Frontal lobe epilepsy and hibernoma: a case report
Hong Kong Med J 2025 Apr;31(2):159–61 | Epub 3 Apr 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Frontal lobe epilepsy and hibernoma: a case
report
Rongjun Zhang, M Med#; Zhigang Gong, PhD#; Wenbing Jiang, M Med
Department of Neurosurgery, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, China
# Equal contribution
Corresponding author: Dr Rongjun Zhang (zhangrongjun2000@163.com)
Case presentation
A 69-year-old female presented to the clinic
with a 2-year history of intermittent headaches.
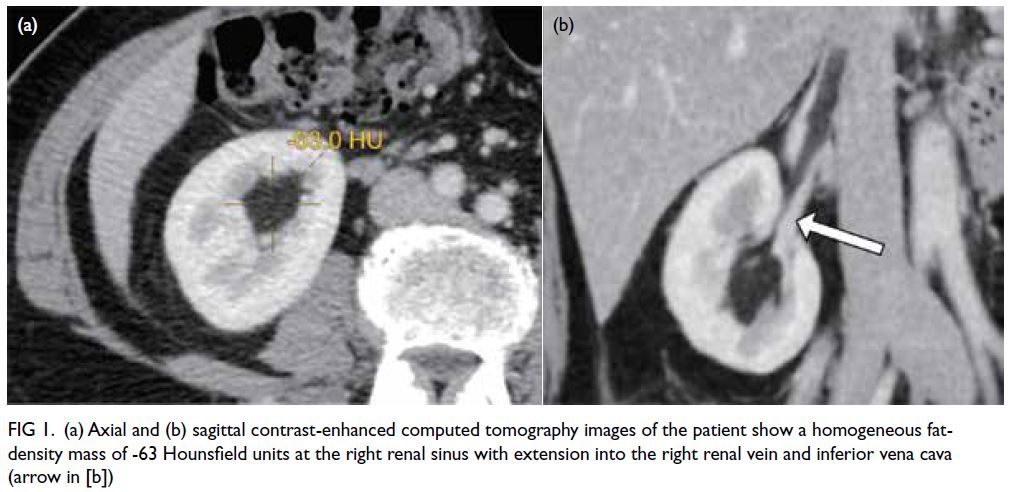
Cranial computed tomography (CT) and magnetic
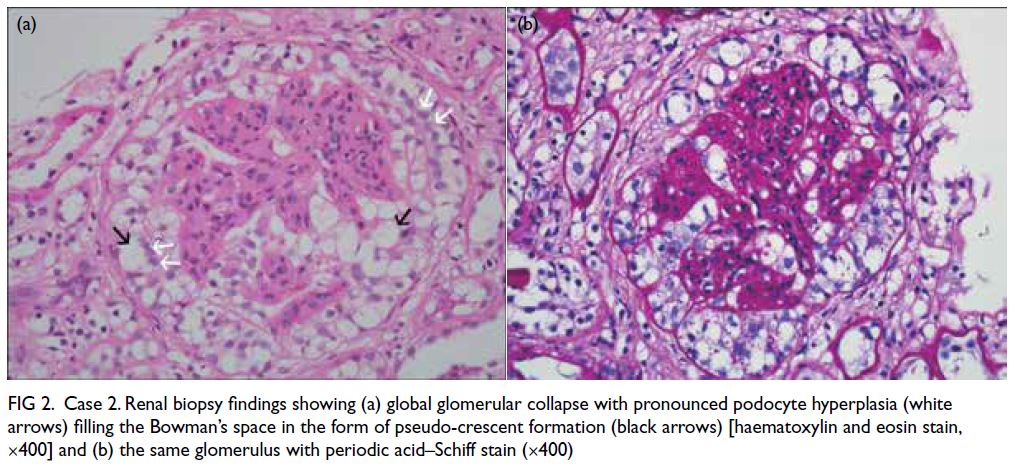
resonance imaging (MRI) examination (Fig 1a and b)
revealed a space-occupying lesion in the right frontal
lobe, suggestive of a meningioma. Preoperatively,
the patient experienced a single episode of epilepsy
lasting for 2 minutes, relieved by antiepileptic
medication.
