Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Immune-mediated necrotising myopathy is a rare
statin-associated adverse effect: a case report
KF Lee, FRCP, FHKAM (Medicine); Maria WH Mak, MRCP, FHKAM (Medicine); Virginia WN Lao, MRCP, FHKAM (Medicine);
Helen LK Yip, MRCP, FHKAM (Medicine); WY Lau, MRCP, FHKAM (Medicine); Victor TL Wong, MRCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, Kwong Wah Hospital, Hong Kong
Corresponding author: Dr KF Lee (leekf1@ha.org.hk)
Case report
The patient was a 60-year-old woman with a 14-year
history of type 2 diabetes mellitus and dyslipidaemia
with a complication of background diabetic
retinopathy. In December 2016, during a routine
follow-up examination, the patient was found to have
asymptomatic 5-fold rise in liver aminotransferases.
The patient’s glycosylated haemoglobin level was
8.5% and her low-density lipoprotein cholesterol
(LDL-C) level was 2.0 nmol/L. She was taking
metformin 500 mg 3 times daily, gliclazide 160 mg
and vildagliptin 50 mg twice daily, and atorvastatin
20 mg once daily. In view of the possibility of
statin-related hepatotoxicity, atorvastatin was
withheld after 22 months of treatment. However,
transaminitis persisted over the following 6 months
after exclusion of viral hepatitis and any structural abnormality. After 2 months, the patient complained
of bilateral thigh weakness (Medical Research
Council grade 4/5) and myalgia that prevented her
from climbing stairs. Blood tests revealed elevated
levels of creatinine kinase (CK) [5426 IU/L; normal
range, 26-192 IU/L], alanine aminotransferase
(294 IU/L; normal range, <47 IU/L), aspartate
aminotransferase (164 IU/L; normal range, <36 IU/L),
and lactate dehydrogenase (722 IU/L; normal
range, 110-210 IU/L). Other inflammatory markers
for myositis including anti-Jo-1 antibodies were
normal. Urine for myoglobulin was negative and
renal function was normal. With persistent clinical
and biochemical abnormalities 9 months after statin
cessation and no history of potential drug or health
products that might induce myositis, immune-mediated
necrotising myopathy (IMNM) associated
with statins was suspected. Electromyography
suggested active myopathic changes while muscle
biopsy revealed atrophy of multiple muscle fibres,
necrosis and regeneration without inflammatory
infiltrates. Diagnosis was finally confirmed following
an enzyme-linked immunosorbent assay by a marked
elevation of anti–3-hydroxy-3-methylglutaryl-CoA
reductase (anti-HMGCR) autoantibody to
>200 IU/mL (normal range, <20 IU/mL). Considering
her reasonable glycaemic control, monotherapy with
intravenous immune globulin (IVIG) was initiated at
a rate of 2 g per kilogram body weight per month.
Muscle power increased and CK decreased (Fig)
so IVIG was stopped after 6 months. Nonetheless
her muscle weakness worsened and extended to
involve the upper limbs as well as her ability to
swallow, and CK rose from 3200 IU/L to almost
8000 IU/L after 2 months. High-dose glucocorticoids
(intravenous methylprednisolone 500 mg/day for
3 days followed by oral prednisolone 45 mg/day) and
cyclosporin were started. A monthly IVIG infusion
was also added in the initial 2 months to enhance
the therapeutic effect for her severe myopathy. After
4 months, her weakness improved and CK dropped
below 1000 IU/L. Owing to deteriorating glycaemic
control (glycosylated haemoglobin level deteriorated
to 9.1%) and acute glaucoma, early tapering of
glucocorticoid dose was considered. However, serum CK level returned to 2000 IU/L when prednisolone
dose was weaned down to 7.5 mg daily, although
she retained full muscle power. Another steroid-sparing
agent, either methotrexate or rituximab, was
considered.
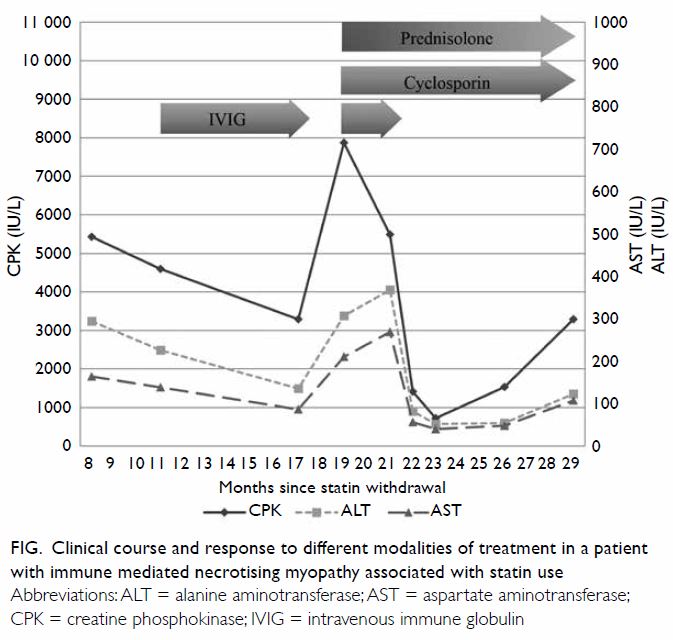
Figure. Clinical course and response to different modalities of treatment in a patient with immune mediated necrotising myopathy associated with statin use
Discussion
Statins are well-proven lipid-lowering drugs that
reduce LDL-C and hence cardiovascular morbidity
and mortality, in both primary and secondary
prevention. Their use is recommended in a wide
range of patients and high intensity therapy (LDL-C
reduction ≥50%) is indicated in a significant
proportion.1
Despite their acceptable side-effect profile,
about 10% of patients report statin-associated
muscle symptoms (SAMS) such as myalgia and/or
weakness.2 Toxic myopathy, defined as SAMS with
marked elevation (>10 times the upper limit of normal)
of CK, occurs in approximately 1 in 10 000 patients
treated with statins per year. Typically, this condition
remits spontaneously with cessation of statin use.
