Hong Kong Med J 2023 Oct;29(5):453–5 | Epub 27 Sep 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Calcific myonecrosis misdiagnosed as right leg abscess: a case report
CY Lee, MB, BS1; CH Lin, MB, BS2
1 Department of Medical Imaging, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
2 Department of Medical Imaging, Chi Mei Medical Center, Tainan, Taiwan
Corresponding author: Mr CH Lin (chienhung0822@gmail.com)
Case presentation
A 63-year-old man attended hospital with a 2-year
history of a slow-growing painful mass in his right
leg. He had undergone successful surgical drainage
of an abscess. Plain X-ray of the right leg at that time
(Fig 1a) revealed a longitudinally distributed fusiform
mass (29.4 cm×5.1 cm) with plaque-like amorphous
calcifications at the right anterolateral aspect. No
obvious periosteal reaction or cortical destruction
of the tibia or fibula was noted. There was varus
deformity of the tibia with medial cortex thickening
and angulation that suggested old fracture. He had
sustained a crush injury to the right leg 10 years
previously and developed peroneal nerve injury with
foot drop.
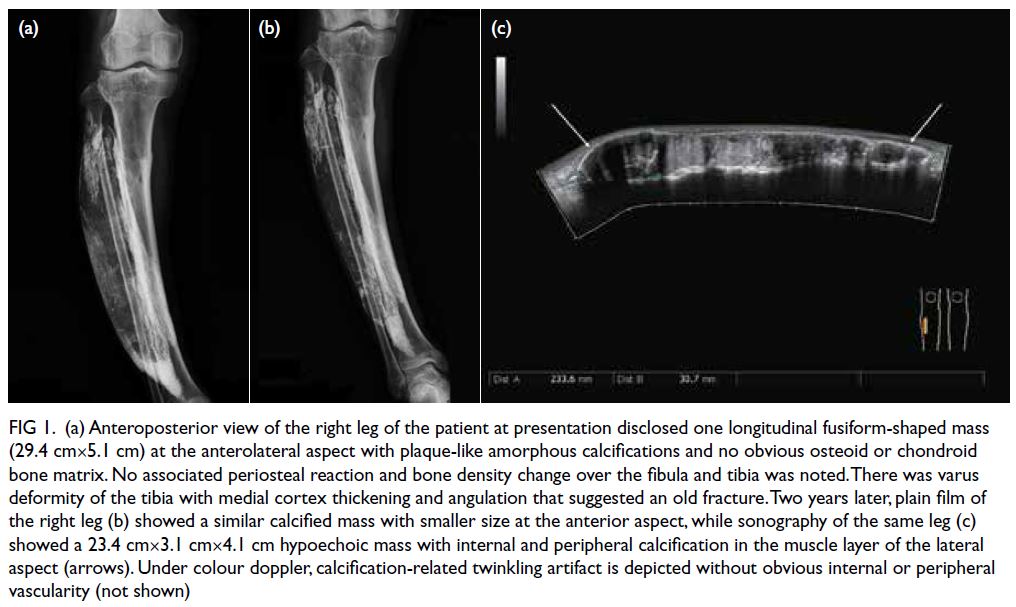
Figure 1. (a) Anteroposterior view of the right leg of the patient at presentation disclosed one longitudinal fusiform-shaped mass (29.4 cm×5.1 cm) at the anterolateral aspect with plaque-like amorphous calcifications and no obvious osteoid or chondroid bone matrix. No associated periosteal reaction and bone density change over the fibula and tibia was noted. There was varus deformity of the tibia with medial cortex thickening and angulation that suggested an old fracture. Two years later, plain film of the right leg (b) showed a similar calcified mass with smaller size at the anterior aspect, while sonography of the same leg (c) showed a 23.4 cm×3.1 cm×4.1 cm hypoechoic mass with internal and peripheral calcification in the muscle layer of the lateral aspect (arrows). Under colour doppler, calcification-related twinkling artifact is depicted without obvious internal or peripheral vascularity (not shown)
Physical examination of the patient revealed a
mass at the anterolateral aspect of the right leg. There
were no skin changes, redness or tenderness. The
patient was afebrile. Biochemical and haematological investigations were normal. The range of motion of
the right lower limb was not impaired but obvious
muscle atrophy at the posterolateral compartment
of the right leg was noted. Further imaging was
requested due to concerns about malignancy.
The plain film of the right leg 2 years later
(Fig 1b) showed a similar but smaller calcified mass
at the anterior aspect. A 23.4 cm×3.1 cm×4.1 cm
corresponding hypoechoic mass with internal and
peripheral calcification foci in the muscle layer
of the lateral aspect of the same leg and paucity of
vascularity was observed under lower extremity
sonography examination using Toshiba Aplio 500
(Canon Medical Systems, Tustin [CA], US) [Fig 1c].
