKAM
(Pathology)14; Martin CS Wong, MD, FHKAM (Family Medicine)15;
TY Wong, MB, BS, FHKAM (Medicine)14; CF Yeung, MB, BS, FHKAM
(Paediatrics)16; Joyce HS You, PharmD, BCPS (AQ Infectious
Diseases)17; Raymond WH Yung, MB, BS, FHKAM (Pathology)18
1 Hong Kong College of Family
Physicians, Hong Kong
2 Infection Control Branch, Centre for
Health Protection, Department of Health, Hong Kong
3 Department of Family Medicine and
Primary Health Care, United Christian Hospital, Hospital Authority, Hong
Kong
4 Hong Kong Medical Association, Hong
Kong
5 Hong Kong Society for Infectious
Diseases, Hong Kong
6 Primary Care Office, Department of
Health, Hong Kong
7 Quality HealthCare Medical Services
Limited, Hong Kong
8 Professional Development and Quality
Assurance, Department of Health, Hong Kong
9 IMPACT Editorial Board, Reducing
bacterial resistance with IMPACT, 5th edition, Hong Kong
10 Department of Family Medicine, New
Territories East Cluster, Hospital Authority, Hong Kong
11 Human Health Holdings Limited, Hong
Kong
12 Chief Pharmacist’s Office, Hospital
Authority, Hong Kong
13 Department of Family Medicine and
Primary Care, The University of Hong Kong, Hong Kong
14 Infection Control Branch, Centre for
Health Protection, Department of Health, Hong Kong
15 Hong Kong Academy of Medicine, Hong
Kong
16 Hong Kong Doctors Union, Hong Kong
17 School of Pharmacy, The Chinese
University of Hong Kong, Hong Kong
18 Hong Kong Sanatorium & Hospital,
Hong Kong
Corresponding author: Dr Edman TK Lam (edmanlam@cuhk.edu.hk)
Abstract
The Centre for Health Protection of the
Department of Health has convened the Advisory Group on Antibiotic
Stewardship Programme in Primary Care (the Advisory Group) to formulate
guidance notes and strategies for optimising judicious use of
antibiotics and enhancing the Antibiotic Stewardship Programme in
Primary Care. Acute pharyngitis is one of the most common conditions
among out-patients in primary care in Hong Kong. Practical
recommendations on the diagnosis and antibiotic treatment of acute
streptococcal pharyngitis are made by the Advisory Group based on the
best available clinical evidence, local prevalence of pathogens and
associated antibiotic susceptibility profiles, and common local
practice.
Introduction
The Government of the Hong Kong Special
Administrative Region attaches great importance to the threat of
antimicrobial resistance. Under the authority of the Food and Health
Bureau, and with collaborative efforts from stakeholders, the Hong Kong
Strategy and Action Plan on Antimicrobial Resistance (2017-2022) was
established in July 2017. Recommendations in six key areas and 19
objectives were included in this Action Plan, aiming to slow the emergence
of antimicrobial resistance and prevent its spread.
In connection with this Action Plan, the Centre for
Health Protection of the Department of Health convened the Advisory Group
on Antibiotic Stewardship Programme in Primary Care (the Advisory Group)
comprising key stakeholders in the public and private sectors, academia,
and major professional societies. Its objective is to formulate guidance
notes and strategies for optimising the judicious use of antibiotics and
enhancing the Antibiotic Stewardship Programme in Primary Care (https://www.chp.gov.hk/en/features/49811.html).
Guidance notes on antibiotic treatments for common infections seen by
primary care doctors have been developed based on the best available
clinical evidence, local prevalence of pathogens and associated antibiotic
susceptibility profiles, and local practice. Clinical evidence has mainly
referred to international practices, the latest guidelines from
international organisations, and systematic review articles. In addition,
simple information sheets for out-patients are prepared to raise awareness
and enable them to use antibiotics appropriately. Primary care doctors
play an important role in antimicrobial resistance containment measures by
not only practising rational antibiotic prescriptions but also educating
and engaging out-patients about the safe use of antibiotics during
clinical encounters.
Acute pharyngitis is the acute inflammation of the
oropharynx. It is characterised by sore throat and pharyngeal erythema. It
is one of the most common conditions among out-patients in primary care in
Hong Kong.1 2
Acute pharyngitis is usually a benign,
self-limiting illness with an average length of illness of 1 week. It is
often caused by respiratory viruses (eg, rhinovirus, coronavirus,
adenovirus, influenza virus, parainfluenza virus, respiratory syncytial
virus and metapneumovirus). The other viruses of concern are enterovirus,
herpes simplex virus, Epstein-Barr virus, cytomegalovirus, and human
immunodeficiency virus (HIV). Viral pharyngitis is a condition for which
antibiotics are not necessary. Out-patients with a sore throat and
associated symptoms and signs, including conjunctivitis, coryza, cough,
discrete ulcerative stomatitis, hoarseness, diarrhoea, and viral
exanthema, are most likely to have a viral illness, such as common cold,
influenza, herpangina, and oral herpes.