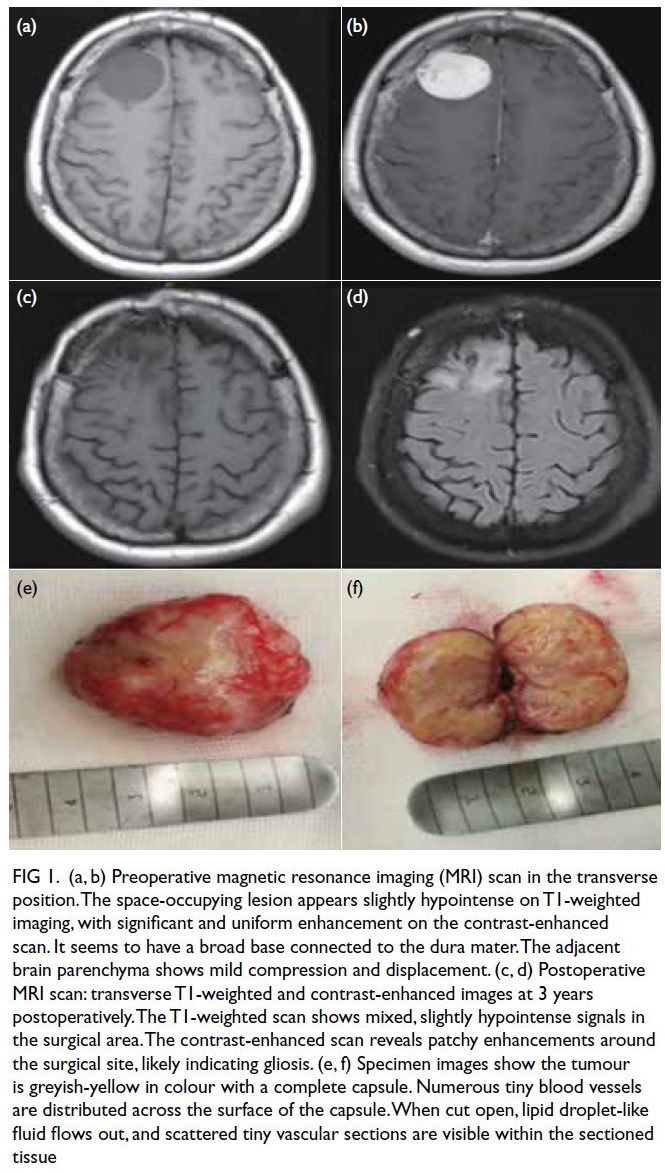
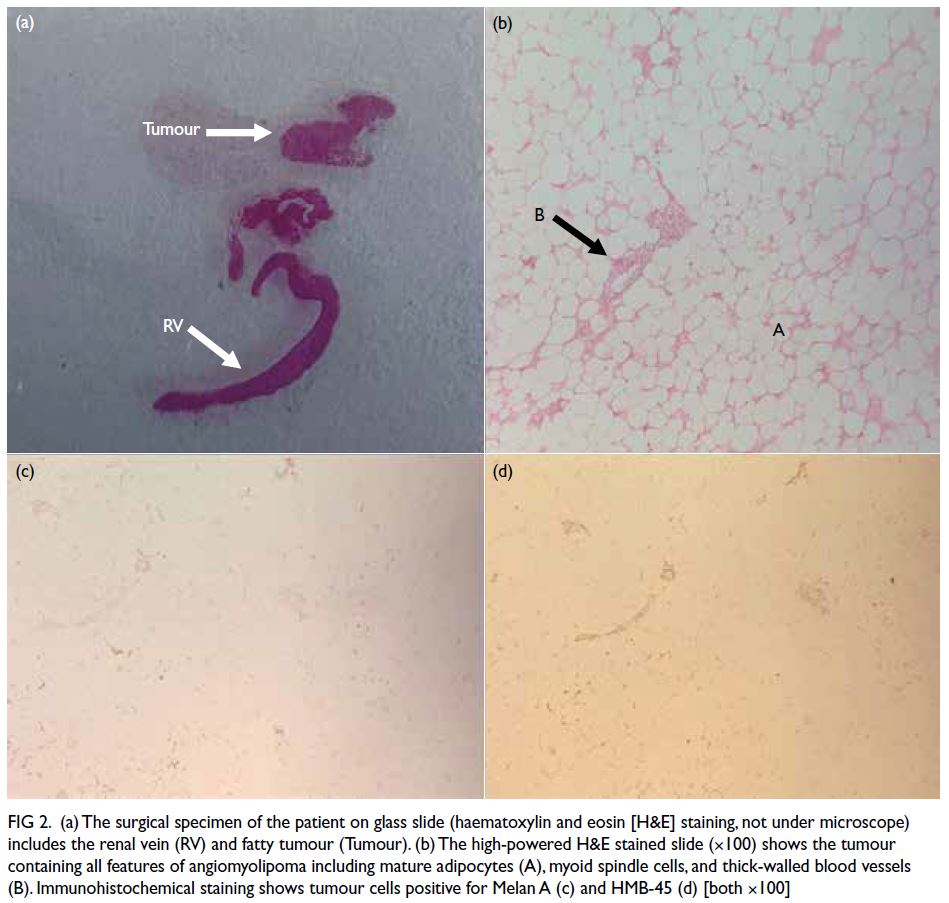
Figure 1. (a, b) Preoperative magnetic resonance imaging (MRI) scan in the transverse position. The space-occupying lesion appears slightly hypointense on T1-weighted imaging, with significant and uniform enhancement on the contrast-enhanced scan. It seems to have a broad base connected to the dura mater. The adjacent brain parenchyma shows mild compression and displacement. (c, d) Postoperative MRI scan: transverse T1-weighted and contrast-enhanced images at 3 years postoperatively. The T1-weighted scan shows mixed, slightly hypointense signals in the surgical area. The contrast-enhanced scan reveals patchy enhancements around the surgical site, likely indicating gliosis. (e, f) Specimen images show the tumour is greyish-yellow in colour with a complete capsule. Numerous tiny blood vessels are distributed across the surface of the capsule. When cut open, lipid droplet-like fluid flows out, and scattered tiny vascular sections are visible within the sectioned tissue