Further magnetic resonance imaging study using
Discovery MR750 (GE Healthcare, Chicago [IL], US)
revealed an elongated and lobulated intramuscular
cystic mass with variable internal T1- or T2-weighted signal intensity and low-signal peripheral rim (Fig 2a and 2b). Mild peripheral enhancement
after intravenous gadolinium administration was
noted (Fig 2c). Additional findings included varus
deformity of the right tibia and severe muscle
atrophy with muscle fibrosis involving the deep
posterior and lateral compartments of the right leg.
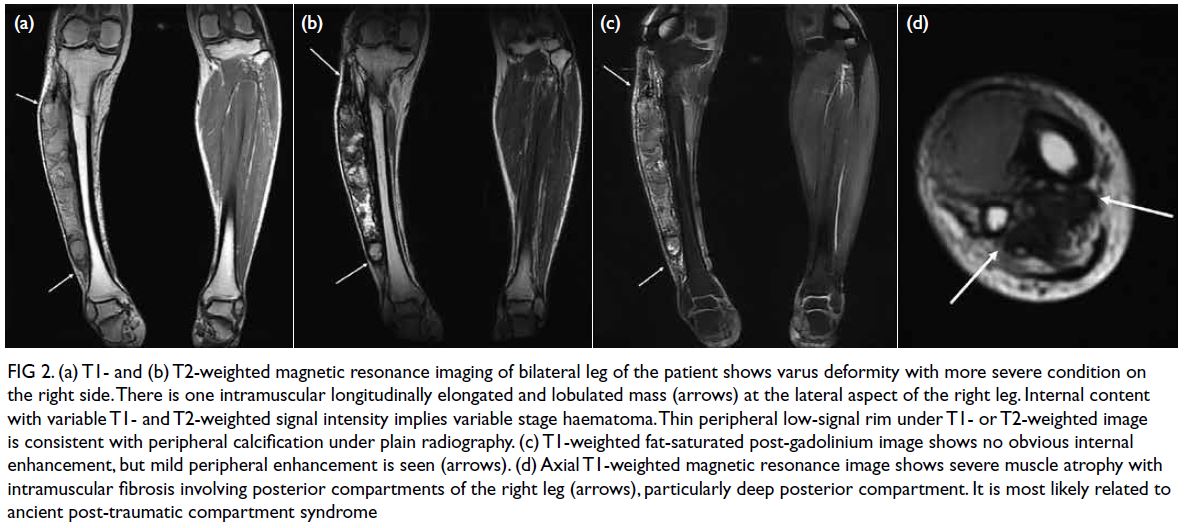
Figure 2. (a) T1- and (b) T2-weighted magnetic resonance imaging of bilateral leg of the patient shows varus deformity with more severe condition on the right side. There is one intramuscular longitudinally elongated and lobulated mass (arrows) at the lateral aspect of the right leg. Internal content with variable T1- and T2-weighted signal intensity implies variable stage haematoma. Thin peripheral low-signal rim under T1- or T2-weighted image is consistent with peripheral calcification under plain radiography. (c) T1-weighted fat-saturated post-gadolinium image shows no obvious internal enhancement, but mild peripheral enhancement is seen (arrows). (d) Axial T1-weighted magnetic resonance image shows severe muscle atrophy with intramuscular fibrosis involving posterior compartments of the right leg (arrows), particularly deep posterior compartment. It is most likely related to ancient post-traumatic compartment syndrome
The tibial and fibular bones were intact and
without involvement. No regional inflammatory
change around the calcified cystic mass, prominent
vascularity and significant peripheral enhancement
was observed. The findings were consistent with
calcific myonecrosis and muscle fibrosis, which
is most likely related to a remote post-traumatic
compartment syndrome (Fig 2d). In the absence of
any symptoms that impacted daily living, the patient
declined invasive treatment and opted for regular
follow-up.
Discussion
Calcific myonecrosis is a rare, delayed manifestation of post-traumatic muscle injury or sequelae of
neurovascular injury that occurs almost exclusively
in the lower limbs.1 It is likely due to previous muscle
injury complicated by compartment syndrome.1
Muscle injury may be a result of trauma, nerve
injury with denervation, snake bite complicated by
compartment syndrome,2 chronic inflammation (eg,
dermatomyositis),3 or repeated contraction due to
static epilepsy.4
The most common site of compartment
syndrome in the leg is the anterior compartment
followed by the lateral and deep posterior compartment. Compartment syndrome results
in chronic vascular insufficiency and ischaemic
change to muscular tissue. Following long-term
hypoperfusion, muscles become necrotic and
fibrosed with a consequent reduction in mass and
development of contracture. If extensive necrosis
with cystic change, haematoma formation, and
calcification are present, calcific myonecrosis may
develop, evident as a slow-growing mass.
In most cases, calcific myonecrosis presents as a
painful or painless mass. Pain is likely due to pressure
on surrounding tissue. Signs of inflammation (skin
erythema, tenderness, and warmth) are often absent.
Cosmetic problems and concerns of malignancy (eg,
soft tissue sarcoma) are common reasons for seeking
medical advice after decades of the disease course.
