Update to the Hong Kong Epilepsy Guideline:
evidence-based recommendations for clinical
management of women with epilepsy throughout
the reproductive cycle
Richard SK Chang, FHKCP, FHKAM (Medicine)1; Kate HK Lui, FHKCP, FHKAM (Medicine)2; William Ip, MRCP (UK)2; Eric Yeung, FHKCP, FHKAM (Medicine)3; Ada WY Yung, FHKCP, FHKAM (Paediatrics)4; Howan Leung, FHKCP, FHKAM (Medicine)5; Eva LW Fung, FHKCPaed, FHKAM (Paediatrics)6;
Ben BH Fung, FHKCP, FHKAM (Medicine)4; Eric LY Chan, FHKCP, FHKAM (Medicine)7; TL Poon, FCSHK, FHKAM (Surgery)8; HT Wong, FCSHK, FHKAM (Surgery)9; Deyond Siu, FHKCR, FHKAM (Radiology)10; Kevin Cheng, FCSHK, FHKAM (Surgery)11; Cannon XL Zhu, FRCS, FHKAM (Surgery)12; Gardian CY Fong, FHKCP, FHKAM (Medicine)4; Jonathan Chu, FHKCP, FHKAM (Medicine)1; Colin HT Lui, FHKCP, FHKAM (Medicine)2; Maggie Yau, FHKCP, FHKAM (Paediatrics)
1 Department of Medicine, Queen Mary Hospital, Hong Kong
2 Department of Medicine, Tseung Kwan O Hospital, Hong Kong
3 Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong
4 Private Practice, Hong Kong
5 Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong
6 Department of Paediatrics, Prince of Wales Hospital, Hong Kong
7 Department of Medicine and Geriatrics, Tuen Mun Hospital, Hong Kong
8 Department of Neurosurgery, Queen Elizabeth Hospital, Hong Kong
9 Department of Neurosurgery, Kwong Wah Hospital, Hong Kong
10 Department of Radiology, Kwong Wah Hospital, Hong Kong
11 Department of Neurosurgery, Queen Mary Hospital, Hong Kong
12 Department of Surgery, Prince of Wales Hospital, Hong Kong
 Full
paper in PDF
Full
paper in PDF
Abstract
Since the publication of the Hong Kong Epilepsy
Guideline in 2009, there has been significant
progress in antiepileptic drug development. New
AEDs have emerged, and data about their uses have
been published. Women require special attention
in epilepsy care. Drug teratogenicity, pregnancy,
breastfeeding, contraception, reproduction
technology, menopause, and catamenial epilepsy
are major topics. Antiepileptic drugs should be
chosen individually for patients who are pregnant
or may become pregnant with consideration of their
teratogenicity and seizure control properties. Folate
is commonly prescribed for women of childbearing
age who are taking antiepileptic drugs. Spontaneous
vaginal delivery and breastfeeding are not
contra-indicated in most cases but need to be
considered individually based on the patient’s
medical condition and wishes. Serum drug level
monitoring of certain antiepileptic drugs during
pregnancy and puerperium can guide dosage
adjustment. For catamenial epilepsy, intermittent
benzodiazepines such as clobazam during the
susceptible phase of the menstrual cycle could be a
treatment option.
Introduction
Women need special attention in epilepsy care. They
face various challenges related to their reproductive
cycles, including pregnancy, breastfeeding,
contraception, menopause, and catamenial epilepsy.
Antiepileptic drug (AED) options have increased
exponentially in recent decades. New data on
epilepsy management have emerged since the 2009
publication of the Hong Kong Epilepsy Guideline
by the Hong Kong Epilepsy Society.
1 This article
aims to update the Society’s guideline with a focus
on epilepsy management in women. This project received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Preconception counselling and
teratogenicity of antiepileptic drugs
Women with epilepsy, preferably with their partner
or parents if appropriate, should receive counselling
on contraception, conception, pregnancy,
breastfeeding, and childcare. Preconception
counselling is especially important for women who will become pregnant, as it may help to ensure
good maternal and fetal outcomes.
2 They should
be reassured that most women with epilepsy have
uneventful pregnancies and deliveries.
2
The risk of epilepsy in the offspring of
women with epilepsy is highly dependent on the
parents’ epilepsy syndrome status.
3 In general, the
risk is slightly higher compared with that of the
background population. In a large population-based
study, the overall cumulative risk of epilepsy up to
age 40 in individuals with epileptic parents was 4.5%,
which is about 3-fold higher than that of the general
population.
4 Referral to appropriate specialists such
as geneticists is appropriate if genetic counselling is
indicated.
Animal studies have shown that AEDs can
decrease the level of serum folate, increasing the risk
of fetal neural tube defects.
5 Neonatal malformations
have also been associated with low maternal serum
folate levels in humans.
6 All women with epilepsy of
childbearing age may be offered folate supplements
while on AEDs.
7 8 Although there is no definite
consensus about folate dosage, it is reasonable to
prescribe oral folate 5 mg daily to women with
childbearing potential.
1 2 9
Many AEDs have the ability to cross the
placenta.
7 Data have emerged on the potential risks
of in-utero AED exposure to offspring. The risk of
major congenital malformations (MCMs) such as
hypospadias, congenital heart defects, club foot,
cleft lip or palate, and spina bifida in children born
to women with epilepsy treated with AEDs during
pregnancy is 2 to 3 times higher than the 2% to
5% occurrence rate in the general population.
10 11
Polytherapy probably has a higher risk of MCMs and
future cognitive adverse drug effects compared with
monotherapy.
12 13 14 The risk of MCMs may increase with higher dosages of AEDs.
12 Antenatal screening
including fetal ultrasound scan can help to detect
major fetal malformations.
2 However, prenatal
screening has limitations in terms of malformation
detection and cannot provide information about
neurodevelopmental complications.
15
The North American Antiepileptic Drug
Pregnancy Registry (NAAPR) reported that
phenobarbital monotherapy was associated with an
increased MCM rate of 6.5% (95% confidence interval
[CI], 2.1%-14.5%) compared with the background
rate of 1.6%.
16 The same registry later reported that
the MCM risk associated with phenobarbital was
5.5% in a larger group of 199 pregnancies.
17 The
teratogenic effects of phenobarbital are probably
related to dosage. Data from the European Registry
of Antiepileptic Drugs and Pregnancy (EURAP)
showed that phenobarbital had an MCM risk of 5.4%
(95% CI, 2.51%-10.04%) in the offspring of women
taking daily doses <150 mg. The risk increased to
13.7% (95% CI, 5.70%-26.26%) at daily doses of
150 mg per day or above. Phenobarbital may also have
deleterious effects on the cognition of offspring.
12 18
A meta-analysis found that phenytoin had an
MCM rate of 7.4% (95% CI, 3.60%-11.11%).
14 The
EURAP reported that the MCM rate associated
with phenytoin was approximately 6.4% (95% CI,
2.8%-12.2%).
19 The NAAPR reported that phenytoin
was associated with a 2.9% risk of MCMs (compared
with a background rate of 1.1%).
17 The UK and
Ireland Epilepsy and Pregnancy Register reported
that the risk of MCMs associated with phenytoin
was 3.7%.
20 21 22 There is also evidence showing that
in-utero phenytoin exposure is associated with an
increased risk of impaired cognition.
23 24 25
Valproate use during pregnancy may be
associated with MCMs and long-term negative
effects on the cognitive and neurological functions
of offspring. Neural tube defects, facial clefts, and
hypospadias may be related to in-utero valproate
exposure.
1 13 27 28 29 A meta-analysis showed that the overall
risk of MCMs associated with in-utero exposure to
valproate was 10.73% (95% CI, 8.16%-13.29%).
14
The European Surveillance of Congenital Anomalies
study found that valproate monotherapy was
associated with significantly increased risks of six
specific malformations, with the following odds
ratios (ORs): spina bifida, 12.7-fold increase (95% CI,
7.7-20.7); atrial septal defect, 2.5-fold (1.4-4.4);
cleft palate, 5.2-fold (2.8-9.9); hypospadias, 4.8-fold
(2.9-8.1); polydactyly, 2.2-fold (1.0-4.5); and
craniosynostosis, 6.8-fold (1.8-18.8).