Cranial surgery was performed based on
the preoperative CT localisation. The tumour was
completely separated and excised, measuring 4 × 3 × 2 cm3, greyish-yellow in colour, and encapsulated
(Fig 1e). The encapsulated surface was rich in blood
vessels. Upon opening, the tumour was yellow, and
lipid droplet-like fluid was observed on the surface
(Fig 1f).
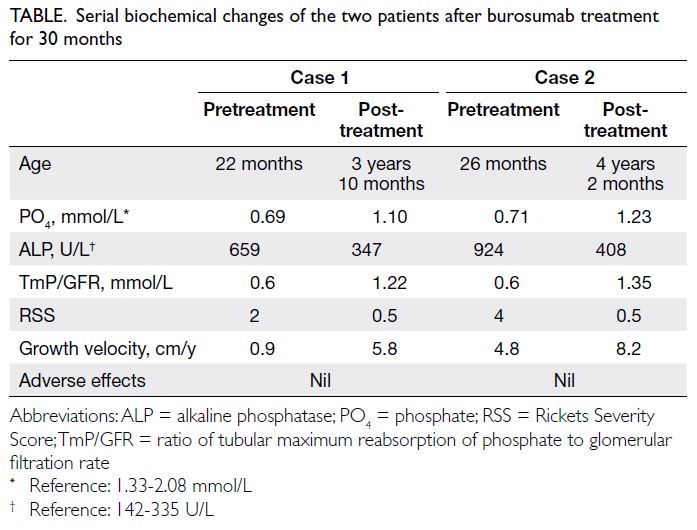
Postoperative pathology indicated a hibernoma.
Haematoxylin and eosin staining revealed a complete
thin capsule attached to the outside of the tumour
that was lobulated. A network of capillaries could
be seen inside. Under the microscope, polygonal/round cells with eosinophilic granular cytoplasm
were seen. The cell nuclei were round and centrally
located. In addition, many small vacuolated brown
adipocytes and unilocular adipocytes were seen,
with a considerable amount of blood vessels, spindle
cells, and collagen fibres infiltrating around the
tumour cells (Fig 2).