Plain radiography and computed tomography
usually reveal lesions confined to muscle
compartments in a longitudinally oriented
pattern with peripheral fusiform and amorphous
calcifications parallel to muscle orientation. Magnetic
resonance imaging usually shows a circumscribed
fusiform intramuscular cystic mass with the
peripheral calcification rim in the longitudinally
oriented pattern as muscle bulk. The internal content
usually shows variable signal intensities in T1- and
T2-weighted images that depend on the internal
content (proteinaceous necrotic fluid, serous fluid,
and different blood stages). Mild peripheral rim
enhancement may be observed following intravenous
gadolinium administration. The central cystic part
lacks enhancement secondary to extensive necrosis.
Post-compartment syndrome–related muscle atrophy, fibrosis, and contracture usually coexist in
these disease entities.1 Other common differential
diagnoses should be excluded including myositis
ossificans, soft tissue sarcoma, and musculoskeletal
tuberculosis.5 6
Calcific myonecrosis is essentially benign
although previous reports have revealed
postoperative complications such as sinus tract
formation,7 poor wound healing, and secondary
infection. This implies that wound healing is difficult,
probably due to previous compartment syndrome
and chronic microvascular insufficiency. In cases
of uninfected calcific myonecrosis, observation
is the recommended management given the high
risk of postoperative complications. Nonetheless
cosmetic problems and symptoms may persist.1 For
calcific myonecrosis with infection, conservative
management with wound dressing and antibiotics
is usually not sufficient. Extensive debridement,
removal of diseased tissue, and flap coverage are
recommended.8 Secondary surgery may be needed
if postoperative complications (poor wound healing,
secondary infection, and sinus tract formation)
are observed. Advanced wound management (eg,
negative pressure wound therapy) facilitates healing
with a moist environment and topical negative
pressure.8 9
In our patient, the calcified mass in his right
leg 2 years previously had been drained since
abscess was suspected. Plain X-ray at that time
suggested calcific myonecrosis or other differential
diagnoses (myositis ossificans, soft tissue sarcoma,
etc.). Thus, further magnetic resonance imaging
surveys and more details about a previous crush
injury would have been helpful to reach a diagnosis
and avoid unnecessary surgery. Knowledge of
calcific myonecrosis is important for clinicians and
radiologists to optimise patient care.
Conclusion
Calcific myonecrosis is a benign disease process.
Generally, ‘do not touch’ is a treatment strategy.
Prompt recognition, proper diagnosis based on
imaging, and detailed history taking are important.
Author contributions
Concept or design: CH Lin.
Acquisition of data: CY Lee.
Analysis or interpretation of data: CY Lee.
Drafting of the manuscript: CY Lee.
Critical revision of the manuscript for important intellectual content: Both authors.
Acquisition of data: CY Lee.
Analysis or interpretation of data: CY Lee.
Drafting of the manuscript: CY Lee.
Critical revision of the manuscript for important intellectual content: Both authors.
Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Both authors declared no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and has provided informed consent for the
treatment/procedures, and consent for publication.
References
1. O’Dwyer HM, Al-Nakshabandi NA, Al-Muzahmi K, Ryan A,
O’Connell JX, Munk PL. Calcific myonecrosis: keys to
recognition and management. AJR Am J Roentgenol
2006;187:W67-76. Crossref
2. Yuenyongviwat V, Laohawiriyakamo T, Suwanno P,
Kanjanapradit K, Tanutit P. Calcific myonecrosis following
snake bite: a case report and review of the literature. J Med
Case Rep 2014;8:193. Crossref
3. Batz R, Sofka CM, Adler RS, Mintz DN, DiCarlo E.
Dermatomyositis and calcific myonecrosis in the leg:
ultrasound as an aid in management. Skeletal Radiol
2006;35:113-6. Crossref
4. Karkhanis S, Botchu R, James S, Evans N. Bilateral
calcific myonecrosis associated with epilepsy. Clin Radiol
2013;68:e349-52. Crossref
5. De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A,
De Schepper AM. Imaging features of musculoskeletal
tuberculosis. Eur Radiol 2003;13:1809-19. Crossref
6. Burrill J, Williams CJ, Bain G, Conder G, Hine AL,
Misra RR. Tuberculosis: a radiologic review. Radiographics
2007;27:1255-73. Crossref
7. Jeong BO, Chung DW, Baek JH. Management of calcific
myonecrosis with a sinus tract: a case report. Medicine
(Baltimore) 2018;97:e12517. Crossref
8. Sreenivas T, Nandish Kumar KC, Menon J, Nataraj AR.
Calcific myonecrosis of the leg treated by debridement
and limited access dressing. Int J Low Extrem Wounds
2013;12:44-9. Crossref
9. Tan AM, Loh CY, Nizamoglu M, Tare M. A challenging
case of calcific myonecrosis of tibialis anterior and hallucis
longus muscles with a chronic discharging wound. Int
Wound J 2018;15:170-3. Crossref

