Indocyanine green fluorescence-guided pulmonary wedge resection in a child: a case report
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Indocyanine green fluorescence-guided
pulmonary wedge resection in a child:
a case report
CH Fung, MB, BS, MRCS; CT Lau, MB, BS, FRCS; Kenneth KY Wong, PhD, FRCS
Department of Surgery, The University of Hong Kong, Hong Kong
Corresponding author: Dr CH Fung (fungchiheng@gmail.com)
Case report
Tissue diagnosis of pulmonary nodules of an
undetermined nature can be achieved with
thoracoscopic wedge resection. However, localisation
of a lesion during surgery can be technically
demanding, especially for small deep-seated lesions.
We report our technique of indocyanine green (ICG)
fluorescence-guided pulmonary wedge resection in
a child.
A 4-year-old boy presented with pyrexia of
unknown origin associated with cough for 2 weeks.
Extensive septic workup including sputum culture,
chest X-ray, nasopharyngeal aspirate and urine
culture were unremarkable. Mantoux test was
positive. Computed tomographic (CT) scan of the
thorax to look for occult chest infection showed
features suggestive of pulmonary tuberculosis and
a 6-mm nodule over the apical segment of the left
lower lobe. He completed a 6-month course of
antitubercular medication but reassessment scan
after 9 months showed a persistent left lower lobe
pulmonary nodule and he was referred to our
surgical unit for tissue diagnosis.
The patient underwent CT-guided localisation
of the pulmonary nodule under general anaesthesia
1 hour prior to thoracoscopic wedge resection.
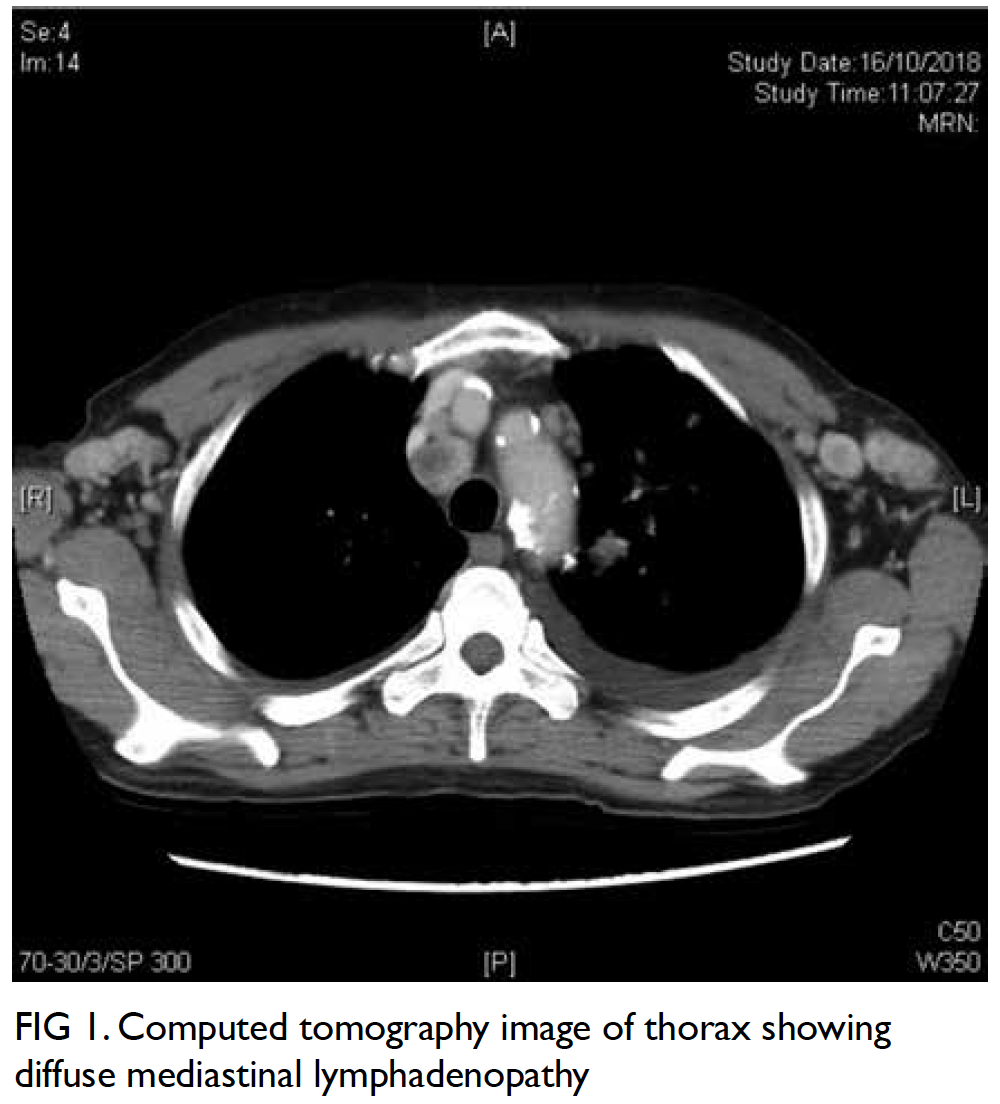
The pulmonary nodule was identified at the apical
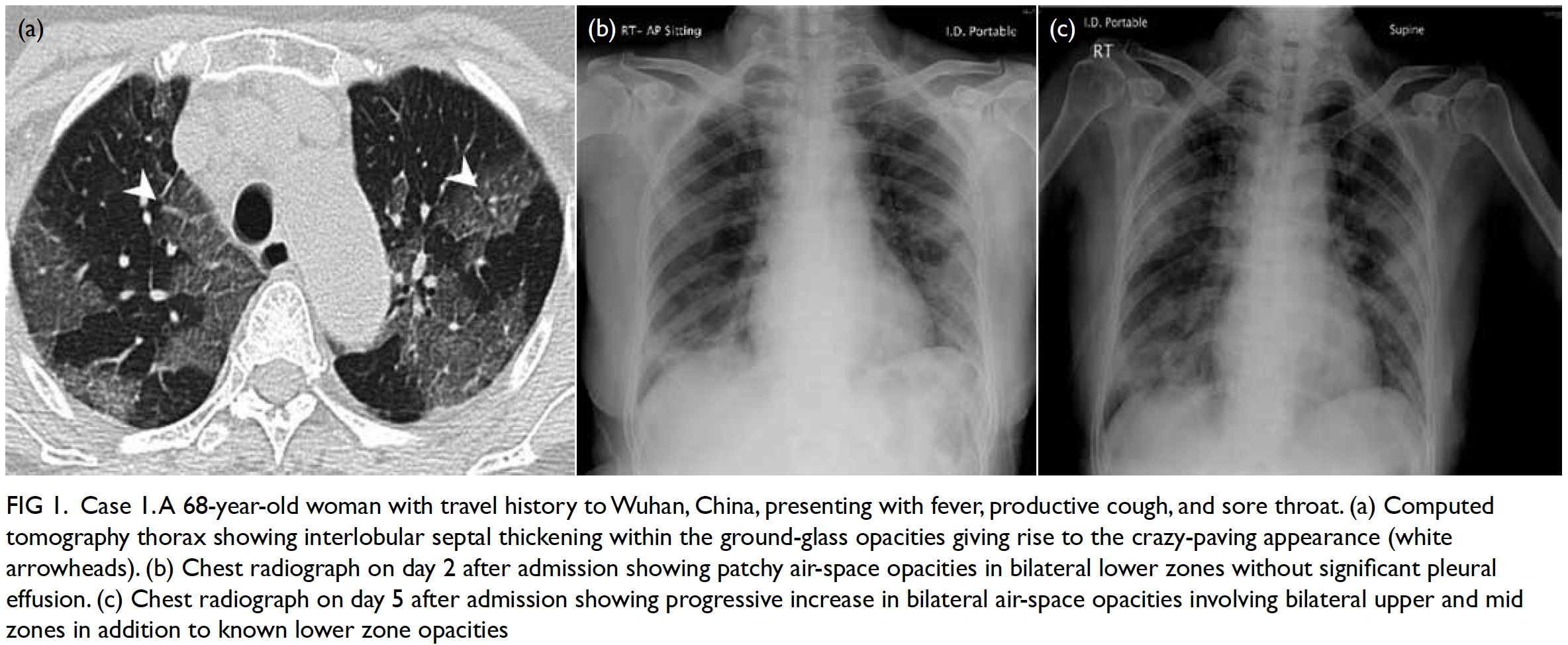
segment of the left lower lobe, 1.3 cm from the
pleural surface (Fig 1). An 18-gauge guiding needle
was inserted by the radiologist to the lesion under
CT guidance. Methylene blue (0.5 mL) and ICG
(0.5 mL) were injected around the lesion via the
guiding needle. A hookwire was also placed for
localisation as safety backup and adjunct to ICG.
