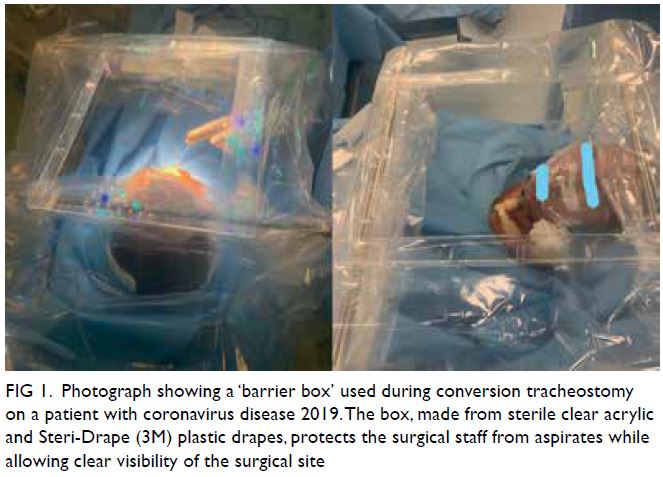
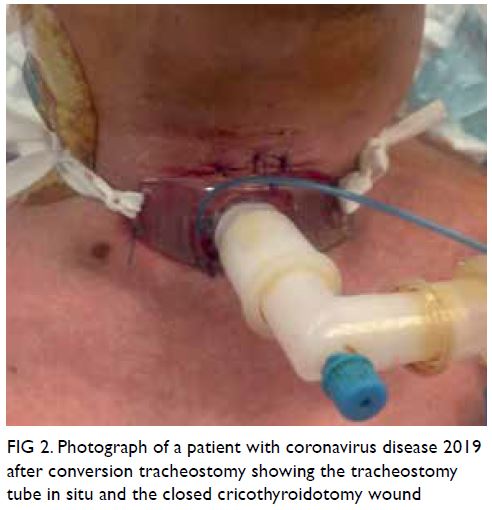
Novel diaphragmatic reconstruction technique for recurrent diaphragmatic hernia: a case report
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Novel diaphragmatic reconstruction technique
for recurrent diaphragmatic hernia: a case report
Teddy HY Wong, MRCS; Simon CY Chow, FRCS; Peter SY Yu, MRCS; Jacky YK Ho, FRCS; Rainbow WH Lau, FRCS; Innes YP Wan, FRCS; Randolph HL Wong, FRCS
Division of Cardiothoracic Surgery, Department of Surgery, Prince of Wales Hospital, Hong Kong
Corresponding author: Dr Randolph HL Wong (wonhl1@surgery.cuhk.edu.hk)
Case report
A 51-year-old lady with a history of congenital
diaphragmatic hernia repair during infancy
presented to the emergency department with
increasing abdominal pain and repeated vomiting.
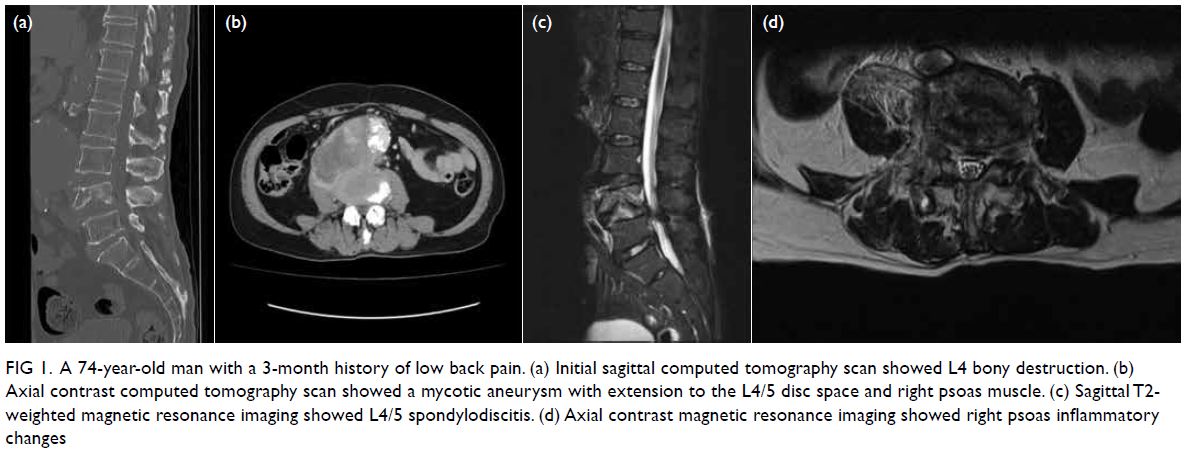
Posteroanterior chest plain radiograph revealed
dilated small bowel loops in the right thoracic cavity.