30 Adverse
effects in terms of mental and motor development
have been shown to be associated with in-utero
valproate exposure in children.
31 32 33 34 35 In 2011,
the US Food and Drug Administration (FDA)
issued a warning about valproate use during
pregnancy because interim results from the NEAD (Neurodevelopmental Effects of Antiepileptic
Drugs) study and other epidemiological studies
showed lower cognitive function in children with
in-utero valproate exposure.
31 36 37 The NEAD study
was a prospective multicentre cohort study conducted
in the US and UK. Interim cognitive assessment at
age 3 years showed that children exposed to valproate
had intelligence quotient (IQ) scores 9 points
lower than those exposed to lamotrigine (95% CI,
3.1-14.6; P=0.009), 7 points lower than those
exposed to phenytoin (95% CI, 0.2-14.0; P=0.04), and
6 points lower than those exposed to carbamazepine
(95% CI, 0.6-12.0; P=0.04). The association between
valproate use and detrimental effects on IQ was
dose-dependent.
31 When 244 newborn children
subsequently completed 6 years of follow-up, mean
IQ at age 6 years was lower in children exposed to
valproate (97; 95% CI, 94-101) than to carbamazepine
(105; 95% CI, 102-108; P=0.0015), lamotrigine
(108; 95% CI, 105-110; P=0.0003), or phenytoin
(108; 95% CI, 104-112; P=0.0006).
32 Children exposed
to valproate were inferior in terms of verbal and
memory abilities to those exposed to the other AEDs
and in terms of non-verbal and executive functions
to those exposed to lamotrigine. High doses of
valproate were negatively associated with IQ, verbal
ability, non-verbal ability, memory, and executive
function.
32 Valproate use during pregnancy may also
increase the risk of childhood autism in offspring
(adjusted hazard ratio: 2.9; 95% CI, 1.4-6.0).
38 39 In
2013, the FDA strengthened its warning regarding
valproate use: it suggested that valproate should
only be used in epileptic pregnant women if other
medications are ineffective or unacceptable, and it
is contra-indicated for migraines during pregnancy.
In women with epilepsy who are currently not
pregnant but have the potential to become pregnant,
valproate should not be considered as the first-line
treatment, especially for focal epilepsies, unless
there is no other alternative. For cases in which
valproate is considered as an appropriate option, like
certain idiopathic generalised epilepsies, the dose
should be maintained at the lowest effective dose,
preferably not exceeding 500 to 800 mg per day.
15 In
special circumstances, the use of valproate as initial
treatment may be justifiable. For example, it could be
used in epilepsies with high likelihood of remission
and drug withdrawal before puberty or in patients
with severe disabilities that make future pregnancy
extremely unlikely.
15 The American Academy of
Neurology has recommended that valproate be
avoided during the first trimester of pregnancy
if possible.
40 However, the use of valproate in
women with epilepsy should be individualised with
consideration of the dosage administered, seizure
control, and potential effects on offspring. In certain
cases, especially those of specific epilepsy syndromes
such as juvenile myoclonic epilepsy and juvenile absence epilepsy, valproate may be the most suitable
choice after balancing its seizure control properties
with the potential adverse effects on both the patient
and her offspring. Valproate may also be used when
clear communication with the patient about the
risk-benefit ratio has been undertaken.
Although the number of women with
childbearing potential who take valproate is
decreasing, there are persistent concerns about
inadequate information provided to valproate users
about its possible adverse effects on their offspring.
Authorities from various countries or regions, such
as the UK and the European Union, have adopted
specific risk minimisation measures surrounding
valproate usage among women with epilepsy.
Examples include the provision of information
leaflets highlighting the potential teratogenic and
neurodevelopmental impacts of valproate exposure in
utero, pregnancy tests before valproate prescription,
a risk acknowledgement form filled upon valproate
initiation and renewed annually by healthcare
professionals, and alert cards provided to women
with epilepsy with childbearing potential. These
could serve as references to healthcare providers
in different clinical settings. Direct adoption of
these measures may not be feasible in some local
situations. Certain practices may be advisable to
enhance communication between prescribers and
potential childbearing female valproate users. The
indications and potential adverse effects of valproate
could be communicated to patients in different
formats, such as on paper or electronically. The
plan of valproate therapy should be reviewed with
women with epilepsy with pregnancy potential when
clinically indicated, but not necessarily on a yearly
basis. Other medical professionals (eg, nurses or
pharmacists) could take a role in AED counselling,
including advising patients regarding their potential
adverse effects. Materials could be provided to
patients regarding the drugs’ potential implications
on pregnancy and fetal outcomes. These could be
in the form of leaflets, warning messages, or QR
codes on drug packaging. Good documentation
of the communication between the clinician and
patient, preferably including the family, cannot be
overemphasised.
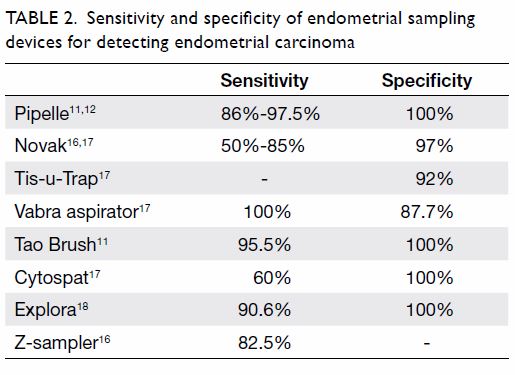
The NAAPR reported that carbamazepine
monotherapy during pregnancy had a 2.9% overall
risk of MCMs.
17 The European Surveillance of
Congenital Anomalies Database reported that
the OR of spina bifida related to carbamazepine
monotherapy was 2.6 versus no AEDs.
41 The EURAP
has also demonstrated a dose-dependent effect of
carbamazepine. Carbamazepine doses of >400 mg
per day were associated with a significantly higher
risk of MCMs (5.3% for ≥400 to <1000 mg; 8.7% for
≥1000 mg) [
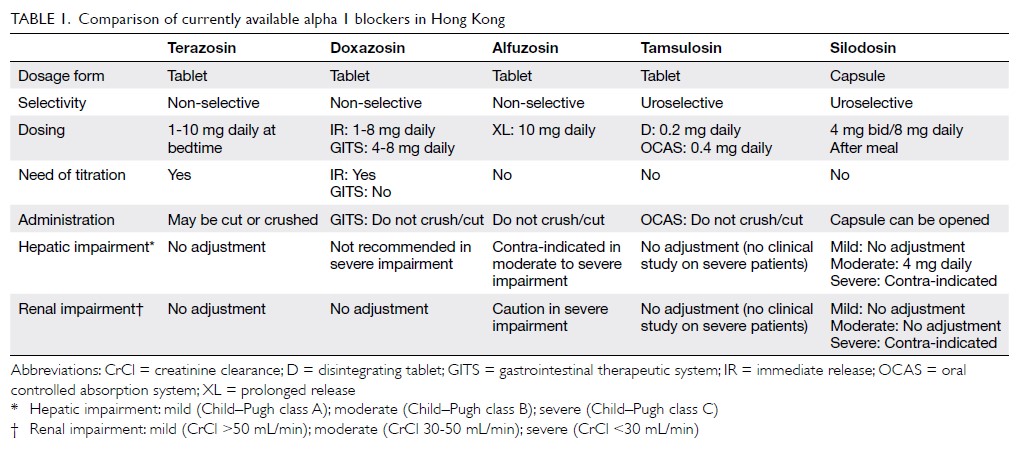
Table 1 17 19 21 42 43].
