© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Malignant otitis externa complicated by multiple
cervical-petrous internal carotid artery pseudoaneurysms: a case
report
James SK Lau, BSc, MB, BS1,2; Jane CY
Wong, MB, BS1; Rebecca YT Ng, FCSHK, FHKAM (Surgery)1;
Vincent KY Pang, FRCS, FHKAM (Surgery)1; CK Wong, FRCS, FHKAM
(Surgery)1
1 Department of Neurosurgery, Pamela
Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Accident and Emergency Department,
Ruttonjee Hospital, Wan Chai, Hong Kong
Corresponding author: Dr Jane CY Wong (janewongcy@gmail.com)
Case report
Pseudoaneurysms that arise from malignant otitis
externa (MOE) are a rare and potentially fatal condition. This is the
first reported case of cervical-petrous internal carotid artery (ICA)
pseudoaneurysm due to secondary MOE.
A 59-year-old man with poorly controlled diabetes
mellitus and end-stage renal failure presented with a 1-week history of
right ear hearing loss and tinnitus. Tympanic examination was normal and
he was treated with intratympanic steroids. Three weeks later he was
diagnosed with chronic suppurative otitis media and completed a course of
oral ciprofloxacin and ototopic ofloxacin. Despite treatment, his
condition deteriorated and he developed otitis externa that was
complicated by a grade IV facial nerve palsy after 6 weeks. A pus swab
from the ear grew Pseudomonas aeruginosa and biopsy of an aural
polyp was compatible with infection. Computed tomography of the temporal
bone excluded the presence of osteomyelitis. Over the following 3 months
of out-patient consultations, he complained of intermittent otorrhea and
was prescribed multiple courses of ciprofloxacin, the longest course
lasting 3 weeks.
The patient developed fifth and twelfth cranial
nerve palsies in addition to facial nerve palsy that persisted for 16
weeks. Computed tomography of the brain revealed lytic changes along the
skull base (Fig a and b) and he was diagnosed with grade II MOE.
Six weeks of meropenem was commenced but he developed epistaxis and
blood-stained otorrhea after 2 weeks. Computed tomography angiogram
revealed multiple pseudoaneurysms at the right ICA situated between the
subpetrous and proximal cavernous segments (Fig c). His blood pressure was stable throughout
with no drop in haemoglobin. Balloon occlusion test (BOT) demonstrated
sufficient crosss-hunting to the right anterior circulation via the
anterior communicating artery as well as from the right external carotid
artery even under hypotensive challenge. The patient remained