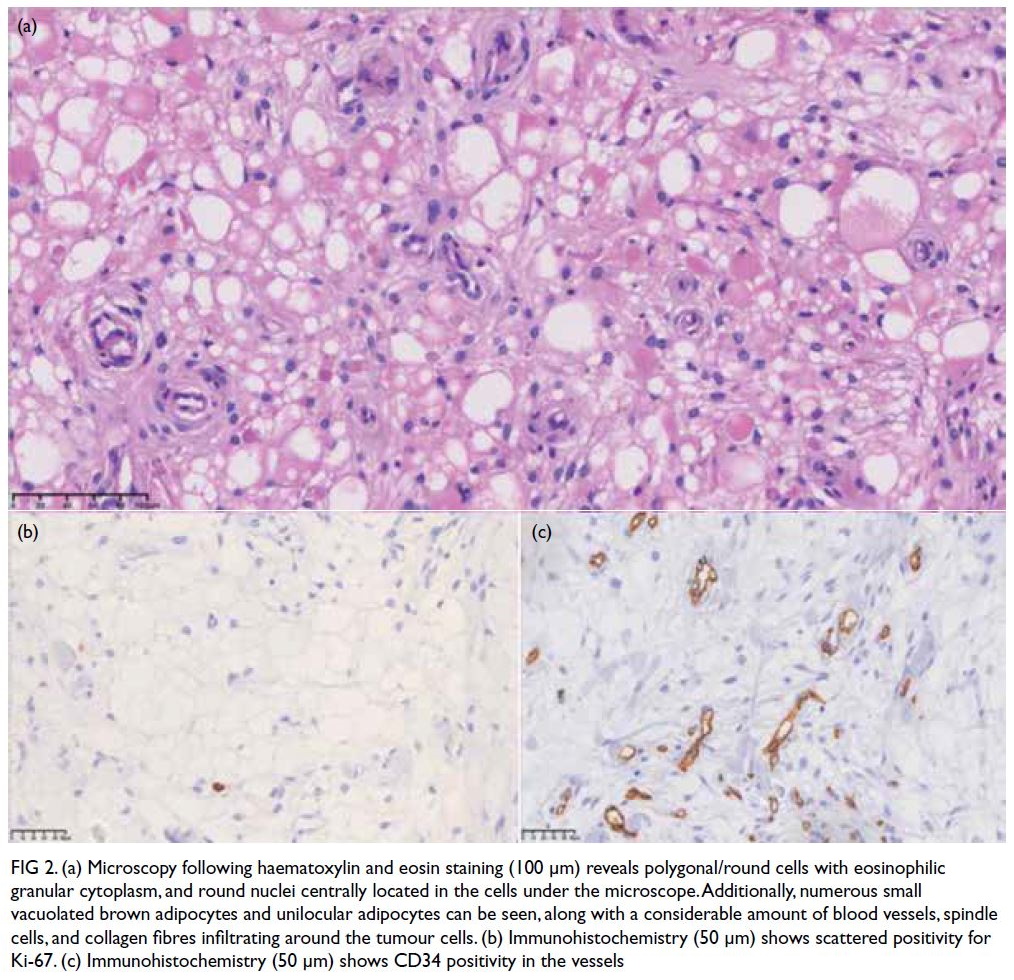
Figure 2. (a) Microscopy following haematoxylin and eosin staining (100 μm) reveals polygonal/round cells with eosinophilic granular cytoplasm, and round nuclei centrally located in the cells under the microscope. Additionally, numerous small vacuolated brown adipocytes and unilocular adipocytes can be seen, along with a considerable amount of blood vessels, spindle cells, and collagen fibres infiltrating around the tumour cells. (b) Immunohistochemistry (50 μm) shows scattered positivity for Ki-67. (c) Immunohistochemistry (50 μm) shows CD34 positivity in the vessels
Immunohistochemistry showed CD34 to be
positive in the vessels, and Ki-67 scattered positive
with a proportion of <1%. Immunohistochemistry
results were as follows: CK (-), S-100 (-), MDM2 (-),
CDK4 (-), P53 (-), CD34 (vascular +), CD117 (-), and
Ki -67 (+, <1%) [Fig 2].
Postoperative re-examination confirmed
complete removal of the tumour, and the patient had
no further epileptic seizures. Three years later, cranial
MRI follow-up revealed no tumour recurrence (Fig 1c and d).
Discussion
Hibernomas are rare benign tumours composed of
brown adipose tissue.1 They are asymptomatic and slow-growing. Compared with lipomas originating
from white adipose tissue, hibernomas are
exceedingly rare, with <200 cases reported
in the literature.2 These tumours most commonly
occur in the proximal axial skeleton, where fetal
brown adipose tissue exists and continues into
adulthood. They are most frequently found in the
interscapular region, upper mediastinum, axilla,
retroperitoneum, and neck. According to literature
reports, hibernomas are extremely rare in the
cranial cavity. In 1972, Vagn-Hansen et al3 reported
a case of intracranial hibernoma. Due to medical
constraints at the time, detailed case descriptions of
CT and MRI images were not available. Therefore,
the diagnosis of this patient has significant clinical
significance and academic value. The rarity of
intracranial hibernomas in clinical diagnosis can
easily be overlooked or misdiagnosed as other more
common intracranial tumours such as meningiomas.