Beta-haemolytic streptococci, particularly group A
Streptococcus (GAS), are the most common bacterial pathogens of
acute pharyngitis. Group A Streptococcus is estimated to be
responsible for approximately 10% of cases of acute pharyngitis in adults
and 15% to 30% of those in children.3
A local study at an accident and emergency department in Hong Kong showed
that for those presenting with a sore throat and without symptoms of
common cold or influenza, the prevalence rates of GAS pharyngitis were
2.65% in adults and adolescents aged >14 years and 38.6% in children
aged 3 to 14 years; none of the children aged <3 years had GAS
pharyngitis.4 Group A Streptococcus
pharyngitis can lead to suppurative (eg, quinsy, otitis media, and other
invasive infections) and non-suppurative (eg, acute rheumatic fever,
poststreptococcal glomerulonephritis) complications. However, acute
rheumatic fever has not been described as a complication of either group C
Streptococcus or group G Streptococcus pharyngitis.
Streptococcal pharyngitis is the most common form of acute pharyngitis, in
which antibiotic treatment is indicated.
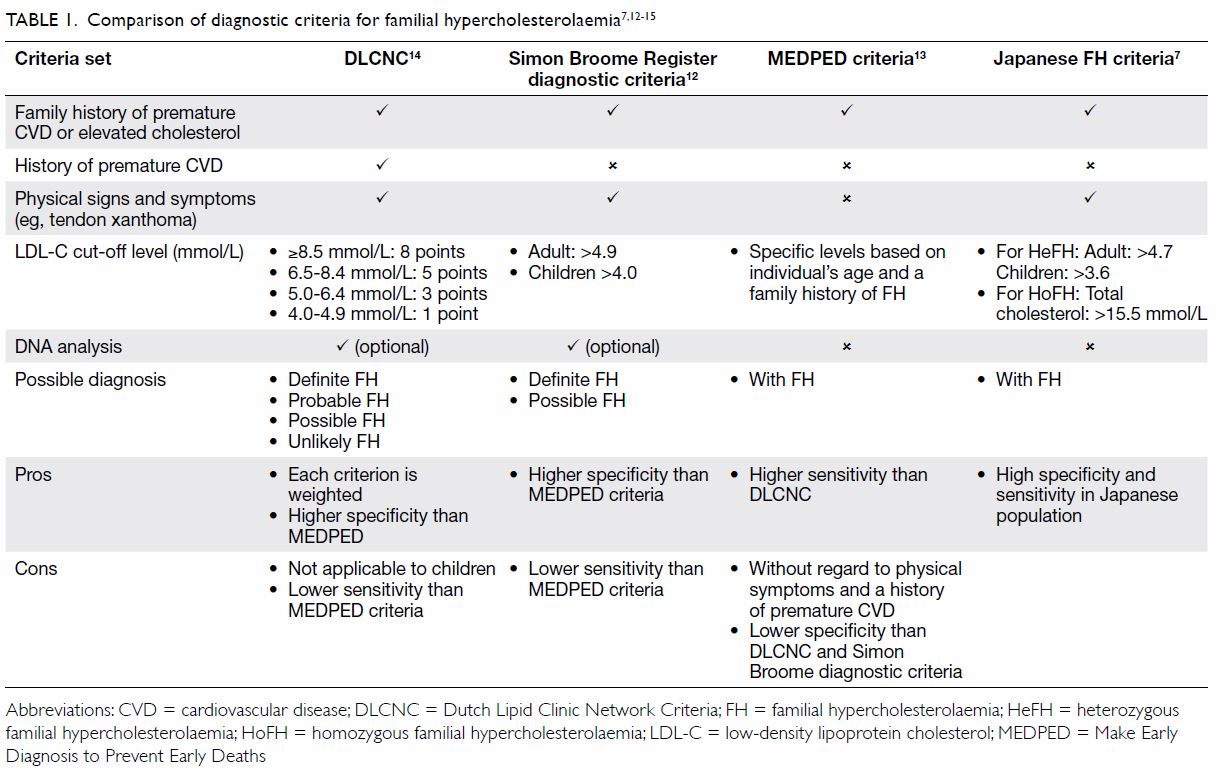
Diagnosis of acute streptococcal pharyngitis
There are different recommendations on the
diagnostic strategy of acute streptococcal pharyngitis. Ideally, to obtain
a definitive diagnosis, out-patients with symptoms and signs suggestive of
a bacterial cause (eg, sudden onset of fever, anterior cervical
lymphadenopathy, tonsillopharyngeal exudates) should be tested for GAS
with a rapid antigen detection test (RADT) and/or throat culture.5 6 7 8 A negative
RADT should be backed up by a throat culture in children and adolescents,
but not in adults. Practically and clinically, different various clinical