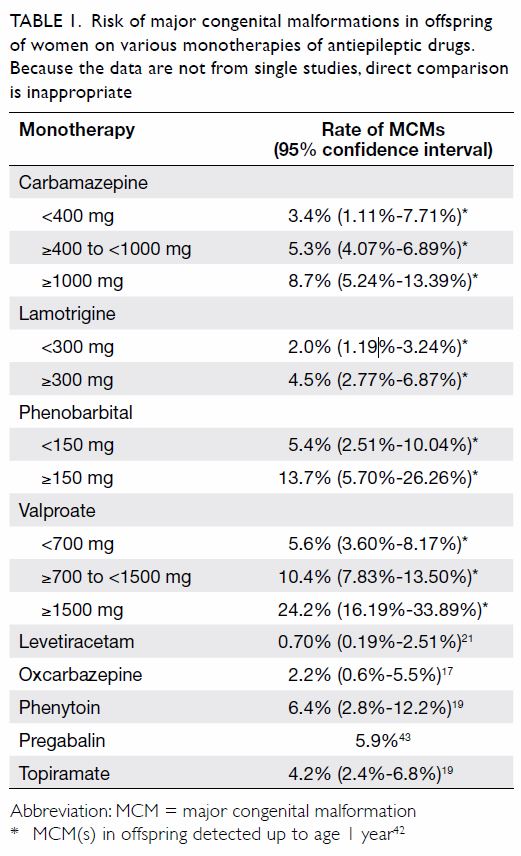 Table 1.
Table 1. Risk of major congenital malformations in offspring
of women on various monotherapies of antiepileptic drugs.
Because the data are not from single studies, direct comparison
is inappropriate
A 2008 meta-analysis showed that lamotrigine monotherapy had an MCM risk of 2.91% (95% CI,
2.00%-3.82%).
14 The NAAPR has indicated that lamotrigine monotherapy carried a 1.9% risk of
MCMs.
44 The EURAP pregnancy registry data show a relatively low risk of malformations when using
lamotrigine monotherapy at doses <300 mg per day
as compared with other AEDs (
Table 1).
42 When
used as polytherapy, the combination of lamotrigine
and valproate may have a relatively higher risk of
MCMs (9.1%; OR=5.0; 95% CI, 1.5%-14%) compared
with combinations of lamotrigine and other AEDs
(2.9%; OR=1.5; 95% CI, 0.7%-3.0%).
44
The UK and Ireland Epilepsy and Pregnancy
Register reported that levetiracetam monotherapy
has a 0.70% risk of MCMs (95% CI, 0.1%-2.51%).
21
The MCM risk of levetiracetam as polytherapy was
relatively higher at 6.47% (95% CI, 4.31%-9.60%).
The MCM rates of polytherapy including
levetiracetam varied with different regimens: the
combination of levetiracetam and lamotrigine had a
risk of 1.77% (95% CI, 0.49%-6.22%) compared with
6.90% (95% CI, 1.91%-21.96%) for the combination
of levetiracetam and valproate and 9.38% (95% CI,
4.37%-18.98%) for the combination of levetiracetam
and carbamazepine.
The FDA issued a warning regarding an
increased risk of oral cleft in children born to mothers
taking topiramate based on data from the NAAPR
and the UK Epilepsy and Pregnancy Register. The UK
registry reported the risk of oral cleft as 29 per 1000
(95% CI, 5-91 per 1000), which is more than 10 times
the background risk.
22 The NAAPR reported that
the risk of cleft lip associated with topiramate
monotherapy during pregnancy was 14 per 1000
(95% CI, 5.1-31 per 1000).
17 The overall MCM rate
of topiramate monotherapy has been reported to be
4.2% (95% CI, 2.4%-6.8%).
Pregabalin use in human pregnancy is
relatively less studied. As pregabalin is also used
for controlling neuralgic pain, clinical studies on
pregabalin may involve confounding factors, such as
concomitant medications and co-morbidities, which
may be different from those of patients with epilepsy
only. This may cause confounding factors in clinical
studies, as the concomitant medications and disease
spectrum may be different from those of patients
with epilepsy only. A multicentre study found that
the risk of MCMs associated with pregabalin use in
the first trimester of pregnancy was 6.0%, compared
with an unexposed risk of 2.1% (OR=3.0; 95% CI,
1.2%-7.9%).
45 However, another larger study did not
find any significant association between pregabalin
use in the first trimester and MCMs. The exposed
risk was 5.9%, compared with an unexposed risk
of 3.3% (OR=1.78; 95% CI, 1.26%-2.58%) and the
adjusted OR was 1.16 (95% CI, 0.81-1.67) according
to that study.
43
Oxcarbazepine is similar to carbamazepine in
terms of chemical structure. The NAAPR reported
that oxcarbazepine use in pregnancy was associated
with a 2.2% risk of MCMs (95% CI, 0.6%-5.5%).
17
The EURAP registry data show a 3.0% MCM risk
associated with in-utero oxcarbazepine exposure
(95% CI, 1.4%-5.4%).
19
Pregnancy
Most women with epilepsy have uneventful
pregnancies.
2 However, they should be informed
about their risks of pregnancy complications. For
example, the risks of such complications as needing
Caesarean section, spontaneous abortion, pre-eclampsia
or pregnancy-induced hypertension,
pregnancy-related bleeding complications,
fetal growth restriction, and premature uterine
contractions, labour, and delivery may be higher
than those of women without epilepsy, especially if
they are on AEDs.
9 46 47 48 Seizure freedom for 9 months
to 1 year prior to pregnancy is associated with a high
likelihood of seizure freedom during pregnancy.
2 9 49 50 51 Neither increased seizure frequency nor status
epilepticus has been substantially associated with
pregnancy.
9 There is no evidence that focal seizures
with or without impairment of consciousness, absence, or myoclonic seizures affect pregnancy or
the developing fetus adversely unless a fall causes
injury or other serious complications. Generalised
tonic-clonic seizures may carry a relatively higher
risk to the fetus, although their absolute risk level
remains very low, and their risk may depend on
seizure frequency.
52 The majority of women with
epilepsy experience no change in seizure frequency
during pregnancy, whereas a minority of them
experience either an increase or a decrease in attack
frequency.
14 53 The increase in seizure frequency
may be caused by a pregnancy-induced drop in
AED concentration, sleep deprivation, or lack of
drug adherence. Women who contemplate going
without AEDs during pregnancy should undergo
discussions about the potential risk of inadvertent
status epilepticus or sudden unexpected death in
epilepsy.
9 52 A balanced discussion may be held with
the patient to facilitate informed decision making
and provide access to various options. Early and
serial fetal ultrasound scans should be offered to
pregnant women who are taking AEDs to screen for
fetal structural anomalies.
2 54 Additional screening or
diagnostic tests, such as maternal serum tests and
fetal echocardiography, should proceed according
to clinical indications.
2 55 Pregnancy in women
with epilepsy optimally involves collaborative,
multidisciplinary planning and management. The
multidisciplinary team should involve specialists,
such as obstetricians and neurologists, who have
experience providing care for pregnant women
with epilepsy.
56 Monitoring of AED levels during
pregnancy may help to guide dosage adjustment.
For example, both total and free clearance of
lamotrigine may increase substantially during
pregnancy, with a peak in the third trimester.
57 58 A
decreased lamotrigine level could result in increased
seizure frequency. Pregnancy may also affect serum
levels of carbamazepine, phenytoin, levetiracetam,
and oxcarbazepine to various extents.
7 59 60 61 Serum
level monitoring may be helpful to inform dosage
adjustment during pregnancy. In pregnant women
presenting with seizures in the second half of
pregnancy that cannot be clearly attributed to
epilepsy alone, consideration is usually given to
pre-eclampsia, in which case definitive diagnosis
should be sought by further assessment.
2
Fetal complications may occur at slightly higher
frequencies in pregnancies of women with epilepsy.
A systematic review and meta-analysis that included
over 2.8 million pregnancies found that spontaneous
miscarriage (OR=1.54; 95% CI, 1.02-2.32), preterm
birth before 37 weeks of gestation (OR=1.16; 95% CI,
1.01-1.34), and fetal growth restriction (OR=1.26;
95% CI, 1.20-1.33) were more common in women
with epilepsy.
48 However, the risks of early preterm
birth before 34 weeks, gestational diabetes, fetal
death or stillbirth, perinatal death, or admission to neonatal intensive care unit did not differ between
women with and without epilepsy. In another
large US cohort study, the risks of preterm labour
(OR=1.54; 95% CI, 1.50-1.57) and stillbirth (adjusted
OR=1.27; 95% CI, 1.17-1.38) were also higher in
pregnancies of women with epilepsy.
