Neonatal haemoperitoneum due to splenic rupture: a case report
Hong Kong Med J 2024 Jun;30(3):245–7 | Epub 6 Jun 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Neonatal haemoperitoneum due to splenic rupture: a case report
Stephanie LH Cheung, MB, ChB, MRCS1,2; Bess SY Tsui, FRCSEd (Paed), FHKAM (Surgery)1,2; Rosanna MS Wong, FHKAM (Paediatrics)3; YH Tam, FRCSEd (Paed), FHKAM (Surgery)1,2
1 Department of Paediatric Surgery, Hong Kong Children's Hospital, Hong Kong SAR, China
2 Division of Paediatric Surgery and Paediatric Urology, Department of Surgery, Prince of Wales Hospital, Hong Kong SAR, China
3 Department of Paediatrics and Adolescent Medicine, Hong Kong Children's Hospital, Hong Kong SAR, China
Corresponding author: Dr Stephanie LH Cheung (clh106@ha.org.hk)
Case presentation
Our female neonate presented in May 2023 was born full term at 39+4 weeks in a local private hospital with
a birth weight of 3.2 kg. There were no abnormalities
on antenatal check-up. She was born by vacuum-assisted
vaginal delivery due to non-reassuring
cardiotocography tracings. The Apgar score was
9 at 1 minute and 10 at 5 minutes of life. The first
24 hours postnatally was normal with meconium
passage and feeding initiated.
She was noted to have apnoea, pallor
and abdominal distension at 26 hours of life.
Haemoglobin level had dropped to 3 g/dL. Urgent
fluid boluses and packed cell transfusions were
given. Post-transfusion haemoglobin level was raised
to 10.3 g/dL. Her initial platelet counts were normal
but clotting profile was deranged (international
normalised ratio=1.49) and she was given fresh
frozen plasma transfusion. Clinically, the patient did
not require inotropic support. Urgent ultrasound
of the abdomen revealed ascites with echogenicity
suspicious of haemoperitoneum, with an echogenic
heterogeneous lesion over the left suprasplenic area
measuring 5 × 2 × 4 cm3 which was suspicious of a