scoring criteria have been developed to estimate the likelihood of acute
streptococcal pharyngitis, and we recommend that practitioners make
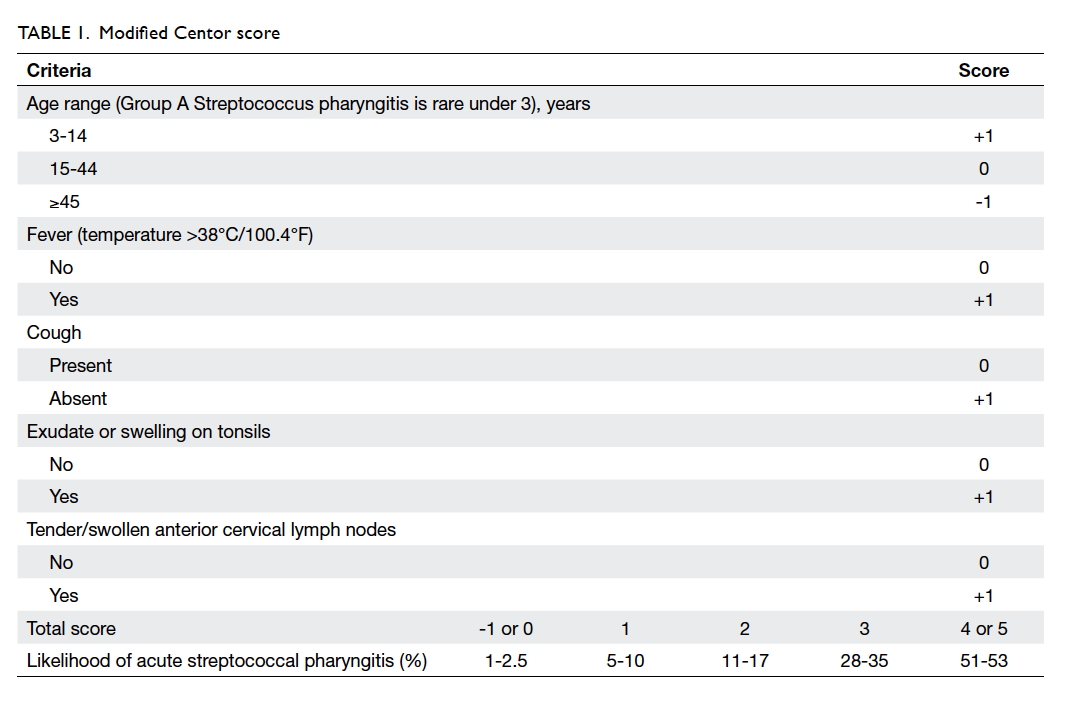
clinical decisions about laboratory testing and/or antibiotic prescribing.9 10
11 The FeverPAIN score was
developed in the primary care setting in the United Kingdom in 2013.12 The Centor criteria were developed in the emergency
department setting in the United States in 1981; the modified Centor
criteria add age to the original Centor criteria.13
14 The FeverPAIN score criteria
are Fever (during the previous 24 hours), Purulence (pus on tonsils),
Attend rapidly (within 3 days after onset of symptoms), severely Inflamed
tonsils, and No cough or coryza; each of the criteria is worth 1 point
(maximum score of 5). A score of 0 or 1 is associated with a 13% to 18%
likelihood of isolating Streptococcus. A score of 2 or 3 is
associated with a 34% to 40% likelihood of isolating Streptococcus.
A score of 4 or 5 is associated with a 62% to 65% likelihood of isolating
Streptococcus. In contrast, the modified Centor criteria are age 3
to 14 years, history of fever (over 38°C), absence of cough, exudate or
swelling on tonsils, and tender/swollen anterior cervical lymph nodes;
each of the criteria is worth 1 point (maximum score of 5); note that 0
points are assigned for age 15 to 44 years, whereas -1 point is given for
age ≥45 years. A score of -1, 0 or 1 is associated with a 1% to 10%
likelihood of isolating Streptococcus. A score of 2 or 3 is
associated with an 11% to 35% likelihood of isolating Streptococcus.
A score of 4 or 5 is associated with a 51% to 53% likelihood of isolating
Streptococcus. There is currently uncertainty about which clinical
scoring tool is more effective.