Clinicians and pathologists need to remain highly
vigilant for this rare tumour to ensure timely and
accurate diagnosis and treatment.
Imaging characteristics of hibernomas
In radiology, differentiation between hibernomas and
meningiomas is challenging. Hibernomas typically
present as low- to medium-intensity on T1-weighted
MRI and high intensity on T2-weighted MRI. Due
to their origin from adipose tissue, hibernomas
may show signal suppression in the fat-suppressed
sequence of MRI. Due to the rich vascular supply
inside the tumour, MRI post-contrast enhancement
is evident. Also, due to the high fat content of
hibernomas, they may appear as low density or iso-density
lesions on CT scans.
Meningiomas typically present as iso- to high-intensity
on both T1- and T2-weighted MRI and show
significant uniform enhancement following contrast
administration. Meningiomas often accompany the
dural tail sign, an important distinguishing feature in
imaging. Furthermore, the density of meningiomas
on CT scans is quite uniform, with significant
enhancement following contrast administration.
Therefore, while there is overlap in imaging between
hibernomas and meningiomas, detailed radiological
analysis, especially the combination of fat-suppressed sequences and tumour enhancement characteristics,
can help distinguish the two and increase diagnostic
accuracy.
Pathological characteristics of hibernomas
Pathological diagnosis is the gold standard for
confirming hibernomas. Histological features include
small multilocular brown adipocytes that have a rich
granular cytoplasm and central or eccentric round or oval nuclei. Unlike the single large lipid droplet in
white adipocytes, brown adipocytes contain multiple
small lipid droplets and abundant mitochondria,
giving the cytoplasm a granular appearance. In
addition, hibernomas usually have a rich vascular
network. Immunohistochemical staining showing
vascular CD34 positivity can further confirm the
diagnosis. Hibernoma cells have varying degrees of
S-100 protein and CD34 expression according to the
literature.4 5 Combining these pathological features
can effectively distinguish hibernomas from other
intracranial tumours such as meningiomas.
Conclusion
The diagnostic process of this case of intracranial
hibernoma emphasises the importance of clinicians
and pathologists when facing uncommon intracranial
tumours. Detailed imaging analysis and pathological
examination facilitate accurate differential diagnosis,
providing the best treatment plan for patients. More
case reports and research are needed to further
enrich the understanding and treatment strategies
of intracranial hibernomas.
Author contributions
All authors contributed to the concept or design, drafting of
the manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgement
The authors thank Dr Yanqing
Li and Dr Qinyi Wang from the Department of Pathology
at Suzhou TCM Hospital Affiliated to Nanjing University of
Chinese Medicine for their expertise and assistance in the
diagnosis and analysis of the patient’s condition.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Ethics Committee of Suzhou
TCM Hospital Affiliated to Nanjing University of Chinese
Medicine, China (Ref No.: 2024-005). The patient was treated in
accordance with the Declaration of Helsinki. The patient
provided written consent for publication of this case report.
References
1. Klevos G, Jose J, Pretell-Mazzini J, Conway S. Hibernoma.
Am J Orthop (Belle Mead NJ) 2015;44:284-7.
2. Furlong MA, Fanburg-Smith JC, Miettinen M. The
morphologic spectrum of hibernoma: a clinicopathologic
study of 170 cases. Am J Surg Pathol 2001;25:809-14. Crossref
3. Vagn-Hansen PL, Osgård O. Intracranial hibernoma.
Report of a case. Acta Pathol Microbiol Scand A
1972;80:145-9. Crossref
4. Vassos N, Lell M, Hohenberger W, Croner RS, Agaimy A.
Deep-seated huge hibernoma of soft tissue: a rare differential diagnosis of atypical lipomatous tumor/well differentiated
liposarcoma. Int J Clin Exp Pathol 2013;6:2178-84.
5. Suster S, Fisher C. Immunoreactivity for the human
hematopoietic progenitor cell antigen (CD34) in
lipomatous tumors. Am J Surg Pathol 1997;21:195-200. Crossref