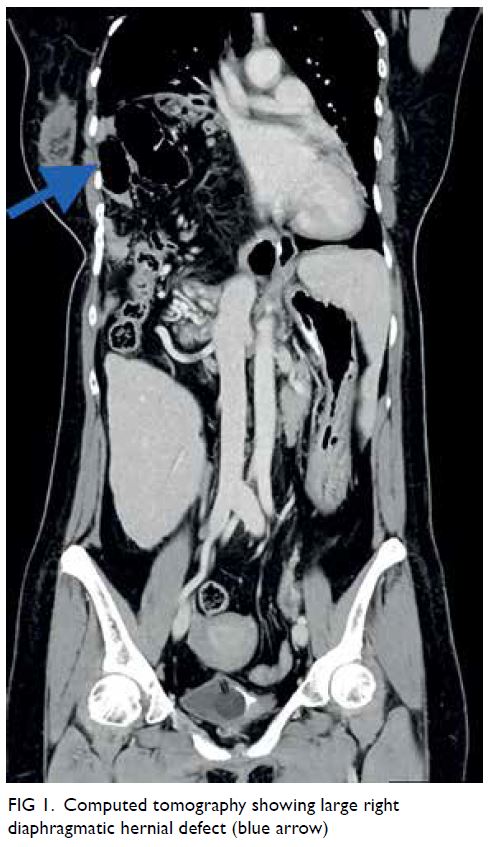
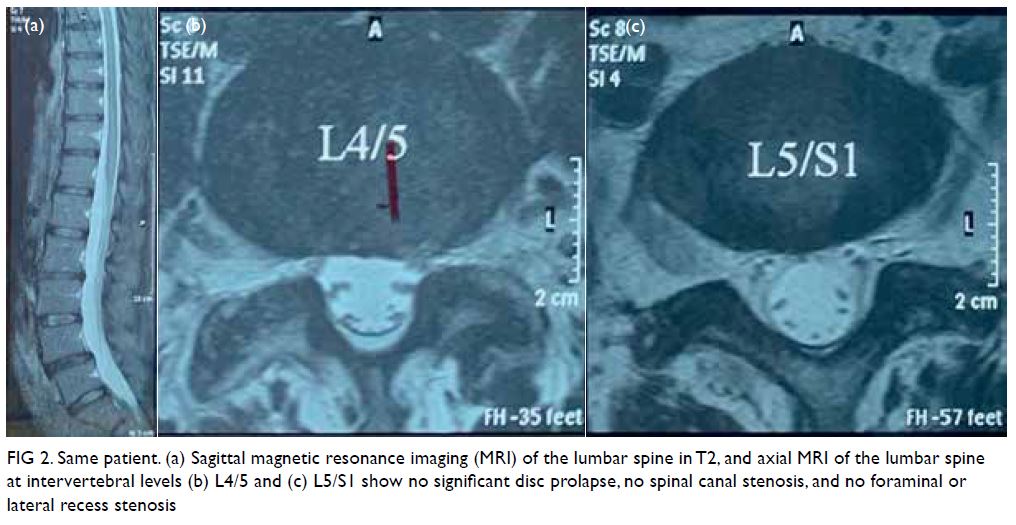
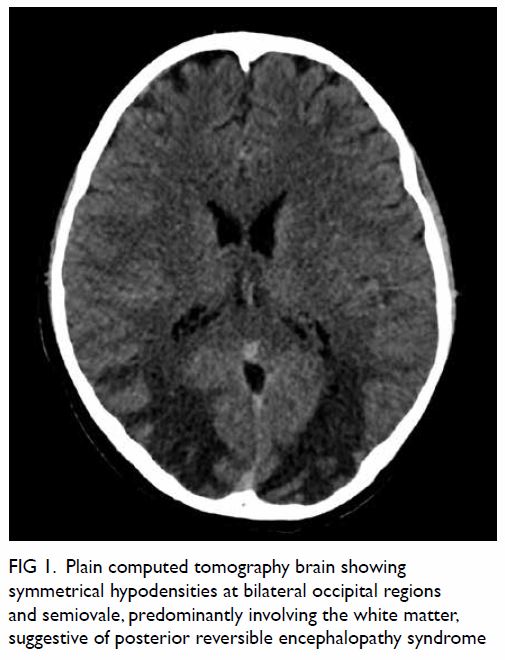
Computed tomography scan revealed two closely
related small bowel loops trapped within a narrow
hernia neck orifice across the diaphragm, suggestive
of closed loop intestinal obstruction (Fig 1). Blood
test results revealed metabolic acidosis and elevated
lactate level.
