© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Pulmonary metastasis from a World Health
Organization grade I intracranial parasagittal meningioma: a case report
Peter YM Woo, MB, BS, FRCS; Remy SL Hung, MB, BS,
MRCS; Saori Takemura, MB, ChB; KY Chan, MB, ChB, FRCS; John CK Kwok, MB,
ChB, FRCS
Department of Neurosurgery, Kwong Wah Hospital,
Yaumatei, Hong Kong
Corresponding author: Dr Peter YM Woo (wym307@ha.org.hk)
Case report
A 37-year-old woman presented to our neurosurgical
centre in January 2003 with a 2-month history of progressive blurred
vision and was found to have papilloedema. Magnetic resonance imaging
(MRI) scan of the brain revealed a large left frontal parasagittal
extra-axial dural-based tumour with homogenous gadolinium
contrast-enhancement (6.3 cm × 4.3 cm × 3.7 cm) and Sindou grade II
invasion (ie, into the lateral recess) into the junction of the
anterior-to-middle third superior sagittal sinus (SSS). The patient
underwent preoperative polyvinyl alcohol particle catheter tumour
embolisation and a subsequent craniotomy was performed for Simpson’s grade
III excision (macroscopic complete excision without resection of the
tumour’s extra-dural extension into the SSS). The histological diagnosis
was a World Health Organization (WHO) grade I meningothelial meningioma
with a Ki-67 proliferation index of 5%. A 2-year surveillance MRI scan (Fig 1a) revealed an asymptomatic local recurrence
with further invasion into the SSS (Sindou grade IV, ie, involvement of
the roof and lateral wall). The patient was asymptomatic and reluctant to
undergo further treatment, opting for regular observation of the lesion. A
new MRI scan performed 11 years after the first operation revealed
interval tumour growth with complete occlusion of the SSS (Sindou grade V)
that was confirmed with MR venography (Fig 1b and c). A second craniotomy was performed in
October 2014, 11 years after the first, but only subtotal excision could
be achieved because of dense tumour adhesions to a large posterior frontal
cortical draining vein. The histology remained that of a WHO grade I
meningioma.
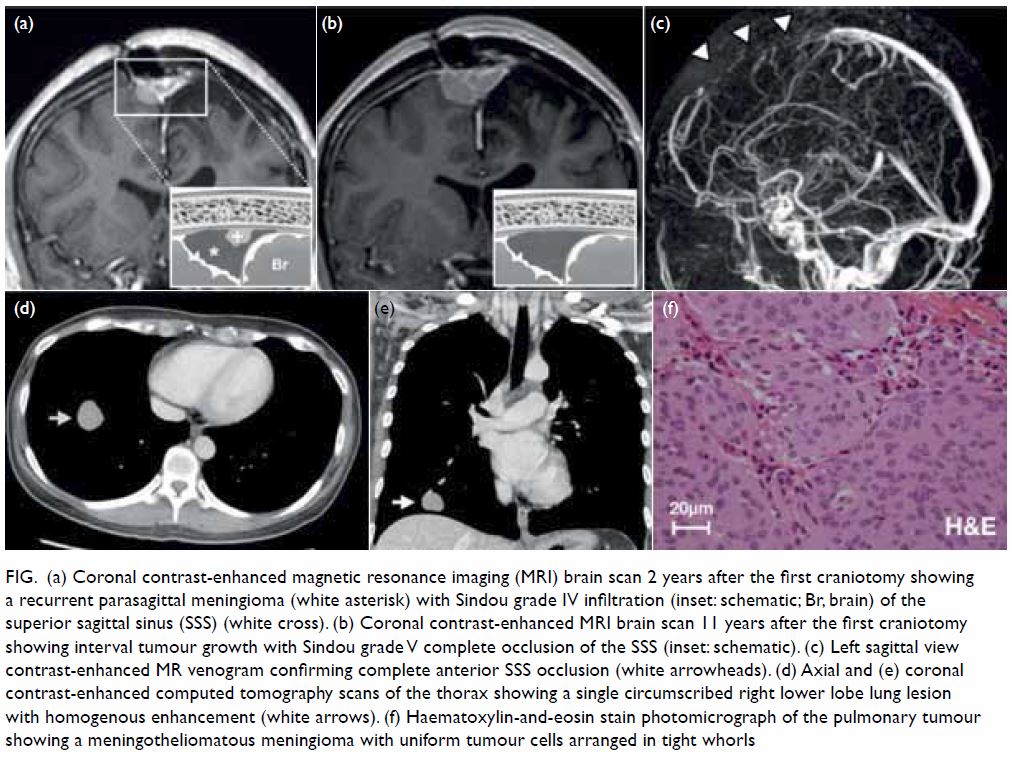