Based on the clinical experience that RADT is not
commonly available, and throat culture is time consuming, requiring a 2-
to 3-day turnaround time, the Advisory Group agreed that using clinical
scoring criteria is preferential to not using any laboratory tests or
clinical scoring criteria, and the modified Centor criteria are more
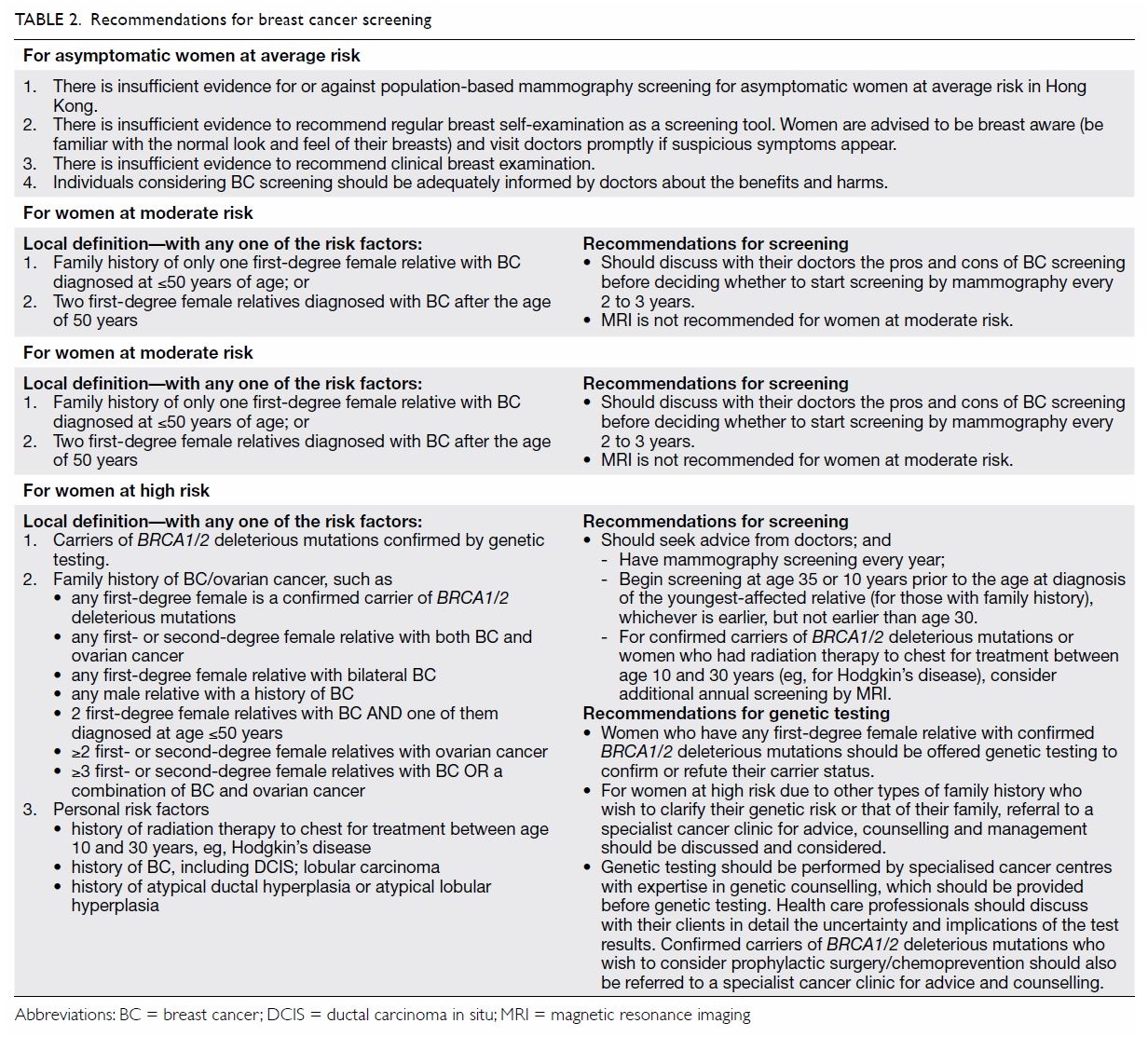
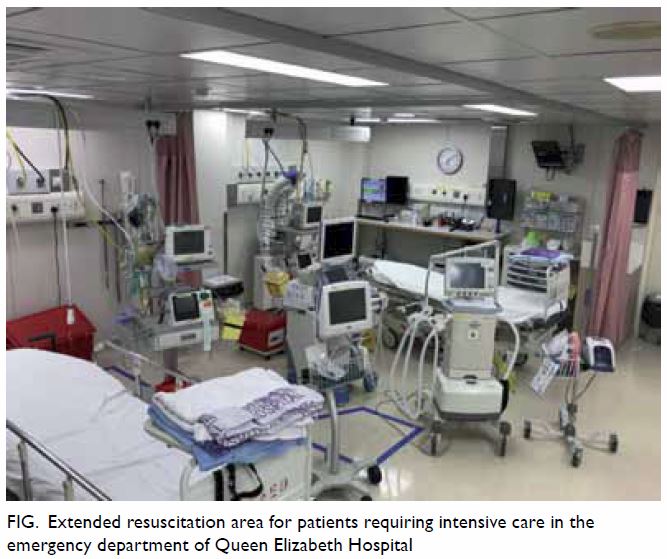
widely and easily used (Table 1).