haematoma. The spleen was not enlarged and the
liver and kidneys were normal. Elective intubation
and urgent transferral were arranged to the neonatal
intensive care unit for further management.
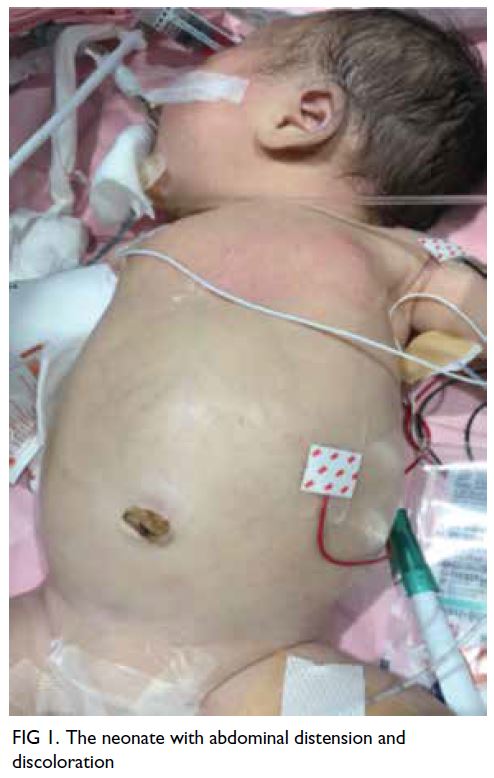
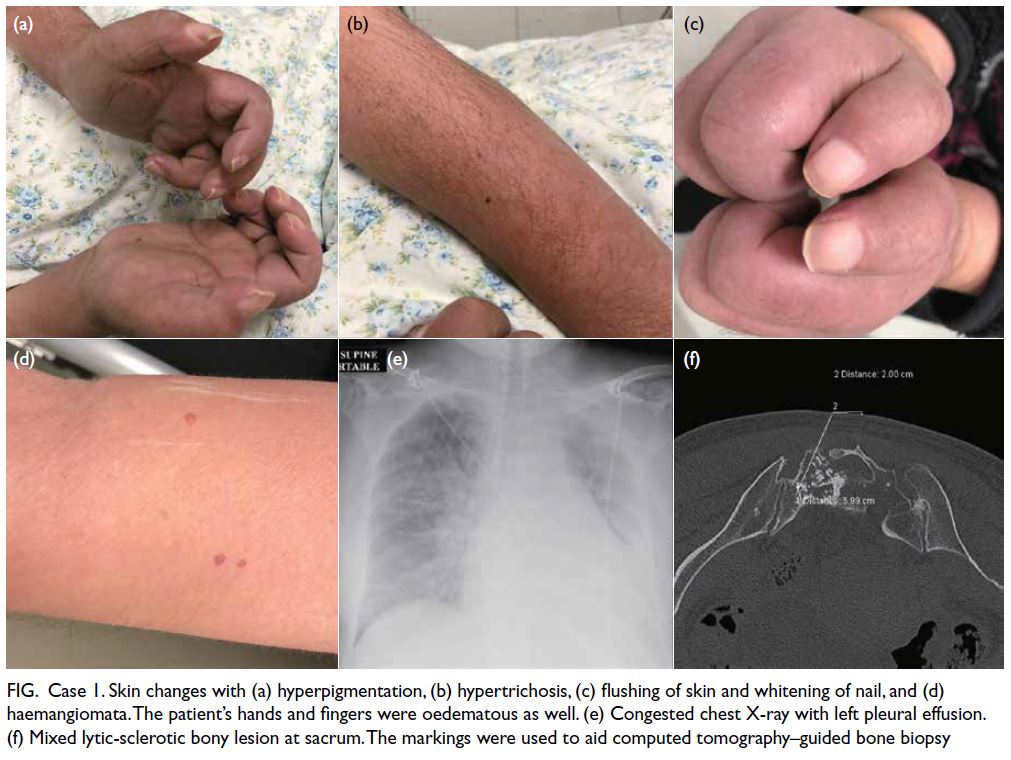
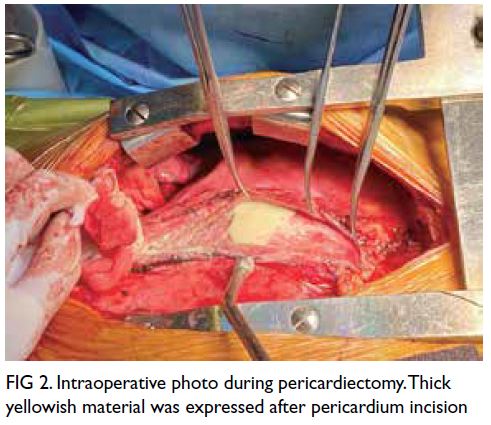
Upon arrival, the baby was noted to be pale
with gross abdominal distension (Fig 1). She was
tachycardic with pulse of 190 bpm and mean
arterial pressure of 40 mm Hg. She responded to
fluid resuscitation with a total of 180 mL of normal
saline boluses and packed cell transfusion and did
not require inotropic support. Haemoglobin level
on arrival was 6 g/dL. Urgent computed tomography
of the abdomen was arranged which revealed
haemoperitoneum with a large left subphrenic
haematoma (6.3 × 3.6 × 6.9 cm3) likely arising from
the spleen. Contrast extravasation was noted over
the medial aspect of the spleen suggestive of active
bleeding. The size and enhancement of the spleen
was normal. The rest of the abdomen was normal.
Urgent surgical exploration was performed in
view of active bleeding and haemodynamic instability of the patient despite resuscitation. Laparotomy
revealed large amount of old blood clots. The spleen
had a normal configuration and orientation and
was located over the left upper quadrant. A 1.5-cm
linear tear was noted over the medial side of the
lower pole of the spleen (Fig 2). Secure haemostasis
could not be achieved despite the use of packing,
oxidised regenerated cellulose and suturing, hence
splenectomy was performed. The short gastric
vessels were divided by cautery and splenic hilum
transfixed and over-sewn with 5-0 polydioxanone
sutures (Ethicon, Cincinnati [OH], United States). A
small spleniculi was noted and it was left untouched. The surgery lasted for 1 hour 34 minutes and total
blood loss including the drained old blood was 750 mL.