Figure. (a) Coronal contrast-enhanced magnetic resonance imaging (MRI) brain scan 2 years after the first craniotomy showing a recurrent parasagittal meningioma (white asterisk) with Sindou grade IV infiltration (inset: schematic; Br, brain) of the superior sagittal sinus (SSS) (white cross). (b) Coronal contrast-enhanced MRI brain scan 11 years after the first craniotomy showing interval tumour growth with Sindou grade V complete occlusion of the SSS (inset: schematic). (c) Left sagittal view contrast-enhanced MR venogram confirming complete anterior SSS occlusion (white arrowheads). (d) Axial and (e) coronal contrast-enhanced computed tomography scans of the thorax showing a single circumscribed right lower lobe lung lesion with homogenous enhancement (white arrows). (f) Haematoxylin-and-eosin stain photomicrograph of the pulmonary tumour showing a meningotheliomatous meningioma with uniform tumour cells arranged in tight whorls
From a preoperative chest X-ray, performed in
preparation for the patient’s second craniotomy, a new opacity in the
right lower lobe was incidentally discovered. Computed tomography scan of
the thorax revealed a single right lower lobe lung nodule (2.4 cm × 2.8 cm
× 2.3 cm) with a well-defined border and vivid homogenous contrast
enhancement (Fig 1d and e). Video-assisted thoracoscopic wedge
resection of the right lower lobe was performed 8 weeks after the
craniotomy with gross total excision achieved. The final pathological
diagnosis was a metastatic WHO grade I meningioma with a Ki-67
proliferation index of 1% and clear margins (Fig 1f).
In view of residual intracranial disease, the
patient underwent adjuvant fractionated radiotherapy (50.4 Gy). At 2 years
after the second craniotomy, surveillance MRI brain scans and chest X-rays
showed no detectable tumour.
Discussion
Meningiomas are the most frequently diagnosed
primary brain tumour in adults, accounting for 13% to 26% of all lesions.1 The population incidence is
estimated to be four to six per 100 000 with a female:male ratio of 2:1.
Despite this high prevalence, distant (extracranial) metastasis is
extremely rare with fewer than 120 cases reported.1
The grading of meningiomas is principally
determined by light microscopy of haematoxylin-eosin sections in
accordance with WHO criteria. Grade I intracranial meningiomas comprise
80% of tumours and are generally considered benign, slow-growing lesions
that have no demonstrable malignant behaviour such as distant metastasis.