Antibiotic treatment of acute streptococcal pharyngitis
Although the symptoms of acute streptococcal
pharyngitis resolve without antibiotic treatment, there are arguments that
justify antibiotic treatment for acute symptom relief, prevention of
suppurative and non-suppurative complications, and reduction of
communicability. A recent systematic review on antibiotics for sore throat
found that the clinical benefits were modest and required treatment of
many with antibiotics for one to benefit (the number of people with sore
throat who must be treated to resolve the symptoms of one by day 3 was
about 3.7 for those with positive throat swabs for Streptococcus;
6.5 for those with a negative swab, and 14.4 for those in whom no swab had
been taken).15 Antibiotic
treatment may shorten the duration of sore throat by 1 to 2 days.
Antibiotics may prevent complications of GAS infection, including acute
rheumatic fever or suppurative complications.15
Out-patients are considered no longer contagious after 24 hours of
antibiotic treatment. However, little evidence supports the prevention of
poststreptococcal glomerulonephritis by antibiotic treatment.15
Although scarlet fever occurs throughout the year,
there has been a seasonal pattern in Hong Kong, with higher activity
observed from May to June and from November to March in the past few
years.16 Scarlet fever is a
bacterial infection caused by GAS, and it classically presents with fever,
sore throat, red and swollen tongue (known as strawberry tongue), and
erythematous rash with a sandpaper texture. It is mainly a clinical
diagnosis and can be treated by appropriate antibiotics effectively.
After considering the benefits and risks (eg,
allergies and side-effects) of antibiotic treatment, the Advisory Group
agreed that antibiotic treatment is indicated for out-patients presenting
with a sore throat and a modified Centor score of 4 or 5 and for
out-patients with positive laboratory results or certain special reasons
(eg, clinical scarlet fever, household contact with scarlet fever, or
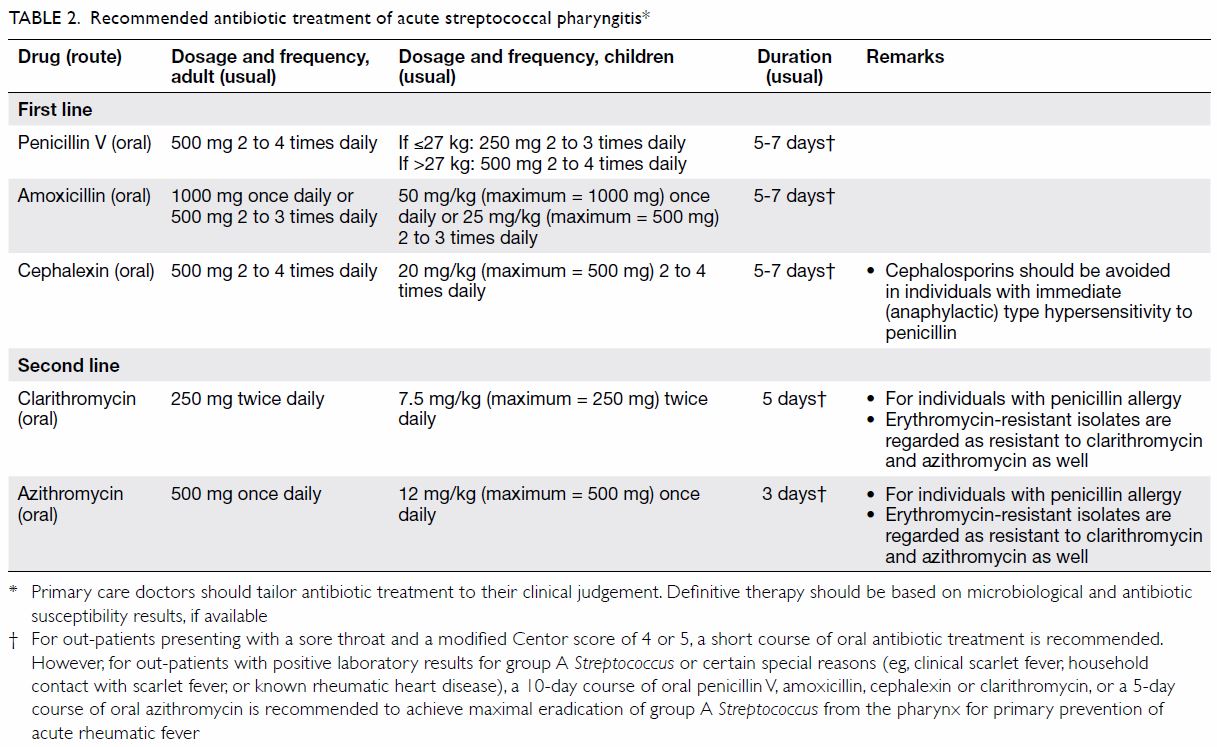
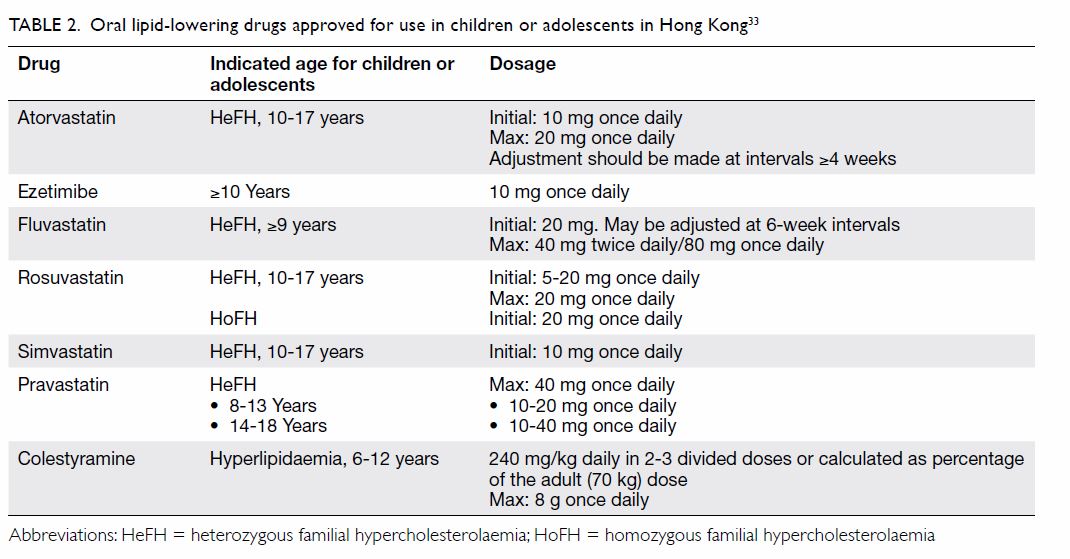
known rheumatic heart disease) [Table 2].
Oral penicillin V or amoxicillin are the
recommended antibiotics of choice for out-patients who are not allergic to
these agents. Resistance of GAS to penicillins and other beta-lactams has
not been reported.17
First-generation cephalosporins (eg, oral cephalexin) are the first-line
agents for out-patients with penicillin allergies who are not
anaphylactically allergic. Other cephalosporins (eg, oral cefaclor,
cefuroxime) are alternatives, but they are not favoured as the first-line
agents because of their broad spectrum of activity. Resistance of GAS to
macrolides (eg, oral azithromycin, clarithromycin, erythromycin) is known
to be common in Hong Kong. Erythromycin-resistant isolates of GAS are
regarded as resistant to clarithromycin and azithromycin as well.17 According to data from the Microbiology Division of