47 A 1-minute
Apgar score of <7 in the neonate may be associated
with maternal AED use.
12 62
Contraception
The interactions between AEDs and oral
contraceptive pills should be discussed with all
women with epilepsy of childbearing age. Enzyme-inducing
AEDs can hinder the effectiveness of
oral contraceptives
63 (
Table 2 64 65 66 67 68 69 70 71 72 73), and women
with epilepsy on enzyme-inducing AEDs should
avoid combined oral contraceptive pills, combined
contraceptive patches, progestogen-only pills,
progestogen-only implants, and vaginal ring
contraceptives.
2 74 75 Older-generation AEDs such
as phenytoin, carbamazepine, phenobarbital, and
primidone may belong to the enzyme-inducing
category, but some newer-generation AEDs such
as oxcarbazepine also have enzyme-inducing
properties.
76 It may be advisable for women with
epilepsy to use other contraceptive methods than
the above-mentioned hormone-containing pills and
devices because of the risk of contraception failure.
If the use of oral contraceptives is unavoidable,
hormonal contraception may be adjusted in women
taking enzyme-inducing AEDs, although there is little
evidence supporting this (
Box 75 77). As different cases
are unique, it is advisable to consult specialists who
have experience managing these conditions. Copper
intrauterine devices, the levonorgestrel-releasing
intrauterine system, and medroxyprogesterone
acetate injections are less affected by enzyme-inducing
AEDs.
2 Some opinions suggest more
frequent injections of medroxyprogesterone acetate
(every 10 weeks instead of every 12 weeks) in
women with epilepsy taking enzyme-inducing AEDs
because of potential interactions.
64 For emergency
contraception, a copper intrauterine device should
be advised. If the intrauterine device is not suitable
or acceptable, a double dose of a total of 3 mg
levonorgestrel can be given.
2 78 79 In contrast, serum
lamotrigine levels can be reduced by simultaneous
use of any oestrogen-based contraceptive, leading to
deteriorated seizure control.
2 When the concomitant
use of the contraceptive is stopped, the lamotrigine
dose may need to be adjusted.
46
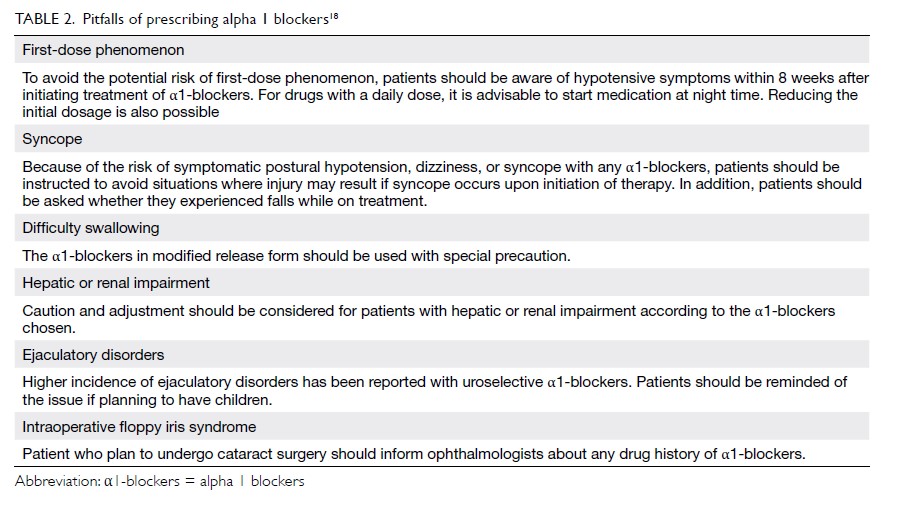
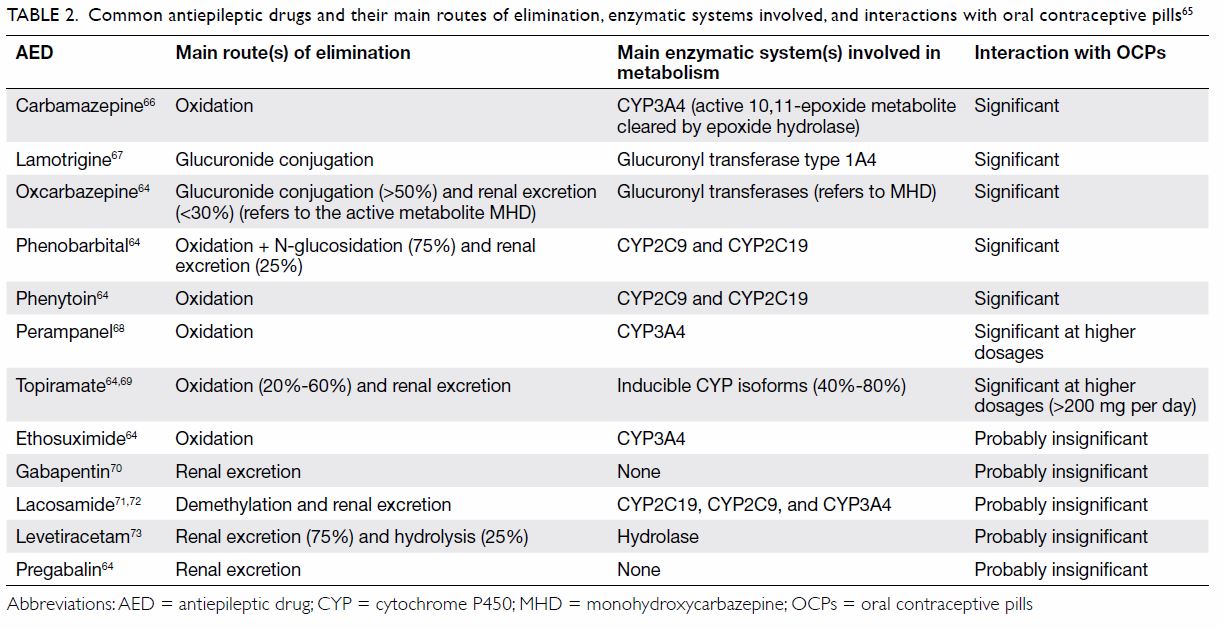 Table 2.
Table 2. Common antiepileptic drugs and their main routes of elimination, enzymatic systems involved, and interactions with oral contraceptive pills
65
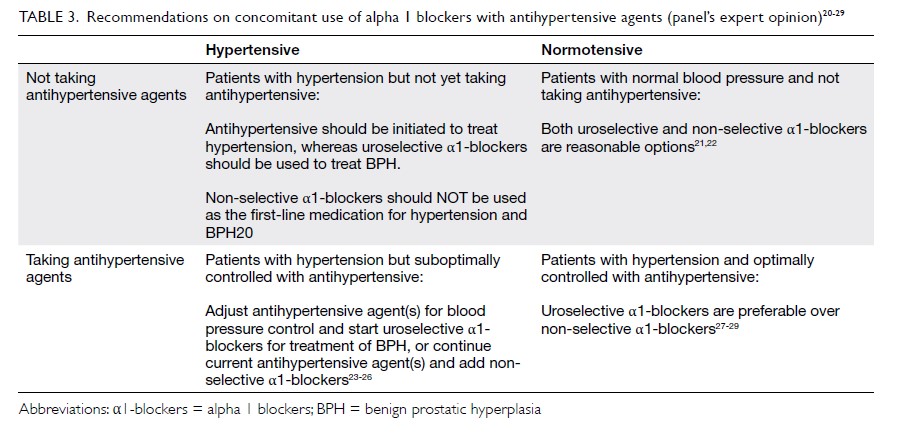
 Box.
Box. Possible measures for women taking enzyme-inducing antiepileptic drugs if oral hormonal contraceptives need to be used
75 77
Labour
Spontaneous vaginal delivery is not absolutely
contra-indicated in most women with epilepsy. Only
about 1% to 2% of women with epilepsy develop
generalised tonic-clonic seizures during labour.