The patient was then transferred back to the
operating theatre. The target lesion was identified
thoracoscopically with guidance of methylene blue
dye and ICG fluorescence (KARL STORZ OPAL1®).
Wedge resection of the target lesion was performed
with Endo GIA™ Ultra Universal 30-mm staplers
(Medtronic). Complete excision was confirmed by
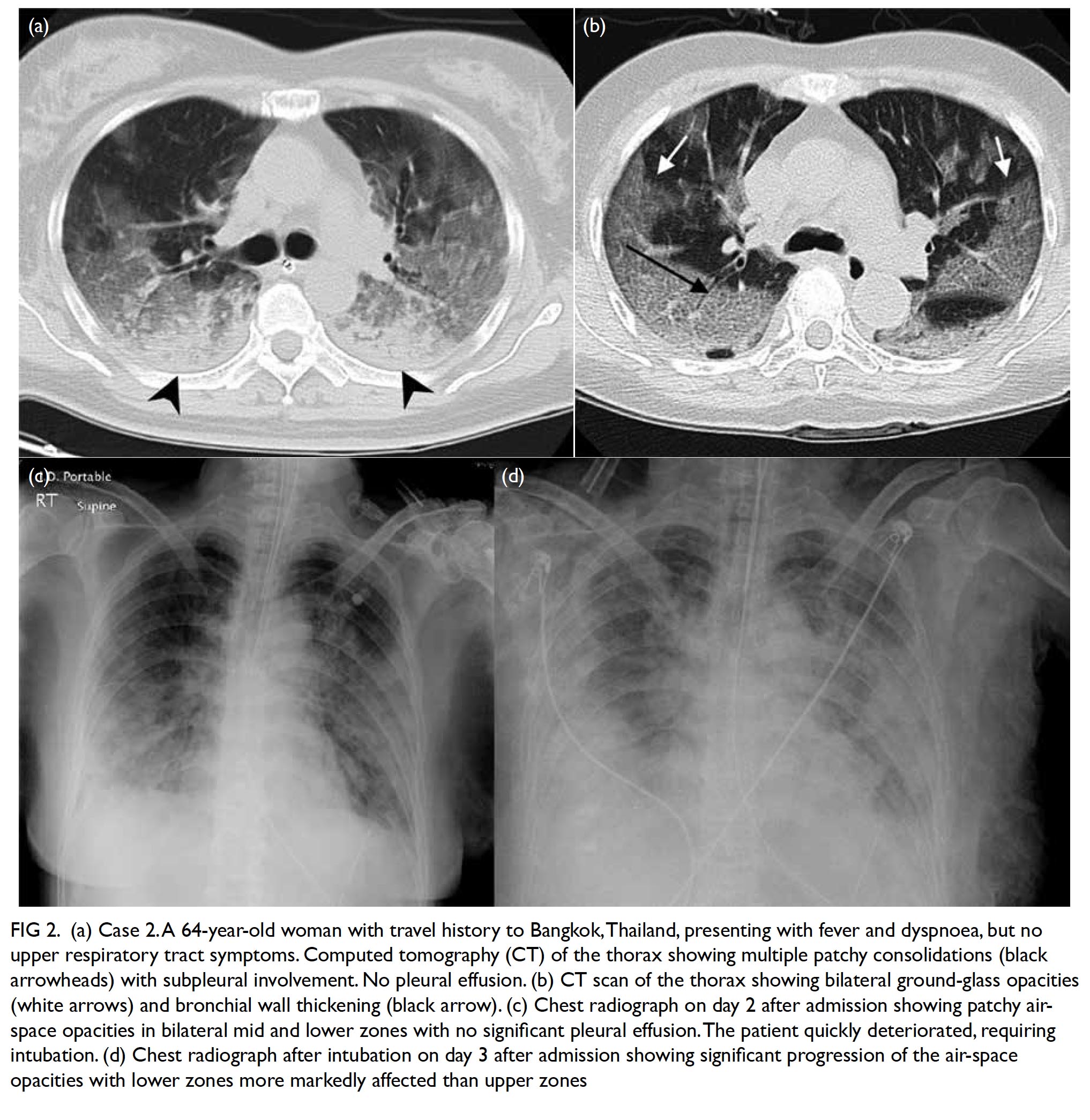
absence of fluorescence in the remaining left lower
lobe on ICG fluorescence imaging (Fig 2). The use
of ICG fluorescence enhanced intra-operative
localisation of the small pulmonary nodule and facilitated a minimally invasive operation. The patient
made an uneventful recovery and was discharged
2 days after surgery. Histology of the wedge-resected
specimen confirmed complete excision and showed
granulomatous inflammation with focal necrosis and
no evidence of malignancy. The patient had resumed
full activity at follow-up 1 week after surgery.