the Public Health Laboratory Services Branch of the Centre for Health
Protection, which undertakes bacterial isolation and antibiotic
susceptibility testing in public and private out-patient settings in Hong
Kong, the erythromycin resistance rates of beta-haemolytic streptococci
(in which GAS contributed to majority of them) in throat swab specimens
has risen to 59.1% in the last few years.18
Studies have shown that the erythromycin resistance rates of GAS isolates
were 4% in the United States, 3.2% in France, 32.8% in Spain, and 65% in
Taiwan.19 20 21 22 Respiratory fluoroquinolones (eg, oral levofloxacin)
are active against GAS, but they have an unnecessarily broad spectrum of
activity and are not recommended for routine treatment of acute
streptococcal pharyngitis.6
Excessive use of respiratory fluoroquinolones may lead to delay in the
diagnosis of tuberculosis and increased fluoroquinolone resistance among Mycobacterium
tuberculosis in Hong Kong.23
Trimethoprim-sulfamethoxazole should not be used because it does not
eradicate GAS from out-patients with acute pharyngitis.6
After considering the basic principle that
narrow-spectrum antibiotics should be used as the first-line agents to
treat an infection that is not life-threatening, the Advisory Group agreed
that oral penicillin V, amoxicillin or cephalexin are the first-line
agents to treat acute streptococcal pharyngitis (Table 2). Treatment with oral macrolides or
respiratory fluoroquinolones requires sound justifications, including
documented history of beta-lactam allergy or intolerance, positive throat
culture results, and associated antibiotic susceptibility profiles.
A 10-day course of oral penicillin V, amoxicillin,
cephalexin or clarithromycin, or a 5-day course of oral azithromycin is
recommended by the Infectious Diseases Society of America, the American
College of Physicians, and the American Academy of Pediatrics to achieve
maximal eradication of GAS from the pharynx for primary prevention of
acute rheumatic fever.6 7 8 However, a
recent systematic review comparing a 3- to 6-day course of oral
antibiotics (primarily cephalosporins) with a conventional 10-day course
of oral penicillin found similar effectiveness in children, but no
conclusions could be drawn on the complication rates of acute rheumatic
fever and poststreptococcal glomerulonephritis.24
Furthermore, a 5-day course of antibiotic treatment is sufficient to
mitigate the clinical course of group C Streptococcus and group G
Streptococcus pharyngitis, as acute rheumatic fever is not a
complication of infections due to these organisms.8
Based on the clinical experience that the
prevalence of acute rheumatic fever is very low in Hong Kong nowadays, the
Advisory Group agreed that a 5- to 7-day course of oral penicillin V,
amoxicillin or cephalexin, or a 5-day course of oral clarithromycin, or a
3-day course of oral azithromycin is sufficient to treat out-patients
presenting with a sore throat and a modified Centor score of 4 or 5.