2 The EURAP registry reported seizure occurrence in
3.5% of women with epilepsy in labour.
80 An epilepsy
diagnosis per se is not an indication for elective
Caesarean section or labour induction.
2 Antiepileptic
drugs should be continued orally or intravenously.
Measures such as adequate analgesia, including
transcutaneous electrical nerve stimulation, nitrous
oxide and oxygen, and regional analgesia, are
recommended to reduce pain and emotional distress,
but they may trigger seizures.
2 Pethidine, also known
as meperidine, should be used with caution for
analgesia during labour in women with epilepsy.
Its metabolites may reduce the seizure threshold.
81
Caesarean section may be necessary if seizures are
frequent or prolonged.
2 Seizures during labour
should be terminated as quickly as possible to avoid
maternal and fetal complications. Benzodiazepines
are the drugs of choice,
2 and phenytoin can also
be given intravenously.
82 Magnesium sulphate is a
treatment option if a diagnosis of eclampsia is made.
2 Some clinicians may prescribe vitamin K to women
with epilepsy on enzyme-inducing AEDs in the
last month of pregnancy to prevent haemorrhagic
disease in the newborn. The usual dose is about 10
to 20 mg/day of oral vitamin K. There are insufficient
data to fully support or refute maternal vitamin K
prophylaxis.
2 7 83 It has been recommended that
newborns of women with epilepsy be given 1 mg of
vitamin K1 parenterally at delivery.
2
Breastfeeding
There is no absolute contra-indication to
breastfeeding by women with epilepsy who are
taking AEDs.
2 84 However, mothers who take AEDs
should be counselled about the potential risks of
breastfeeding. Many AEDs are excreted in breast milk.
The amount of drug absorbed by the infant depends
on various factors, including the maternal plasma
concentration, the degree of drug transfer to breast
milk, and the amount of breast milk consumed by
the infant. The degree of drug transfer to breast milk
is inversely dependent on the drug’s protein binding
ability.
76 Primidone, levetiracetam, barbiturates,
benzodiazepines, lamotrigine, gabapentin,
topiramate, ethosuximide, and zonisamide probably
have significant penetration into breast milk.
7 76 85 86
However, there is no evidence to show that indirect
AED exposure through breastfeeding has clinically
significant effects on offspring.
2 87 88 The potential
risks of breastfeeding should be balanced by the
benefits of breastfeeding for both the neonate and
the mother.
84
Puerperium
Women with epilepsy who have just given birth
might face significant anxiety concerning the risk
of accidents should they develop breakthrough
seizures when they take care of their infants. While
there is risk of injury to the infant, reassurance
should be provided to the parents, as the actual
risk level is probably low.
89 Safety precautions that
are simple to implement could further reduce such
risks significantly.
2 Mothers should breastfeed
their babies while sitting on floor cushions to avoid
dropping their babies should a seizure occur. If
there is an aura, the mother should lay the baby
down.
2 The mother bathing the baby in a bathtub
by herself could potentially carry some drowning
risk if a seizure occurs. The same risk also applies
to the mother carrying the baby single-handedly.
46
Supervision by the partner or a caregiver may
help to prevent injury to the baby should a seizure
occur.
2 Information may also be given to parents early to facilitate preparation or home modification
to provide an optimal environment to care for the
newborn. Seizure frequency may be exacerbated
during the postpartum period for reasons such as
lack of sleep and drug non-compliance.
90 Serial
serum AED level monitoring of the mother may be
useful, as there may be physiological changes during
the puerperal period, especially with certain agents
such as lamotrigine.
57 91 Maintaining the serum drug
level at approximately the pre-conception level is
advisable, provided that the woman had good seizure
control before the pregnancy.
51 92 Dosages of AEDs
should be adjusted accordingly to avoid postpartum
toxicity or deteriorated seizure control.
2
Reproduction technology
With advancements in the field of reproduction
technology, clinicians may receive questions on
such technologies from women with epilepsy.
Reproductive intervention may have implications for women with epilepsy and their offspring. For
example, ovarian stimulation with gonadotropin
may induce seizure exacerbation in women with
epilepsy.
93 Epilepsy is a heterogeneous disease entity,
and although genetics play a part in its aetiology, it
can have implications for many bodily functions.
Single gene (ie, Mendelian) disorders account for
only a small proportion of patients. The implications
of these genetic factors in the offspring’s life and
development are often variable and complicated by
many different factors. Reproductive options and
application of reproductive technology, such as
pre-implantation diagnosis, remain controversial.
Whenever possible, cases should be referred to
appropriate specialists, including geneticists and
obstetricians, for proper counselling.
Menopause
Hormonal profile changes can affect seizure control
in women with epilepsy.
94 Clinicians should remind
patients that hormone replacement therapy can
significantly increase seizure frequency during
menopause, particularly in women with catamenial
epilepsy.
95 Hormone replacement therapy (which
entails the administration of exogenous female sex
hormones) and contraceptive pills probably have
similar drug interactions with AEDs.
96
Catamenial epilepsy
Epileptic seizures may be triggered by serum hormonal
changes. Catamenial epilepsy is characterised by
an increased seizure frequency during certain
phase(s) of the menstrual cycle compared with
baseline. However, there is no uniform definition
for it. The seizures can cluster in different phases,
such as the perimenstrual, periovulatory, and luteal
phases of the menstrual cycle.
97 Recognition of the
catamenial seizure pattern can be challenging, as
irregular menstrual cycles and anovulatory cycles
are common. Women whose seizure occurrence is
suspected to be related to the menstrual cycle should
be advised to record the seizure attacks together
with the phase of the menstrual cycle in their seizure
diaries. Prolonged observation may be needed
before a catamenial epilepsy pattern is recognised.
Treatments include intermittent benzodiazepines
(eg, clobazam) during the susceptible phase of the
menstrual cycle.
98 99 Increasing the anticonvulsant
dosage during susceptible periods of the menstrual
cycle may also be feasible. Hormonal therapy has not
yet been proven to be effective in general.
100 It may
be beneficial in women with more frequent seizures
around the perimenstrual period, especially in
women with regular menstrual cycles.
101 102 However,
further studies are probably needed before routine
clinical use can be suggested.
100 Acetazolamide, used
either daily or perimenstrually, may have benefits for catamenial epilepsy, although the evidence to
support its use is limited.
93 94 103 104
Conclusion
New information has accumulated recently,
providing new insight into the care of women with
epilepsy and the implications for their offspring
(
Table 3). Thorough discussions with women with
epilepsy may increase patients’ levels of comfort and
care, especially regarding major treatment decision
on the issues of drug teratogenicity, neurobehavioral
adverse effects on offspring, pregnancy,
breastfeeding, contraception, reproduction
technology, menopause, and catamenial epilepsy.