Emergency laparotomy via a subcostal
incision revealed a right-sided large diaphragmatic
defect with bowel herniating into the thoracic
cavity. Transabdominal reduction of the bowel was
difficult owing to adhesion of bowel to the lung
so an additional posterolateral thoracotomy was
performed by a cardiothoracic team. Adhesiolysis
was performed and the small bowel freed and
reduced to the abdominal cavity via the transthoracic
approach. Examination through the thoracotomy
revealed minimal residual diaphragmatic tissue
(Fig 2a). Primary closure was not possible and repair
with a patch posed a technical challenge in the
absence of sufficient residual diaphragmatic tissue
to enable secure anchorage. For the anterolateral
portion, multiple pledgetted 3-O sutures were
passed through the intercostal space for anchorage
(Fig 2b). A neo-diaphragm was reconstructed
using a porcine dermal collagen implant of size
150 mm × 200 mm × 1 mm (PermacolTM; Medtronic,
Minneapolis [MN], United States). The patch was
then parachuted down to cover the defect and
secured. A 4-cm flutter-valve patch was directed
downwards and medially to avoid suturing and
injury to the oesophagus and inferior vena cava
(Fig 2c). We believe this design permits peristalsis of
oesophageal content without causing stricture while
also preventing future intestinal herniation.
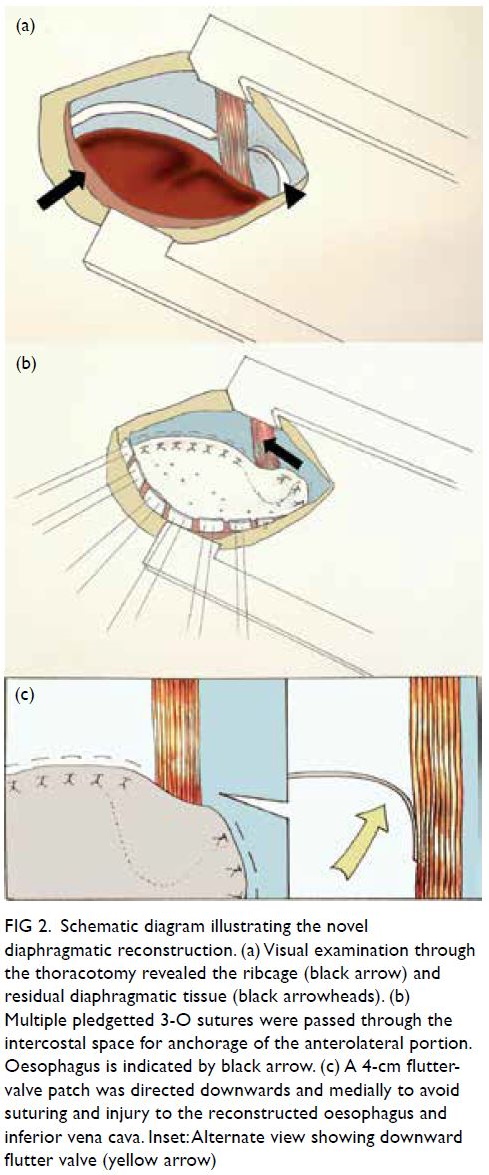
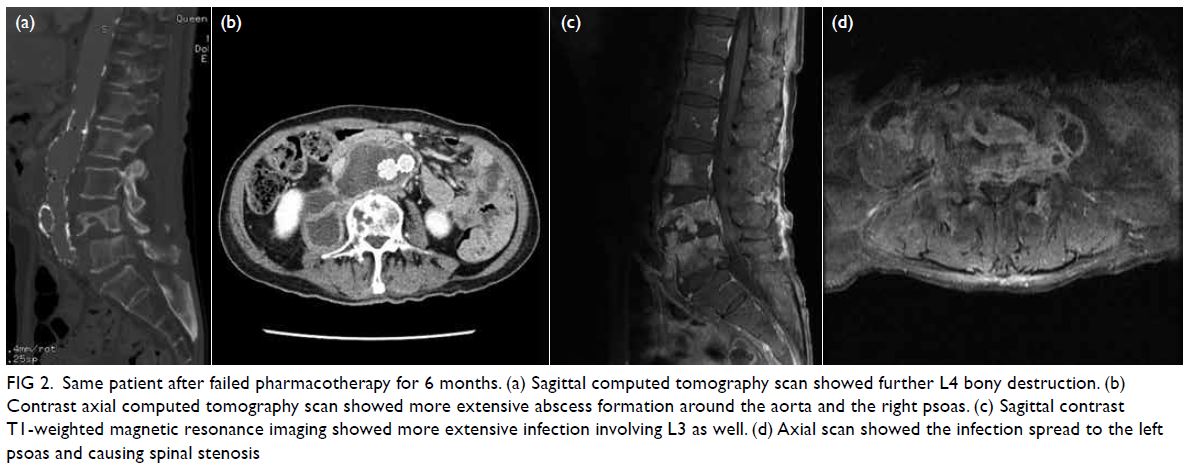
Figure 2. Schematic diagram illustrating the novel diaphragmatic reconstruction. (a) Visual examination through the thoracotomy revealed the ribcage (black arrow) and residual diaphragmatic tissue (black arrowheads). (b) Multiple pledgetted 3-O sutures were passed through the intercostal space for anchorage of the anterolateral portion. Oesophagus is indicated by black arrow. (c) A 4-cm flutter-valve patch was directed downwards and medially to avoid suturing and injury to the reconstructed oesophagus and inferior vena cava. Inset: Alternate view showing downward flutter valve (yellow arrow)
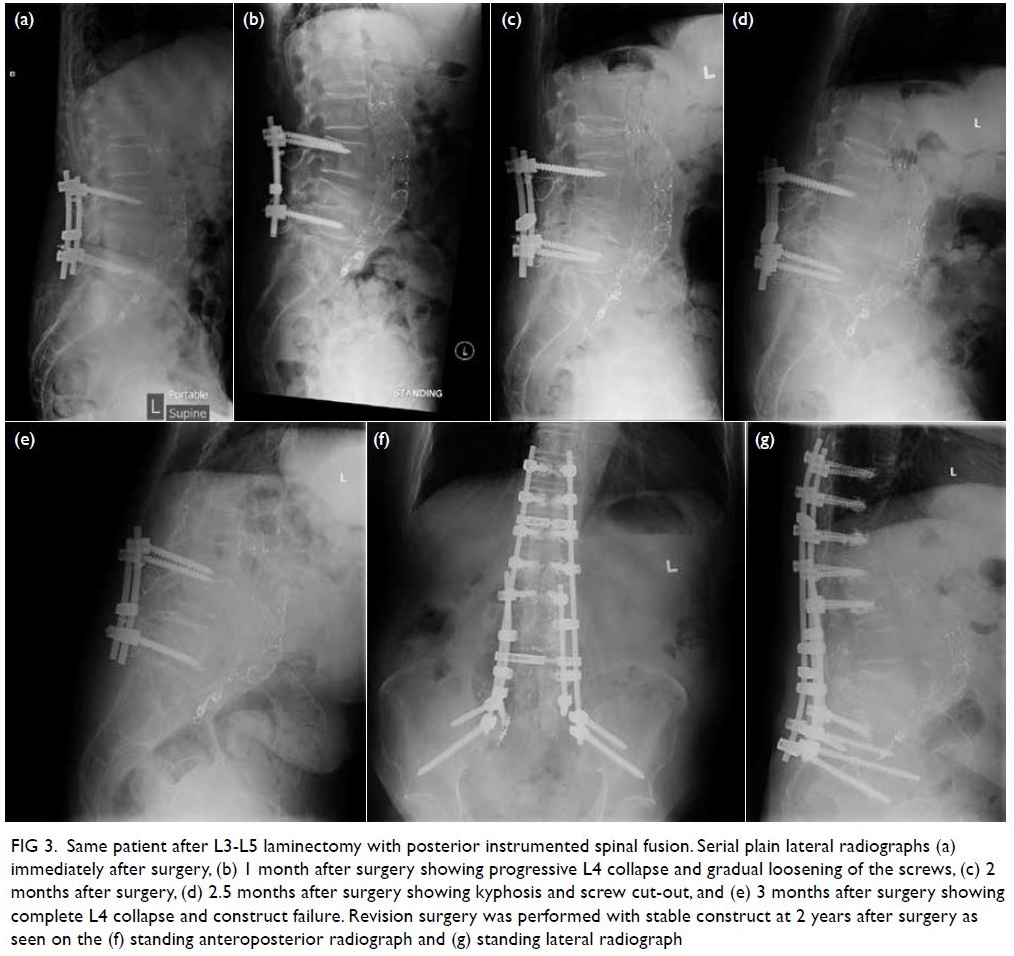
Postoperatively the patient was prescribed
total parenteral nutrition for 10 days and gradually