However, for out-patients with positive laboratory results for GAS or
certain special reasons (eg, clinical scarlet fever, household contact
with scarlet fever, or known rheumatic heart disease), a 10-day course of
oral penicillin V, amoxicillin, cephalexin or clarithromycin, or a 5-day
course of oral azithromycin is recommended to achieve maximal eradication
of GAS from the pharynx for primary prevention of acute rheumatic fever (Table 2).
Other issues
Alternative diagnosis should be considered for
out-patients who present with unusually severe signs and symptoms, such as
difficulty swallowing, drooling, neck tenderness or swelling, or systemic
unwellness. They should be evaluated for potentially dangerous infections
(eg, peritonsillar abscess, retro-/para-pharyngeal abscess, acute
epiglottitis and systemic infections). Out-patients who do not improve
within 5 to 7 days or who have worsening symptoms should be evaluated for
a previously unsuspected diagnosis (eg, infectious mononucleosis, primary
HIV infection, or gonococcal pharyngitis). Infectious mononucleosis is a
clinical syndrome characterised by fever, severe pharyngitis (which lasts
longer than GAS pharyngitis), cervical or diffuse lymphadenopathy, and
prominent constitutional symptoms. Out-patients who have infectious
mononucleosis and are treated with amoxicillin may develop a generalised,
erythematous, maculopapular rash, and this should not be regarded as a
penicillin allergy. A properly taken sexual history may hint at
possibility of sexually transmitted infections like HIV and gonorrhoea.
Management of out-patients with infections should
be individualised. Primary care doctors should check, document, and inform
out-patients well about antibiotic treatment (eg, indications,
side-effects, allergies, contra-indications, potential drug-drug
interactions). Out-patients should take antibiotics exactly as prescribed
by their doctors. If their symptoms change, persist, or get worse, they
should seek medical advice promptly.
Primary care doctors are invited to show their
commitment on judicious use of antibiotics by visiting the “I Pledge”
website (https://www.chp.gov.hk/en/static/100755.html) and signing a certificate on
pledging to use antibiotics responsibly. Furthermore, this invitation is
open to general public. Primary care doctors can engage their out-patients
on “I Pledge” during clinical encounters to facilitate shared decision
making on antibiotic prescribing.
Conclusion
Acute pharyngitis is one of the most common
conditions among out-patients in primary care in Hong Kong. Practical
recommendations on the diagnosis and antibiotic treatment of acute
streptococcal pharyngitis are made in consultation with key stakeholders
in primary care settings such that the recommendations can be tailored to
their needs. The recommendations are under regular review, in
consideration of the latest research, together with local prevalence of
pathogens and associated antibiotics susceptibility profiles, and common
local practice.
Author contributions
All authors have made substantial contributions to
the viewpoints of this study, literature review, and critical revision for
important intellectual content. ETK Lam was responsible for literature
search and drafting of the manuscript. All authors had full access to the
data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As editors of this journal, DVK Chao and MCS Wong
were not involved in the peer review process of this article. All other
authors have no conflicts of interest to disclose.
Declaration
An earlier version of this article was published
online in the Centre for Health Protection website, November 2017
(https://www.chp.gov.hk/files/pdf/guidance_notes_acute_pharynitis_full.pdf).
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
References
1. Lam TP, Ho PL, Lam KF, Choi K, Yung R.
Use of antibiotics by primary care doctors in Hong Kong. Asia Pac Fam Med
2009;8:5. Crossref
2. Kung K, Wong CK, Wong SY, et al. Patient
presentation and physician management of upper respiratory tract
infections: a retrospective review of over 5 million primary clinic
consultations in Hong Kong. BMC Fam Pract 2014;15:95. Crossref
3. Alcaide ML, Bisno AL. Pharyngitis and
epiglottitis. Infect Dis Clin North Am 2007;21:449-69. Crossref
4. Wong MC, Chung CH. Group A streptococcal
infection in patients presenting with a sore throat at an accident and
emergency department: prospective observational study. Hong Kong Med J
2002;8:92-8.
5. Chan JY, Yau F, Cheng F, Chan D, Chan B,
Kwan M. Practice recommendation for the management of acute pharyngitis.
Hong Kong J Paediatr 2015;20:156-62.