neurologically stable throughout the test that lasted 40 minutes. Trapping
of the right ICA was then performed with coil embolisation of the cervical
segment and the horizontal part of the cavernous segment separately (Fig
d and e), in order to minimise deposition of any foreign body at the
infected region.
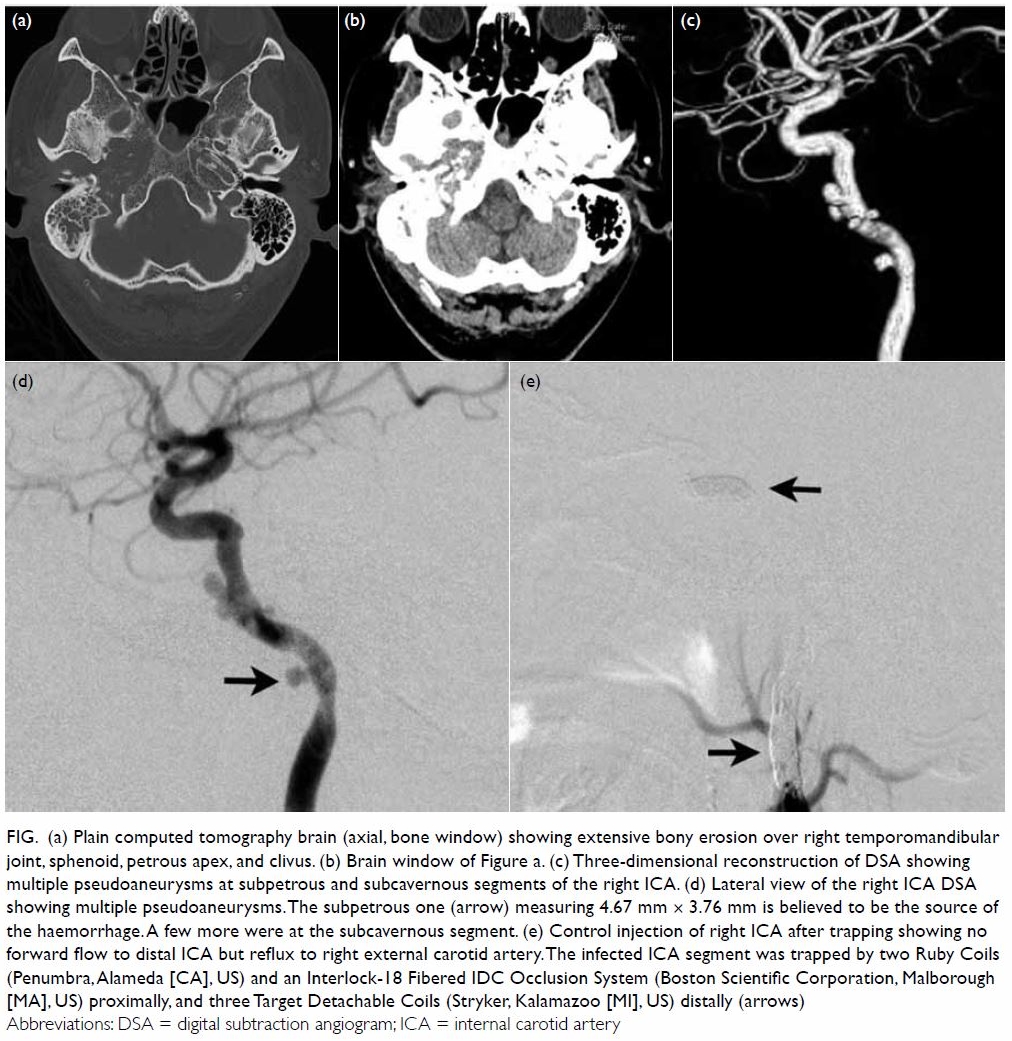
Figure. (a) Plain computed tomography brain (axial, bone window) showing extensive bony erosion over right temporomandibular joint, sphenoid, petrous apex, and clivus. (b) Brain window of Figure a. (c) Three-dimensional reconstruction of DSA showing multiple pseudoaneurysms at subpetrous and subcavernous segments of the right ICA. (d) Lateral view of the right ICA DSA showing multiple pseudoaneurysms. The subpetrous one (arrow) measuring 4.67 mm × 3.76 mm is believed to be the source of the haemorrhage. A few more were at the subcavernous segment. (e) Control injection of right ICA after trapping showing no forward flow to distal ICA but reflux to right external carotid artery. The infected ICA segment was trapped by two Ruby Coils (Penumbra, Alameda [CA], US) and an Interlock-18 Fibered IDC Occlusion System (Boston Scientific Corporation, Malborough [MA], US) proximally, and three Target Detachable Coils (Stryker, Kalamazoo [MI], US) distally (arrows)
Postoperatively, he was stable and was discharged
home with an 8-week course of ciprofloxacin and amoxillin clavulanate.
Gallium scan was repeated at 3 and 8 weeks postoperatively and revealed
further interval decrease in uptake over the right skull with mild
residual gallium activity. His otalgia and fifth and twelfth cranial nerve
palsy subsided subsequently. Three months after embolisation, he continues
to suffer residual grade III 7th nerve palsy and otorrhea.
Discussion
Cervical-petrous internal carotid artery
pseudoaneurysms can arise from different aetiologies including congenital,
trauma, malignancy, radiation therapy, and infection. Cases of ruptured
ICA pseudoaneurysm due to otogenic infection are rare with only four cases
reported1 2 3 4; these cases were MOE complicated by pseudoaneurysm on
only one ICA segment. This is the first reported case of pseudoaneurysm
occurring on multiple segments of the ICA with rupture due to MOE, and
demonstrates the features that predict development of complex vascular
complications of unresolved otogenic infection, the timing of appropriate
imaging modalities, and appropriate algorithm and duration of treatments
to prevent or rescue a fatal carotid blowout.