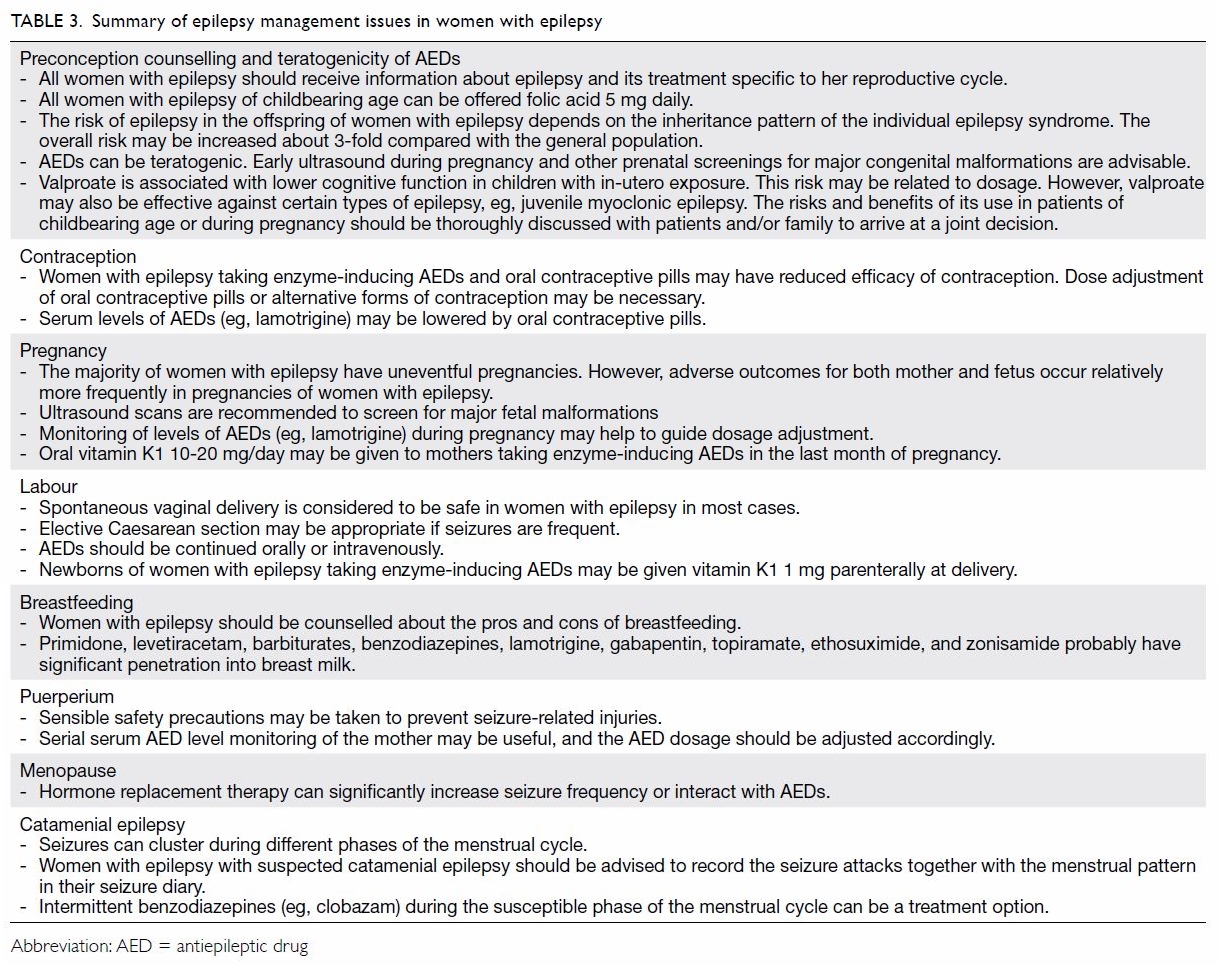 Table 3.
Table 3. Summary of epilepsy management issues in women with epilepsy
Author contributions
Concept or design: All authors.
Acquisition of data: RSK Chang.
Analysis and interpretation of data: RSK Chang.
Drafting of the manuscript: RSK Chang, KHK Lui, AWY Yung.
Critical revision of the manuscript for important intellectual
content: RSK Chang, H Leung.
All authors had full access to the data, contributed to the
study, approved the final version for publication and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This project was supported in part by an unrestricted grant from the Hong Kong Epilepsy Society. All authors are
members of the Hong Kong Epilepsy Society.
Disclaimer
This consensus statement is designed to assist clinicians by
providing an analytical framework for treatment of women
with epilepsy. It is not intended to establish a community
standard of care, replace a clinician’s medical judgement, or
establish a protocol for all patients.
References
1. Guideline Development Group, Hong Kong Epilepsy
Society. The Hong Kong Epilepsy Guideline 2009. Hong
Kong Med J 2009;15 Suppl 5:6-28.
2. Royal College of Obstetricians and Gynaecologists.
Epilepsy in Pregnancy (Green-top Guideline No. 68).
Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg68/. Accessed 19 Jul
2020.
3. Ottman R, Hirose S, Jain S, et al. Genetic testing in the
epilepsies—report of the ILAE Genetics Commission.
Epilepsia 2010;51:655-70.
Crossref4. Peljto AL, Barker-Cummings C, Vasoli VM, et al.
Familial risk of epilepsy: a population-based study. Brain
2014;137:795-805.
Crossref5. Billings RE. Decreased hepatic 5,
10-methylenetetrahydrofolate reductase activity in mice
after chronic phenytoin treatment. Mol Pharmacol 1984;25:459-66.
6. Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology 2003;60:575-9.
Crossref7. Harden CL, Pennell PB, Koppel BS, et al. Management
issues for women with epilepsy—focus on pregnancy (an
evidence-based review): III. Vitamin K, folic acid, blood
levels, and breast-feeding: report of the Quality Standards
Subcommittee and Therapeutics and Technology
Assessment Subcommittee of the American Academy of
Neurology and the American Epilepsy Society. Epilepsia
2009;50:1247-55.
Crossref8. Betts T, Fox C. Proactive pre-conception counselling for
women with epilepsy—is it effective? Seizure 1999;8:322-7.
Crossref9. Harden CL, Hopp J, Ting TY, et al. Management issues for
women with epilepsy-focus on pregnancy (an evidence-based
review): I. Obstetrical complications and change
in seizure frequency: report of the Quality Standards
Subcommittee and Therapeutics and Technology
Assessment Subcommittee of the American Academy of
Neurology and the American Epilepsy Society. Epilepsia
2009;50:1229-36.
Crossref10. Shorvon SD, Tomson T, Cock HR. The management of epilepsy during pregnancy—progress is painfully slow.
Epilepsia 2009;50:973-4.
Crossref11. Fried S, Kozer E, Nulman I, Einarson TR, Koren G.
Malformation rates in children of women with untreated
epilepsy: a meta-analysis. Drug Saf 2004;27:197-202.
Crossref12. Harden CL, Meador KJ, Pennell PB, et al. Management
issues for women with epilepsy-focus on pregnancy (an
evidence-based review): II. Teratogenesis and perinatal
outcomes: report of the Quality Standards Subcommittee
and Therapeutics and Technology Subcommittee of the
American Academy of Neurology and the American
Epilepsy Society. Epilepsia 2009;50:1237-46.
Crossref13. Morrow J, Russell A, Guthrie E, et al. Malformation risks
of antiepileptic drugs in pregnancy: a prospective study
from the UK Epilepsy and Pregnancy Register. J Neurol
Neurosurg Psychiatry 2006;77:193-8.
Crossref14. Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C.
Pregnancy outcomes in women with epilepsy: a systematic
review and meta-analysis of published pregnancy
registries and cohorts. Epilepsy Res 2008;81:1-13.
Crossref15. Tomson T, Marson A, Boon P, et al. Valproate in the treatment of epilepsy in girls and women of childbearing
potential. Epilepsia 2015;56:1006-19.
Crossref16. Holmes LB, Wyszynski DF, Lieberman E. The AED
(antiepileptic drug) pregnancy registry: a 6-year
experience. Arch Neurol 2004;61:673-8.
Crossref17. Hernández-Díaz S, Smith CR, Shen A, et al. Comparative
safety of antiepileptic drugs during pregnancy. Neurology
2012;78:1692-9.
Crossref18. Reinisch JM, Sanders SA, Mortensen EL, Rubin DB. In
utero exposure to phenobarbital and intelligence deficits
in adult men. JAMA 1995;274:1518-25.
Crossref19. Tomson T, Battino D, Bonizzoni E, et al. Comparative risk
of major congenital malformations with eight different
antiepileptic drugs: a prospective cohort study of the
EURAP registry. Lancet Neurol 2018;17:530-8.
Crossref20. Campbell E, Kennedy F, Russell A, et al. Malformation
risks of antiepileptic drug monotherapies in pregnancy:
updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014;85:1029-34.
Crossref21. Mawhinney E, Craig J, Morrow J, et al. Levetiracetam in
pregnancy: results from the UK and Ireland epilepsy and
pregnancy registers. Neurology 2013;80:400-5.
Crossref22. Hunt S, Russell A, Smithson WH, et al. Topiramate in
pregnancy: preliminary experience from the UK Epilepsy
and Pregnancy Register. Neurology 2008;71:272-6.
Crossref23. Vanoverloop D, Schnell RR, Harvey EA, Holmes LB.