However, contrary to this belief, one third of meningiomas with distant
metastases originate from grade I tumours with 31% identified
incidentally.1 In contrast, grade
III lesions, which demonstrate overt aggressive behaviour, represent only
1% of meningiomas and account for 40% of documented metastases.1 The true incidence of metastatic meningiomas is
unknown, but given the frequent occurrence of grade I tumours, that
metastatic lesions are often asymptomatic and that routine whole-body
imaging is seldom performed, the stated figure of 0.1% is likely an
underestimation.1 In our case, the
interval between primary resection and metastasis detection was 11 years,
considerably longer than the cited median duration of 58 months (range, 4
months to 15 years), reflecting the slow-growing nature of grade I
tumours.1
Three quarters of metastatic WHO grade I
meningiomas involve a single organ, primarily the lung (42%) followed by
the spine (12%), bone (10%), liver (10%), and cervical lymph nodes (10%).1 Although conventional histological
studies such as the Ki-67 proliferation index have failed to identify a
subgroup of meningiomas predisposed to metastasis, loss of heterozygosity
of 1p, 9p, 14q and 22q may be characteristic of these lesions.2 Clinical risk factors for metastasis include repeated
surgery, local recurrence and invasion of the dural venous sinuses.1 The non-collapsible and valve-less nature of the dural
venous sinuses, such as the SSS, may permit seeding of tumour cells into
the internal jugular vein and subsequently into the pulmonary
microcirculation, an indication that tumour location is pivotal in
determining haematogenous metastasis.1
Parasagittal meningiomas, comprising 20% to 34% of lesions, are perhaps
most susceptible because of their propensity to invade the SSS,
technically hindering their complete resection.3
4 Our case illustrates the
importance of treating the SSS infiltrating portion of these tumours, but
there is little consensus on the appropriate management strategy. When the
posterior SSS is patent, prohibiting its ligation and excision, some
neurosurgeons prefer subtotal resection followed by adjuvant radiosurgery
or radiotherapy.3 Others advocate
the more technically demanding surgical approach of gross total resection
with sinus reconstruction, to spare the patient the long-term adverse
effects of irradiation.4 Both
strategies offer comparable tumour control rates although multimodality
treatment may be associated with fewer procedure-related complications.3
Bronchogenic carcinoma is the most important
differential diagnosis to exclude in patients with pulmonary meningioma
metastasis, but it is difficult to distinguish on computed tomography
imaging. Meningioma metastases are usually single, non-calcified
well-circumscribed lesions that may display strong homogenous
contrast-enhancement.1 111Indium-octreotide
imaging is useful in identifying meningiomas, exhibiting avid uptake, but
its restricted availability limits its use.5
Excision of the pulmonary lesion is recommended to establish the diagnosis
and in some instances the meningioma metastasis may manifest a more
aggressive grading than the primary lesion warranting adjuvant
radiotherapy.5 When multiple
disseminated metastases preclude surgical excision, systemic treatments
such as octreotide acetate or bevacizumab, an anti-angiogenic therapy
directed against vascular-endothelial growth factor have shown some
promise for tumour control.5
However, in a case series of patients with recurrent meningioma refractory
to surgery, radiotherapy and chemotherapy, pulmonary metastasis was
identified as an unfavourable prognostic factor for overall survival.5
Distant metastasis from a WHO grade I meningioma is
a rare phenomenon and can occur more than a decade after the initial
diagnosis of the primary tumour. This case demonstrates that, regardless
of grading and especially when the patient is young, meningiomas that
infiltrate the dural venous sinuses require proactive management, either
by adjuvant irradiation or by gross total resection.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept of study: PYM Woo, RSL Hung.
Acquisition of data: PYM Woo, RSL Hung.
Analysis of data: PYM Woo, RSL Hung, S Takemura.
Drafting of the article: PYM Woo, RSL Hung, S Takemura.
Critical revision for important intellectual content: All authors.
Acquisition of data: PYM Woo, RSL Hung.
Analysis of data: PYM Woo, RSL Hung, S Takemura.
Drafting of the article: PYM Woo, RSL Hung, S Takemura.
Critical revision for important intellectual content: All authors.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics committee approval was waived because this
is a case report and no personal identifying information was disclosed. A
signed patient consent statement was obtained.
References
1. Surov A, Gottschling S, Bolz J, et al.
Distant metastases in meningioma: an underestimated problem. J Neurooncol
2013;112:323-7. Crossref
2. Gladin CR, Salsano E, Menghi F, et al.
Loss of heterozygosity studies in extracranial metastatic meningiomas. J
Neurooncol 2007;85:81-5. Crossref
3. Gatterbauer B, Gevsek S, Höftberger R,
et al. Multimodal treatment of parasagittal meningiomas: a single-center
experience. J Neurosurg 2017;127:1249-56. Crossref
4. Ricci A, Di Vitantonio H, De Paulis D,
et al. Parasagittal meningiomas: our surgical experience and the
reconstruction technique of the superior sagittal sinus. Surg Neurol Int
2017;8:1. Crossref
5. Alexandru D, Glantz MJ, Kim L,
Chamberlain MC, Bota DA. Pulmonary metastases in patients with recurrent,
treatment-resistant meningioma: prognosis and identification by
111Indium-octreotide imaging. Cancer 2011;117:4506-11. Crossref