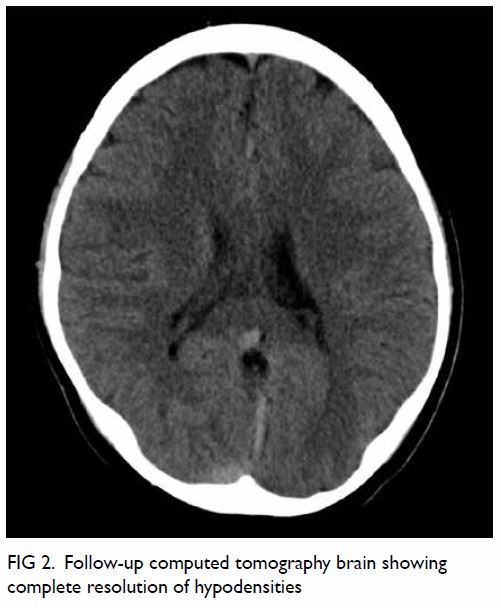
tolerated a normal diet. A computed tomography
scan at 10 days after surgery confirmed an intact neo-diaphragm with no recurrence of hernia. The
patient was discharged home 13 days after surgery.
She was well at follow-up examinations at 1 month
and 12 months after surgery. Chest plain radiograph
confirmed no recurrence of hernia (Fig 3).
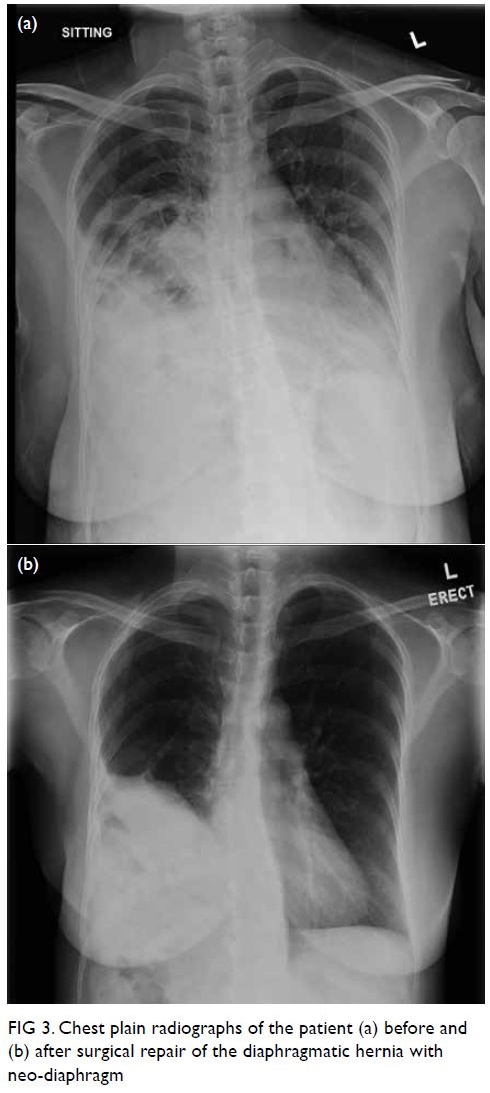
Figure 3. Chest plain radiographs of the patient (a) before and (b) after surgical repair of the diaphragmatic hernia with neo-diaphragm
Discussion
Diaphragmatic hernia is a rare but serious condition
associated with respiratory and gastrointestinal
complications and increased mortality.
Surgical repair is the gold standard treatment
to prevent complications. However, patients with a
previously repaired congenital diaphragmatic hernia
are more prone to respiratory complications during
childhood and occasionally develop recurrences.1
However, the long-term success rate of repair is
good with patients surviving well into adulthood
with a normal life expectancy.