The effects of prenatal exposure to phenytoin and other
anticonvulsants on intellectual function at 4 to 8 years of
age. Neurotoxicol Teratol 1992;14:329-35.
Crossref24. Scolnik D, Nulman I, Rovet J, et al. Neurodevelopment of
children exposed in utero to phenytoin and carbamazepine
monotherapy. JAMA 1994;271:767-70.
Crossref25. Wide K, Henning E, Tomson T, Winbladh B. Psychomotor
development in preschool children exposed to
antiepileptic drugs in utero. Acta Paediatr 2002;91:409-14.
Crossref26. Arpino C, Brescianini S, Robert E, et al. Teratogenic effects
of antiepileptic drugs: use of an International Database on
Malformations and Drug Exposure (MADRE). Epilepsia
2000;41:1436-43.
Crossref27. Bertollini R, Mastroiacovo P, Segni G. Maternal epilepsy
and birth defects: a case-control study in the Italian
Multicentric Registry of Birth Defects (IPIMC). Eur J
Epidemiol 1985;1:67-72.
Crossref28. Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J.
Antiepileptic drug use of women with epilepsy and
congenital malformations in offspring. Neurology
2005;64:1874-8.
Crossref29. Samrén EB, van Duijn CM, Christiaens GC, Hofman A,
Lindhout D. Antiepileptic drug regimens and major
congenital abnormalities in the offspring. Ann Neurol
1999;46:739-46.
Crossref30. Jentink J, Loane MA, Dolk H, et al. Valproic acid
monotherapy in pregnancy and major congenital
malformations. N Engl J Med 2010;362:2185-93.
Crossref31. Meador KJ, Baker GA, Browning N, et al. Cognitive
function at 3 years of age after fetal exposure to
antiepileptic drugs. N Engl J Med 2009;360:1597-605.
Crossref32. Meador KJ, Baker GA, Browning N, et al. Fetal
antiepileptic drug exposure and cognitive outcomes at age
6 years (NEAD study): a prospective observational study.
Lancet Neurol 2013;12:244-52.
Crossref33. Meador KJ, Baker GA, Browning N, et al. Effects of fetal
antiepileptic drug exposure: outcomes at age 4.5 years.
Neurology 2012;78:1207-14.
Crossref34. Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal
intelligence in children with prenatal exposure to
carbamazepine. Neurology 2004;62:28-32.
Crossref35. Bromley R, Weston J, Adab N, et al. Treatment for epilepsy
in pregnancy: neurodevelopmental outcomes in the child.
Cochrane Database Syst Rev 2014;(10):CD010236.
Crossref36. Adab N, Jacoby A, Smith D, Chadwick D. Additional
educational needs in children born to mothers with
epilepsy. J Neurol Neurosurg Psychiatry 2001;70:15-21.
Crossref37. Adab N, Kini U, Vinten J, et al. The longer term outcome
of children born to mothers with epilepsy. J Neurol
Neurosurg Psychiatry 2004;75:1575-83.
Crossref38. Bromley RL, Mawer GE, Briggs M, et al. The prevalence
of neurodevelopmental disorders in children prenatally
exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry 2013;84:637-43.
Crossref39. Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal
valproate exposure and risk of autism spectrum disorders
and childhood autism. JAMA 2013;309:1696-703.
Crossref40. Harden CL, Meador KJ, Pennell PB, et al. Practice
parameter update: management issues for women with
epilepsy—focus on pregnancy (an evidence-based
review): teratogenesis and perinatal outcomes: report of
the Quality Standards Subcommittee and Therapeutics
and Technology Assessment Subcommittee of the
American Academy of Neurology and American Epilepsy
Society. Neurology 2009;73:133-41.
Crossref41. Jentink J, Dolk H, Loane MA, et al. Intrauterine exposure
to carbamazepine and specific congenital malformations:
systematic review and case-control study. BMJ
2010;341:c6581.
Crossref42. Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent
risk of malformations with antiepileptic drugs: an analysis
of data from the EURAP epilepsy and pregnancy registry.
Lancet Neurol 2011;10:609-17.
Crossref43. Patorno E, Bateman BT, Huybrechts KF, et al. Pregabalin
use early in pregnancy and the risk of major congenital
malformations. Neurology 2017;88:2020-5.
Crossref44. Holmes LB, Mittendorf R, Shen A, Smith CR, Hernandez-
Diaz S. Fetal effects of anticonvulsant polytherapies:
different risks from different drug combinations. Arch
Neurol 2011;68:1275-81.
Crossref45. Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome
following maternal exposure to pregabalin may call for
concern. Neurology 2016;86:2251-7.
Crossref46. Datta S, Muñoz-Largacha JA, Li L, Zhao GQ, Litle VR.
Subcutaneous metastases from early stage esophageal
adenocarcinoma case report. Int J Surg Case Rep
2016;29:108-12.
Crossref47. MacDonald SC, Bateman BT, McElrath TF, Hernández-Diáz S. Mortality and morbidity during delivery
hospitalization among pregnant women with epilepsy in
the United States. JAMA Neurol 2015;72:981-8.
Crossref48. Viale L, Allotey J, Cheong-See F, et al. Epilepsy in
pregnancy and reproductive outcomes: a systematic
review and meta-analysis. Lancet 2015;386:1845-52.
Crossref49. Vajda FJ, Hitchcock A, Graham J, O’Brien T, Lander C,
Eadie M. Seizure control in antiepileptic drug-treated
pregnancy. Epilepsia 2008;49:172-6.
Crossref50. Gjerde IO, Strandjord RE, Ulstein M. The course of
epilepsy during pregnancy: a study of 78 cases. Acta
Neurol Scand 1988;78:198-205.
Crossref51. Harden CL, Hopp J, Ting TY, et al. Practice parameter
update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review):
obstetrical complications and change in seizure frequency:
report of the Quality Standards Subcommittee and
Therapeutics and Technology Assessment Subcommittee
of the American Academy of Neurology and American
Epilepsy Society. Neurology 2009;73:126-32.
Crossref52. Pennell PB. Antiepileptic drugs during pregnancy: what
is known and which AEDs seem to be safest? Epilepsia
2008;49 Suppl 9:43-55.
Crossref53. European Registry of Antiepileptic Drugs in Pregnancy
(EURAP). Seizure control and treatment in pregnancy:
observations from the EURAP epilepsy pregnancy
registry. Neurology. 2006;66:354-60.
Crossref54. National Institute for Health and Care Excellence, UK Government. Epilepsies: diagnosis and management.
Clinical guideline [CG137]. 2013. Available from:
https://www.nice.org.uk/guidance/cg137/chapter/1-
Guidance#women-and-girls-with-epilepsy. Accessed 19
Jul 2020.
55. International Society of Ultrasound in Obstetrics and
Gynecology, Carvalho JS, et al. ISUOG Practice Guidelines
(updated): sonographic screening examination of the fetal
heart. Ultrasound Obstet Gynecol 2013;41:348-59.
Crossref56. Bhatia M, Adcock JE, Mackillop L. The management
of pregnant women with epilepsy: a multidisciplinary
collaborative approach to care. The Obstet Gynaecol
2017;19:279-88.
Crossref57. de Haan GJ, Edelbroek P, Segers J, et al. Gestation-induced
changes in lamotrigine pharmacokinetics: a monotherapy
study. Neurology 2004;63:571-3.
Crossref58. Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in
pregnancy: clearance, therapeutic drug monitoring, and
seizure frequency. Neurology 2008;70:2130-6.
Crossref59. Pennell PB. Antiepileptic drug pharmacokinetics during
pregnancy and lactation. Neurology 2003;61:S35-42.
Crossref60. Westin AA, Nakken KO, Johannessen SI, Reimers A,
Lillestølen KM, Brodtkorb E. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia
2009;50:480-5.
Crossref61. Reisinger TL, Newman M, Loring DW, Pennell PB,
Meador KJ. Antiepileptic drug clearance and seizure
frequency during pregnancy in women with epilepsy.
Epilepsy Behav 2013;29:13-8.
Crossref62. Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R.