Postoperative recovery was unremarkable. Her
haemoglobin level stabilised on postoperative day
1, extubated on postoperative day 4, and full enteral
feeding achieved on postoperative day 7. She was
discharged on day 8 after receiving necessary post-splenectomy
vaccinations.
Discussion
Splenic rupture in newborns is a rare disease entity.
There were more mortality cases than survival in
literature. A review of literature from 1970 revealed
37 cases of splenic rupture in a neonate reported in
literature.1 This rupture is usually associated with
traumatic birth or other intrinsic pathology of the
parenchyma such as haemophilia or erythroblastosis
fetalis.2 However, there were also >10 cases of
spontaneous splenic rupture with no preceding risks
or predisposing factors in literature. Splenic injury
usually occurs in two stages, first with subcapsular
haematoma formation and then with urgent
symptoms presenting when the splenic capsule
gives way resulting in haemoperitoneum. The signs
and symptoms of haemoperitoneum are vague and
often easily missed. The common symptoms are
blood loss causing pallor, abdominal distension and
radiological evidence of intraperitoneal effusion
without pneumoperitoneum,3 which resembled our index case. Rarely, haemoperitoneum can also
present with scrotal swelling or haematoma. Splenic
rupture usually occurs within the first day of life
but rarely can present as late as 5 days of life. The
initial haemoglobin level in these neonates is often
very low (<6 g/dL) and require immediately blood
transfusion support.
Due to the friable nature of splenic tissue in
a neonate, more than half of the cases described
in literature proceeded to splenectomy, like our
index case. Ten cases settled with conservative
treatment with transfusion of blood products, but
these cases usually present later (>10 hours of life)
and the neonates had stable haemodynamics. There
were cases where laparotomy was performed and
haemostasis was secured without splenectomy.1 Other methods for haemostasis were employed. In
a case report in 1976 where the spleen was almost
transacted at its lower pole, the laceration was
repaired by chromic catgut mattress sutures and
the patient survived.4 The second case used an
absorbable mesh for haemostasis.5 The third case
reported was in 2002, where the splenic capsule
ruptured with oozing and gel-foam and oxidised
regenerated cellulose was used to pack the spleen.2
Second-look laparotomy was performed 48 hours
later which revealed that the bleeding had stopped
after removal of packing materials. One case in
literature utilised interventional radiology for
splenic artery embolisation to stop the bleeding.3
The choice of conservative versus surgical versus
interventional radiological treatment depends on
the haemodynamic of the patient and availability of
expertise and material. Due to the rare nature of the
condition, no evidence reported superiority of one
treatment method over another.
Conclusion
Neonatal splenic rupture is a rare emergency with high mortality. High index of clinical suspicion and
early establishment of diagnosis is necessary for
timely treatment. Our patient presented classically
with a difficult delivery and typical presentation
of abdominal distension, pallor and hypovolaemic
shock. Splenectomy is still the mainstay of treatment
but other approaches are also feasible.
Author contributions
All authors contributed to the concept or design, acquisition
of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The parents of the patient provided written consent for publication of this case report.
References
1. Hui CM, Tsui KY. Splenic rupture in a newborn. J Pediatr Surg 2002;37:E3. Crossref
2. Matsuyama S, Suzuki N, Nagamachi Y. Rupture of the spleen in the newborn: treatment without splenectomy. J Pediatr Surg 1976;11:115-6. Crossref
3. Raats JW, van Dam L, van Doormaal PJ, van Hengel-Jacobs M,
Langeveld-Benders H. Neonatal rupture of the spleen:
successful treatment with splenic artery embolization. AJR
Rep 2021;11:e58-60. Crossref
4. Chang HP, Fu RH, Lin JJ, Chiang MC. Prognostic factors and clinical features of neonatal splenic rupture/hemorrhage: two cases reports and literature review. Front
Pediatr 2021;9:616247. Crossref
5. Fasoli L, Bettili G, Bianchi S, Dal Moro A, Ottolenghi A.
Spleen rupture in the newborn: conservative surgical
treatment using absorbable mesh. J Trauma 1998;45:642-3. Crossref