Malignant otitis externa is becoming an
increasingly common condition as a consequence of the prevalence of
diabetes and other immunocompromised states. It is an aggressive infection
that involves the external ear canal, either primarily originating from
the external auditory canal or secondary to chronic suppurative otitis
media. Pseudomonas aeruginosa is the most common causative
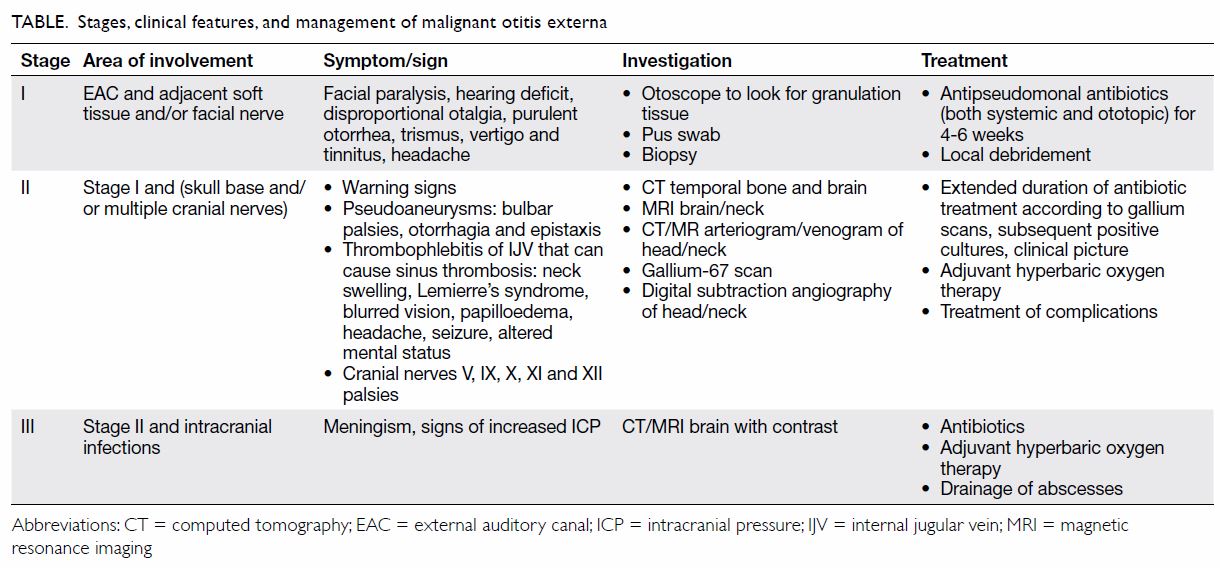
organism.1 Conventionally, MOE is classified according to the structural
involvement (Table).5 In
stage II MOE, complications can arise as it invades the temporal bone and
surrounding nerve and vascular structures causing multiple cranial nerve
palsies and carotid pseudoaneurysms. Ruptured mycotic pseudoaneurysm has
an alarming mortality rate of 54% due to shock, aspiration and ultimately
cardiopulmonary arrest.4
Furthermore, cavernous sinus thrombosis and cerebral venous sinus
thrombosis can occur when the internal jugular vein is affected.
In our patient, the time to diagnosis of MOE was 12
weeks compared with an average of 13 weeks.1
Because the possible mechanisms of the palsies include contiguous
infectious spread or direct compression by the pseudoaneurysm, it is
paramount that we recognise the warning signs of pseudoaneurysm and
instigate appropriate investigations and treatment (Table).
Treatment of malignant otitis externa and its
complications
A prolonged course of culture-directed antibiotics
remains the mainstay treatment for osteomyelitis, especially when surgical
debridement at the skull base is not feasible. Because Pseudomonas
aeruginosa is the most common bacterial organism in MOE, an
antipseudomonal antibiotic such as ceftazidime is preferred. With regard
to duration, 4 to 6 weeks is optimal with the rationale that bone
revascularisation takes 3 to 4 weeks. Gallium-67 citrate scintigraphy and
indium scintigraphy scans are sensitive to active infection and can be
used to monitor treatment progress, guide duration of antibiotic
administration, and prevent recurrence.