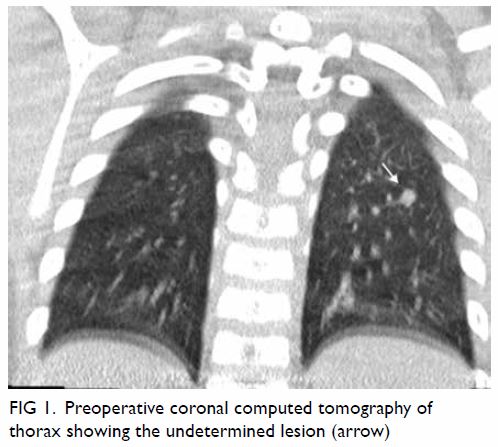
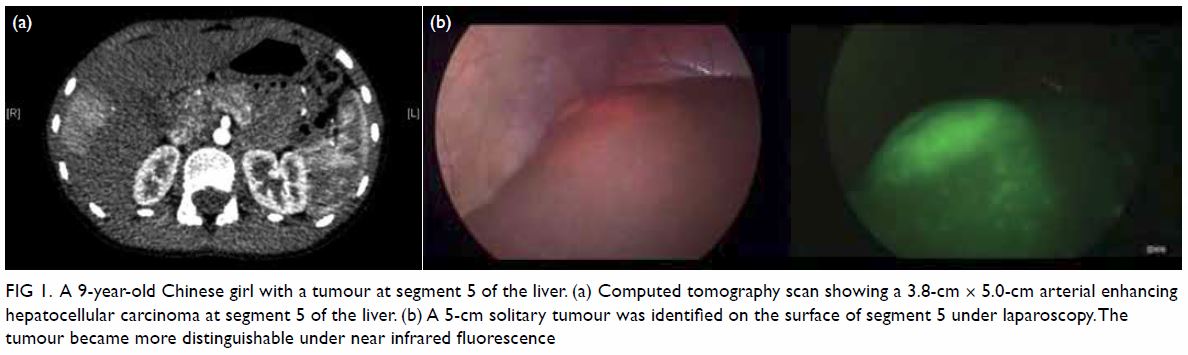
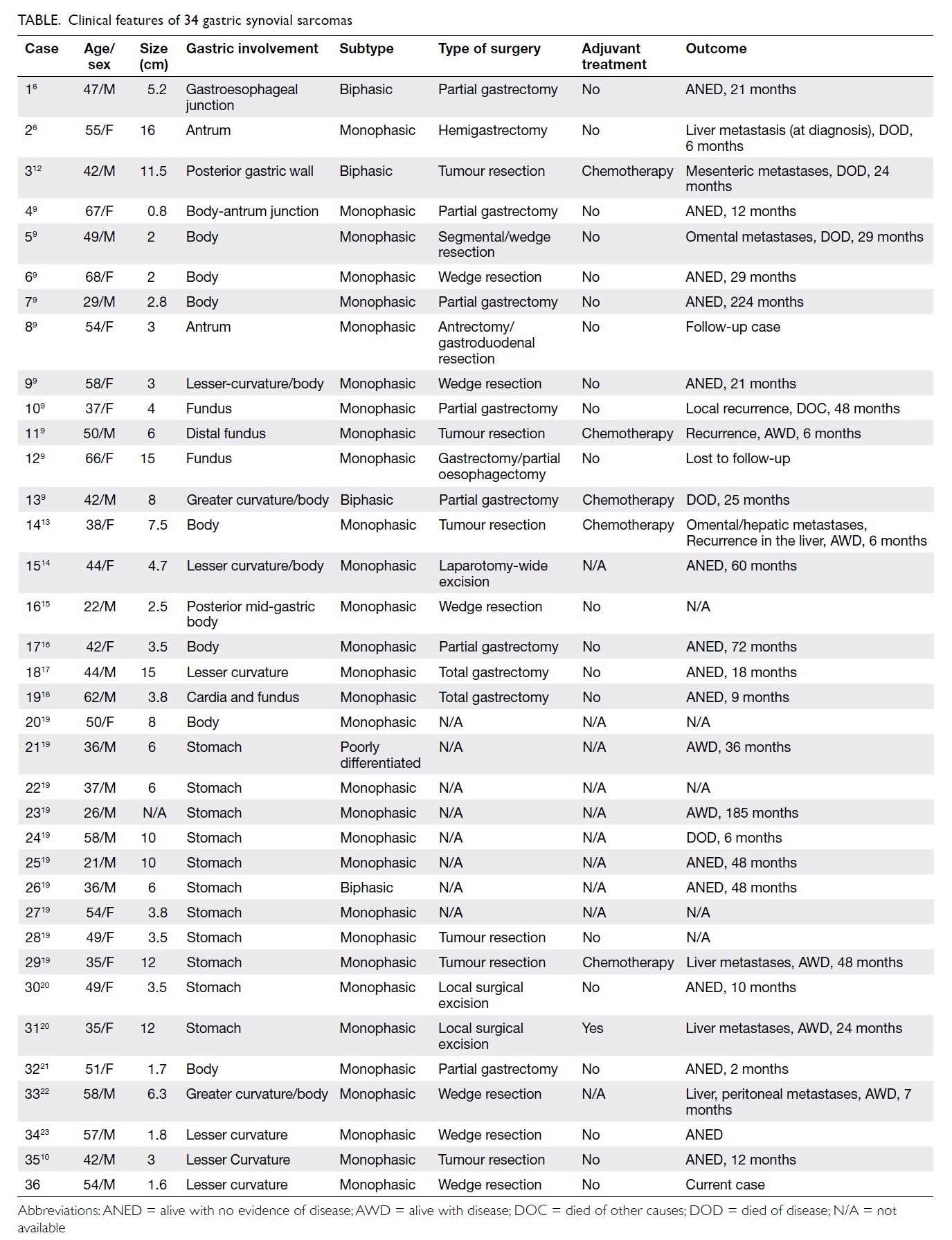
Figure 1. Preoperative coronal computed tomography of thorax showing the undetermined lesion (arrow)
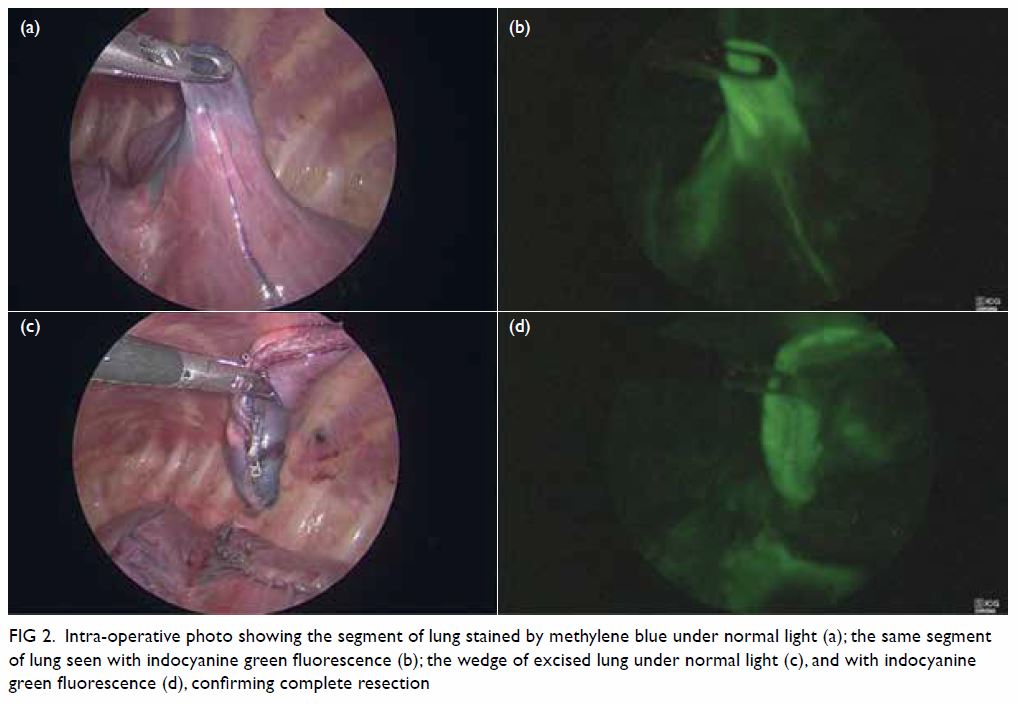
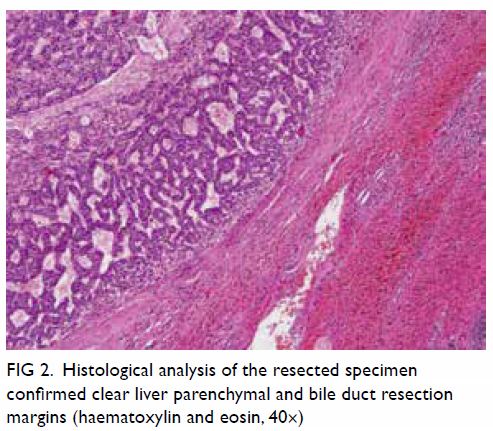
Figure 2. Intra-operative photo showing the segment of lung stained by methylene blue under normal light (a); the same segment of lung seen with indocyanine green fluorescence (b); the wedge of excised lung under normal light (c), and with indocyanine green fluorescence (d), confirming complete resection
Discussion
Intra-operative localisation of small pulmonary
nodules remains a challenge for prompt and
complete resection of lesions. It is recommended
that preoperative localisation should be performed
prior to minimally invasive resection for pulmonary
nodules <10 mm in diameter or >5 mm from the
pleural surface.1 Various means of preoperative
localisation have been reported in children including
micro-coil insertion, methylene blue dye injection,
and radiotracer labelling.2 Hookwire is reported to
be safe and useful for localisation of lung nodules
in children as well as other situations such as
thoracoscopic resection of deep-seated congenital
cystic adenomatoid malformation.2 3 Although these
methods are considered feasible in children, they