6. Shulman ST, Bisno AL, Clegg HW, et al.
Clinical practice guideline for the diagnosis and management of group A
streptococcal pharyngitis: 2012 update by the Infectious Diseases Society
of America. Clin Infect Dis 2012;55:e86-102. Crossref
7. Harris AM, Hicks LA, Qaseem A; High
Value Care Task Force of the American College of Physicians and for the
Centers for Disease Control and Prevention. Appropriate antibiotic use for
acute respiratory tract infection in adults: advice for high-value care
from the American College of Physicians and the Centers for Disease
Control and Prevention. Ann Intern Med 2016;164:425-34. Crossref
8. Gerber MA, Baltimore RS, Eaton CB, et
al. Prevention of rheumatic fever and diagnosis and treatment of acute
streptococcal pharyngitis: a scientific statement from the American Heart
Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee
of the Council on Cardiovascular Disease in the Young, the
Interdisciplinary Council on Functional Genomics and Translational
Biology, and the Interdisciplinary Council on Quality of Care and Outcomes
Research: endorsed by the American Academy of Pediatrics. Circulation
2009;119:1541-51. Crossref
9. ESCMID Sore Throat Guideline Group,
Pelucchi C, Grigoryan L, et al. Guideline for the management of acute sore
throat. Clin Microbiol Infect 2012;18(Suppl 1):1-28. Crossref
10. Public Health England, UK Government.
Management and treatment of common infections. Antibiotic guidance for
primary care: For consultation and local adaptation. 2017. Available from:
https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care.
Accessed 6 Oct 2017.
11. The National Institute for Health and
Care Excellence, Public Health England, UK Government. Sore throat
(acute): antimicrobial prescribing. 2018. Available from:
https://www.nice.org.uk/guidance/ng84. Accessed 13 Jul 2018.
12. Little P, Moore M, Hobbs FD, et al.
PRImary care Streptococcal Management (PRISM) study: identifying clinical
variables associated with Lancefield group A β-haemolytic Streptococci and
Lancefield non–Group A streptococcal throat infections from two cohorts of
patients presenting with an acute sore throat. BMJ Open 2013;3:e003943. Crossref
13. Centor RM, Witherspoon JM, Dalton HP,
Brody CE, Link K. The diagnosis of strep throat in adults in the emergency
room. Med Decis Making 1981;1:239-46. Crossref
14. McIsaac WJ, White D, Tannenbaum D, Low
DE. A clinical score to reduce unnecessary antibiotic use in patients with
sore throat. CMAJ 1998;158:75-83.
15. Spinks A, Glasziou PP, Del Mar CB.
Antibiotics for sore throat. Cochrane Database Syst Rev
2013;(11):CD000023. Crossref
16. Centre for Health Protection,
Department of Health, Hong Kong SAR Government. Letter to doctors: further
increase in scarlet fever activity in Hong Kong. 2017. Available from:
https://www.chp.gov.hk/files/pdf/letters_to_doctors_20171204.pdf. Accessed
13 Jul 2018.
17. Clinical and Laboratory Standards
Institute. Performance standards for antimicrobial susceptibility testing.
27th ed. 2017. Available from:
https://clsi.org/media/1469/m100s27_sample.pdf. Accessed 6 Oct 2017.
18. Centre for Health Protection,
Department of Health, Hong Kong SAR Government. Bacterial pathogen
isolation and percentage of antimicrobial resistance—out-patient setting.
2014-2018. Available from
http://www.chp.gov.hk/en/data/1/10/641/697/3345.html. Accessed 13 Jul
2018.
19. Tanz RR, Shulman ST, Shortridge VD, et
al. Community-based surveillance in the United States of
macrolide-resistant pediatric pharyngeal group A Streptococci during 3
respiratory disease seasons. Clin Infect Dis 2004;39:1794- 801. Crossref
20. d’Humières C, Cohen R, Levy C, et al.
Decline in macrolide-resistant Streptococcus pyogenes isolates
from French children. Int J Med Microbiol 2012;302:300-3. Crossref
21. Rubio-López V, Valdezate S, Alvarez D,
et al. Molecular epidemiology, antimicrobial susceptibilities and
resistance mechanisms of Streptococcus pyogenes isolates resistant
to erythromycin and tetracycline in Spain (1994-2006). BMC Microbiol
2012;12:215. Crossref
22. Chuang PK, Wang SM, Lin HC, et al. The
trend of macrolide resistance and emm types of group A Streptococci from
children at a medical center in southern Taiwan. J Microbiol Immunol
Infect 2015;48:160-7. Crossref
23. Ho PL, Wu TC, Chao DV, et al, editors.
Reducing bacterial resistance with IMPACT—Interhospital Multi-disciplinary
Programme on Antimicrobial ChemoTherapy. 5th version. 2017. Available
from:
https://www.chp.gov.hk/files/pdf/reducing_bacterial_resistance_with_impact.pdf.
Accessed 6 Oct 2017.
24. Altamimi S, Khalil A, Khalaiwi KA,
Milner RA, Pusic MV, Al Othman MA. Short-term late-generation antibiotics
versus longer term penicillin for acute streptococcal pharyngitis in
children. Cochrane Database Syst Rev 2012;(8):CD004872. Crossref