Other than antibiotics and local debridement and
drainage of abscesses, adjuvant hyperbaric oxygen therapy has been
reported to improve the clinical course of MOE. Despite the absence of
randomised controlled trials, adjuvant hyperbaric oxygen therapy has been
shown since the 1980s to be effective in numerous patients. It should be
strongly considered in stage II MOE5
to minimise intracranial involvement that brings high mortality.
Hyperbaric oxygen therapy is postulated to enhance phagocytic action via
free radicals, minimise tissue hypoxia that otherwise leads to further
infection and augment antibiotic activity.
An endovascular approach is frequently adopted in
the treatment of ICA pseudoaneurysm since expertise and accessibility in
open surgery are limited.6
Depending on the anatomy of the aneurysm, collateral flow sufficiency and
the segment involved, either a reparative or destructive approach is used.6 In a life-threatening scenario, we
prefer a more aggressive approach of occluding the parent artery/trapping
as it protects both the aneurysm and the frequently diseased ICA segment.
A BOT can identify those who cannot tolerate permanent carotid occlusion
that has a complication rate of 1.6% for neurological deficits.7 Alternatively, a failure rate of 4.7% and permanent
stroke can occur after passing BOT.7
For patients who fail BOT, a reparative approach such as stenting or
stent-assisted coiling may be considered but there is a risk of further
infection due to foreign body deposition around the infected segment. In
this case, we avoided such infection by not implanting coils in the
diseased ICA segment. Coils were instead deployed at segments of the ICA
both proximal (cervical) and distal (cavernous) to the diseased segment as
illustrated in Figure e. Definitively, performing a high-flow
bypass prior to surgical ligation of the parent artery can avoid the
issues mentioned.
The patient in this case had multiple risk factors
including longstanding uncontrolled diabetes mellitus and end-stage renal
failure. In retrospect, the use of intratympanic steroids along with
intermittent antibiotics could have further worsened his clinical course.
With progression of symptoms despite antibiotics, we should have had a
high clinical suspicion of MOE and treated appropriately before
complications developed. In the presence of multiple cranial nerve palsies
or even facial nerve palsy alone, timely vascular imaging is crucial to
exclude the presence of pseudoaneurysms.
In conclusion, this case highlights the rare but
important complication of MOE and the warning symptoms associated with
pseudoaneurysms. Early involvement of ear, nose, and throat specialists
and neurosurgeons can expedite the time to diagnosis and allow for prompt
investigations and intervention.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: All authors.
Acquisition of data: JSK Lau, JCY Wong, RYT Ng.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JSK Lau, JCY Wong, RYT Ng.
Critical revision for important intellectual content: All authors.
Acquisition of data: JSK Lau, JCY Wong, RYT Ng.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JSK Lau, JCY Wong, RYT Ng.
Critical revision for important intellectual content: All authors.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the
Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. Baker A, Rizk H, Carroll W, Lambert P.
Cervical internal carotid artery pseudoaneurysm complicating malignant
otitis externa: first case report. Laryngoscope 2015;125:733-5. Crossref
2. Oyama H, Hattori K, Tanahashi S, Kito A,
Maki H, Tanahashi K. Ruptured pseudoaneurysm of the petrous internal
carotid artery caused by chronic otitis media. Neurol Med Chir (Tokyo)
2010;50:578-80. Crossref
3. Telmesani LM. Ruptured petrous carotid
pseudoaneurysm complicating malignant otitis externa. J Otolaryngol
2004;33:278-80.
4. Yagci AB, Ardiç FN, Oran I, Bir F,
Karabulut N. Ruptured petrous carotid pseudoaneurysm due to tuberculous
otitis: endovascular treatment. Interv Neuroradiol 2006;12:53-6. Crossref
5. Davis JC, Gates GA, Lerner C, Davis MG
Jr, Mader JT, Dinesman A. Adjuvant hyperbaric oxygen in malignant external
otitis. Arch Otolaryngol Head Neck Surg 1992;118:89-93. Crossref
6. Powitzky R, Vasan N, Krempl G, Medina J.
Carotid blowout in patients with head and neck cancer. Ann Otol Rhinol
Laryngol 2010;119:476-84. Crossref
7. Mathis JM, Barr JD, Jungreis CA, et al.
Temporary balloon test occlusion of the internal carotid artery:
experience in 500 cases. AJNR Am J Neuroradiol 1995:16:749-54.