have their own limitations and risks, such as local trauma and issues of inaccuracy in small lesions
for hookwire localisation; difficulty in revealing
deep lesions and diffuse spillage in small lesions of
methylene blue dye. Efforts have been made to find
a convenient, safe, and clear method for localisation.
The United States Food and Drug
Administration has recently extended the approved
uses of ICG to include sentinel lymph node biopsy
in various types of tumour, assessment of blood
supply in anastomosis and reconstructive flaps and
enhanced visualisation of biliary anatomy during
laparoscopic cholecystectomy.4 In thoracic surgery,
it is a useful aid to sentinel lymph node mapping,
lung mapping, oesophageal conduit vascular
perfusion, and lung nodule identification.5 To date,
no data about the use of ICG in thoracoscopic wedge
resection in paediatric patients have been reported.
In our experience, ICG preoperative localisation is a
safe and feasible means to help achieve prompt and
complete thoracoscopic wedge resection of small
pulmonary nodules. It appeared to be more accurate
than hookwire localisation in this patient, allowing
immediate clear visualisation when guiding the
extent of resection. It also facilitated easy assessment
of completeness of resection.
In conclusion, ICG fluorescence is a safe and
feasible method of localisation prior to thoracoscopic
wedge resection of a pulmonary nodule in children.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
As an editor of the journal, KKY Wong was not involved in
the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This case report received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided written informed consent for
all procedures.
References
1. Suzuki K, Nagai K, Yoshida J, et al. Video-assisted
thoracoscopic surgery for small indeterminate pulmonary
nodules: indications for preoperative marking. Chest
1999;115:563-8. Crossref
2. Polites SF, Fahy AS, Sunnock WA, et al. Use of radiotracer labeling of pulmonary nodules to facilitate excisional
biopsy and metastasectomy in children with solid tumors.
J Pediatr Surg 2018;53:1369-73. Crossref
3. Lau CT, Wong KK. Thoracoscopic resection of congenital
cystic adenomatoid malformation in a patient with
fused lung fissure using hookwire. Innovations (Phila)
2018;13:226-9. Crossref
4. Namikawa T, Sato T, Hanazaki K. Recent advances in
near-infrared fluorescence-guided imaging surgery using
indocyanine green. Surg Today 2015;45:1467-74. Crossref
5. Okusanya OT, Hess NR, Luketich JD, Sarkaria IS. Infrared
intraoperative fluorescence imaging using indocyanine
green in thoracic surgery. Eur J Cardiothorac Surg
2018;53:512-8. Crossref