Community-based, prospective, controlled study of
obstetric and neonatal outcome of 179 pregnancies in
women with epilepsy. Epilepsia 2006;47:186-92.
Crossref63. World Health Organization. Epilepsy: a manual for
physicians. 2004. Available from: https://apps.who.int/
iris/bitstream/handle/10665/205014/B0769.pdf Accessed
19 Jul 2020.
64. Reddy DS. Clinical pharmacokinetic interactions between
antiepileptic drugs and hormonal contraceptives. Expert
Rev Clin Pharmacol 2010;3:183-92.
Crossref65. Perucca E. Clinically relevant drug interactions with
antiepileptic drugs. Br J Clin Pharmacol 2006;61:246-55.
Crossref66. Tolou-Ghamari Z, Zare M, Habibabadi JM, Najafi MR.
A quick review of carbamazepine pharmacokinetics in
epilepsy from 1953 to 2012. J Res Med Sci 2013;18(Suppl
1):S81-5.
67. Sabers A, Ohman I, Christensen J, Tomson T. Oral
contraceptives reduce lamotrigine plasma levels.
Neurology 2003;61:570-1.
Crossref68. Patsalos PN. The clinical pharmacology profile of the new
antiepileptic drug perampanel: a novel noncompetitive
AMPA receptor antagonist. Epilepsia 2015;56:12-27.
Crossref69. Rosenfeld WE, Doose DR, Walker SA, Nayak RK.
Effect of topiramate on the pharmacokinetics of an oral
contraceptive containing norethindrone and ethinyl
estradiol in patients with epilepsy. Epilepsia 1997;38:317-23.
Crossref70. Eldon MA, Underwood BA, Randinitis EJ, Sedman AJ.
Gabapentin does not interact with a contraceptive
regimen of norethindrone acetate and ethinyl estradiol.
Neurology 1998;50:1146-8.
Crossref71. Cawello W, Rosenkranz B, Schmid B, Wierich W.
Pharmacodynamic and pharmacokinetic evaluation of coadministration of lacosamide and an oral contraceptive
(levonorgestrel plus ethinylestradiol) in healthy female
volunteers. Epilepsia 2013;54:530-6.
Crossref72. Cawello W. Clinical pharmacokinetic and pharmacodynamic
profile of lacosamide. Clin Pharmacokinet 2015;54:901-
14.
Crossref73. Ragueneau-Majlessi I, Levy RH, Janik F. Levetiracetam
does not alter the pharmacokinetics of an oral
contraceptive in healthy women. Epilepsia 2002;43:697-702.
Crossref74. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical
eligibility criteria for contraceptive use, 2016. MMWR
Recomm Rep 2016;65:1-103.
Crossref75. Gooneratne IK, Wimalaratna M, Ranaweera AK,
Wimalaratna S. Contraception advice for women with
epilepsy. BMJ 2017;357:j2010.
Crossref76. Pennell PB, Gidal BE, Sabers A, Gordon J, Perucca E.
Pharmacology of antiepileptic drugs during pregnancy
and lactation. Epilepsy Behav 2007;11:263-9.
Crossref77. The Faculty of Sexual & Reproductive Healthcare of
the Royal College of Obstetricians & Gynaecologists.
FSRH CEU guidance: drug interactions with hormonal
contraception. Available from: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed
11 Oct 2018.
78. The Faculty of Sexual & Reproductive Healthcare of
the Royal College of Obstetricians & Gynaecologists.
FSRH Guideline Emergency Contraception. Available
from: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed 10 Oct 18.
79. Black KI, Hussainy SY. Emergency contraception: oral and
intrauterine options. Aust Fam Physician 2017;46:722-6.
80. Pennell PB. EURAP outcomes for seizure control during
pregnancy: useful and encouraging data. Epilepsy Curr
2006;6:186-8.
Crossref81. Marinella MA. Meperidine-induced generalized seizures with normal renal function. South Med J 1997;90:556-8.
Crossref82. ACOG educational bulletin. Seizure disorders in
pregnancy. Number 231, December 1996. Committee
on Educational Bulletins of the American College
of Obstetricians and Gynecologists [editorial]. Int J
Gynaecol Obstet 1997;56:279-86.
83. Yamasmit W, Chaithongwongwatthana S, Tolosa JE.
Prenatal vitamin K1 administration in epileptic women
to prevent neonatal hemorrhage: is it effective? J Reprod
Med 2006;51:463-6.
84. Veiby G, Bjørk M, Engelsen BA, Gilhus NE. Epilepsy and
recommendations for breastfeeding. Seizure 2015;28:57-65.
Crossref85. Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy:
pharmacokinetics during delivery, in the neonate, and
during lactation. Epilepsia 2000;41:709-13.
Crossref86. Davanzo R, Dal Bo S, Bua J, Copertino M, Zanelli E,
Matarazzo L. Antiepileptic drugs and breastfeeding. Ital
J Pediatr 2013;39:50.
Crossref87. Meador KJ, Baker GA, Browning N, et al. Effects of
breastfeeding in children of women taking antiepileptic
drugs. Neurology 2010;75:1954-60.
Crossref88. Meador KJ, Baker GA, Browning N, et al. Breastfeeding
in children of women taking antiepileptic drugs: cognitive
outcomes at age 6 years. JAMA Pediatr 2014;168:729-36.
Crossref89. Fox C, Betts T. How much risk does a woman with active
epilepsy pose to her newborn child in the puerperium? A
pilot study. Seizure 1999;8:367-9.
Crossref90. Thomas SV, Syam U, Devi JS. Predictors of seizures
during pregnancy in women with epilepsy. Epilepsia
2012;53:e85-8.
Crossref91. Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R.
Lamotrigine clearance during pregnancy. Neurology
2002;59:251-5.
Crossref92. Tomson T, Landmark CJ, Battino D. Antiepileptic drug
treatment in pregnancy: changes in drug disposition and
their clinical implications. Epilepsia 2013;54:405-14.
Crossref93. Mostacci B, Esposto R, Lello S, Bisulli F, Licchetta L,
Tinuper P. Estrogen-related seizure exacerbation
following hormone therapy for assisted reproduction in
women with epilepsy. Seizure 2018;61:200-2.
Crossref94. Taubøll E, Sveberg L, Svalheim S. Interactions between hormones and epilepsy. Seizure 2015;28:3-11.
Crossref95. Harden CL, Pulver MC, Ravdin L, Jacobs AR. The effect of
menopause and perimenopause on the course of epilepsy.
Epilepsia 1999;40:1402-7.
Crossref96. Reimers A. Hormone replacement therapy with estrogens
may reduce lamotrigine serum concentrations: a matched
case-control study. Epilepsia 2017;58:e6-9.
Crossref97. Herzog AG. Catamenial epilepsy: definition, prevalence
pathophysiology and treatment. Seizure 2008;17:151-9.
Crossref98. Feely M, Gibson J. Intermittent clobazam for catamenial
epilepsy: tolerance avoided. J Neurol Neurosurg
Psychiatry 1984;47:1279-82.
Crossref99. Foldvary-Schaefer N, Falcone T. Catamenial epilepsy:
pathophysiology, diagnosis, and management. Neurology
2003;61(6 Suppl 2):S2-15.
Crossref100. Herzog AG, Fowler KM, Smithson SD, et al. Progesterone
vs placebo therapy for women with epilepsy: a randomized
clinical trial. Neurology 2012;78:1959-66.
Crossref101. Herzog AG. Catamenial epilepsy: update on prevalence,
pathophysiology and treatment from the findings of the
NIH Progesterone Treatment Trial. Seizure 2015;28:18-25.
Crossref102. Navis A, Harden C. A treatment approach to catamenial
epilepsy. Curr Treat Options Neurol 2016;18:30.
Crossref103. Reddy DS, Gould J, Gangisetty O. A mouse kindling
model of perimenstrual catamenial epilepsy. J Pharmacol
Exp Ther 2012;341:784-93.
Crossref104. Lim LL, Foldvary N, Mascha E, Lee J. Acetazolamide in
women with catamenial epilepsy. Epilepsia 2001;42:746-9.
Crossref