Surgical options for repair include a primary repair and a patch repair. A patch repair is associated
with higher risks of complications and recurrence.2
However, for a hernia with large defects in which a primary repair is not possible, repair with surgical
mesh (xenograft or synthetic) is an effective and
safe method.3 In conventional patch repair, the
hernia sac is repositioned and resected. The hernia
defect is sized and the Permacol trimmed to cover
the defect. The flap is then fixed to the remaining
rim of diaphragmatic tissue with absorbable
sutures.4 The lack of an adequate rim of tissue
in our patient presented a unique challenge for
construction of the neo-diaphragm relative to
most instances of diaphragmatic hernia where
a remnant of diaphragmatic tissue is present for
anchorage. Although the pleura and adjacent
chest wall offer good support for anchoring the neo-diaphragm posteriorly and anterolaterally,
there is often a lack of strong tissue medially with
consequent substantial risk of inadvertent injury
to the oesophagus if sutures are placed too deeply.
Travers et al5 reported an incidence of oesophageal
injury up to 3.9% in their series of surgical repair
of para-oesophageal hernia using porcine dermal
collagen biologic mesh (Permacol). Innovative
designs in the creation of the neo-diaphragm have
been reported, but most studies have been in
paediatric patients.6 The technique described in
this case report is novel and has not been reported
elsewhere. The design of a flutter valve mechanism
in the reconstruction of the medial aspect of the
neo-diaphragm serves to create a tension-free repair
near the mediastinum and oesophagus while also
creating a seal to separate the pleural cavity from the
abdominal contents. No sutures were applied near the
oesophagus or the medial side of the neo-diaphragm.
This technique avoids inadvertent visceral injury
and is currently our preferred technique of neo-diaphragm
construction in patients with a large
diaphragmatic defect and insufficient rim.
In our case, we utilised the acellular dermal
matrix Permacol for hernia repair. Permacol is a
cross-linked graft comprised of acellular collagen
matrix. Compared with other collagen-based
implants, it offers long-lasting dimensional stability
and avoids loss of tensile strength as well as increased
tissue laxity resulting in lower rates of recurrence.
Acellular dermal matrix is a developing technology
and studies have shown promising results for its
efficacy.7
In conclusion, repair of diaphragmatic hernia
with a xenograft composed of dermal collagen
implant and a medial flutter-valve design is safe and
effective. It avoids inadvertent injury to mediastinal
structures while allowing satisfactory prevention of
recurrence of diaphragmatic herniation of intestines.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: THY Wong, SCY Chow, RHL Wong.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: THY Wong, SCY Chow, RHL Wong.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided written
informed consent for all treatments and procedures and
consent for publication.
References
1. Jancelewicz T, Chiang M, Oliveira C, Chiu PP. Late surgical
outcomes among congenital diaphragmatic hernia (CDH)
patients: why long-term follow-up with surgeons is
recommended. J Pediatr Surg 2013;48:935-41. Crossref
2. Jancelewicz T, Vu LT, Keller RL, et al. Long-term surgical
outcomes in congenital diaphragmatic hernia: observations
from a single institution. J Pediatr Surg 2010;45:155-60. Crossref
3. Kuhn R, Schubert D, Wolff St, Marusch F, Lippert H,
Pross M. Repair of diaphragmatic rupture by laparoscopic
implantation of a polytetrafluoroethylene patch. Surg
Endosc 2002;16:1495. Crossref
4. Lingohr P, Galetin T, Vestweber B, Matthaei H, Kalff JC,
Vestweber KH. Conventional mesh repair of a giant
iatrogenic bilateral diaphragmatic hernia with an
enterothorax. Int Med Case Rep J 2014;7:23-5. Crossref
5. Travers HC, Brewer JO, Smart NJ, Wajed SA.
Diaphragmatic crural augmentation utilising cross-linked
porcine dermal collagen biologic mesh (Permacol) in the
repair of large and complex para-oesophageal herniation: a
retrospective cohort study. Hernia 2016;20:311-20. Crossref
6. Loff S, Wirth H, Jester I, et al. Implantation of a cone-shaped
double-fixed patch increases abdominal space
and prevents recurrence of large defects in congenital
diaphragmatic hernia. J Pediatr Surg 2005;40:1701-5. Crossref
7. Buinewicz B, Rosen B. Acellular cadaveric dermis
(AlloDerm): a new alternative for abdominal hernia repair.
Ann Plast Surg 2004;52:188-94. Crossref