On the contrary, statin-associated IMNM, a rarer
adverse effect with an estimated occurrence of
2 to 3 per 100 000 treated patients, is unlikely to
be resolved by statin withdrawal, despite having
similar SAMS and muscle enzyme increment.3 The
IMNM was only suspected in our case 9 months
after cessation of statin therapy, probably due to
an initial lack of SAMS and misinterpretation that
the elevated aminotransferases originated from the liver rather than muscle. It is important to also
check CK in asymptomatic statin users with elevated
aminotransferase levels to enable early diagnosis of
statin-associated myopathy.
The IMNM is now recognised as a distinct
form of myositis, usually presenting with
symmetrical proximal arm or leg weakness with
marked elevation of CK (>10 times the upper limit of
normal), muscle oedema, and atrophy on magnetic
resonance imaging. In addition, muscle cell necrosis
and regeneration along with minimal inflammatory
infiltrates in muscle biopsy is evident and irritable
myopathy on electromyography.3 Our patient had
clinical features compatible with most of these
symptoms. Unlike other SAMS phenotypes, there
are no identifiable risk factors such as lipophilic (vs
hydrophilic) statins, high-dose statins, old age, female
gender, small body frame, liver or renal failure, and
concomitant medications metabolised by the same
hepatic P450 isoforms2 in statin-associated IMNM
(Table). The detection of anti-HMGCR autoantibody
in 2010 revolutionised the pathophysiology,
diagnosis, disease classification, and treatment of
this disease entity. This autoantibody detected by
means of an enzyme-linked immunosorbent assay is
both sensitive and specific; it has been detected in
24 of 26 patients (92%) with a clinical presentation
compatible with statin-associated IMNM although it
has not been detected in statin-treated patients who
do not have SAMS or self-limiting toxic myopathy.
The overall specificity of the commercial enzyme-linked
immunosorbent assays for anti-HMGCR autoantibody may be as high as 99.3%.4 Nevertheless
anti-HMGCR autoantibodies can also be detected
in patients with IMNM who have an underlying
malignancy or who are statin-naïve, particularly with
more widespread use of anti-HMGCR autoantibody
in patients with myopathy. With the detection of
another autoantibody against a signal recognition
particle, in 2017 the European Neuromuscular
Centre classified IMNM into three subtypes: anti–signal recognition particle myopathy, anti-HMGCR
myopathy and antibody-negative IMNM.3 Although
these subtypes share similar clinical features to those
mentioned above, they differ in environmental risk
factors, genetic risk factors, cancer risks, extra-muscular
manifestations, and response to different
treatment modalities and prognoses.
Although spontaneous improvement after
statin cessation has been reported in case studies,
most patients with this condition require one to two
immunosuppressive agents, usually in the form of
high-dose glucocorticoids plus one of the following;
methotrexate, azathioprine, mycophenolate mofetil
or cyclosporine, for initial disease control.4 5 The
IVIG has also been used successfully as first-line
monotherapy and it may be considered in those with
pre-existing diabetes, as in our patient.6 However,
incomplete normalisation of CK and the need for a
prolonged course of treatment suggests its inability
to completely abolish the pathophysiological process
that causes muscle damage. This was illustrated in
our patient with a rebound in CK level 2 months
after completion of a 6-month course of IVIG
monotherapy. Rather, her condition stabilised
following treatment with two immunosuppressants,
prednisolone and cyclosporine, although her
anti-glycaemic treatment needed to be intensified.
Similar to the clinical course of other reported
series, her condition relapsed upon weaning of
glucocorticoid dosage.7 Apart from escalation of
steroid dosage, other steroid sparing agents may
need to be considered. Rituximab has emerged
as a promising rescue agent in this situation.8
Lastly, as statin is a known trigger of anti-HMGCR
autoantibody, re-challenge with any statin should
be avoided and an alternative cholesterol-lowering
agent such as ezetimibe or PCSK9 inhibitors can be
considered.9
In conclusion, IMNM can occur rarely
in patients who present with SAMS. Unlike
toxic myopathy, clinical and biochemical
abnormalities persist upon statin withdrawal and
immunosuppressants are usually required for
disease control.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial or not-for-profit sectors
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided written informed consent for
all treatments and procedures.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/
ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of
Blood Cholesterol: A report of the American College of
Cardiology/American Heart Association Task Force on
Clinical Practice Guidelines. Circulation 2019;139:e1082-143. Crossref
2. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res
2019;124:328-50. Crossref
3. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL.
Immune-mediated necrotizing myopathy. Curr Rheumatol
Rep 2018;20:21. Crossref
4. Mammen AL. Statin-associated autoimmune myopathy. N
Engl J Med 2016;374:664-9. Crossref
5. Tiniakou E, Christopher-Stine L. Immune-mediated
necrotizing myopathy associated with statins: history and
recent developments. Curr Opin Rheumatol 2017;29:604-
11. Crossref
6. Mammen AL, Tiniakou E. Intravenous immune globulin
for statin-triggered autoimmune myopathy. N Engl J Med
2015;373:1680-2. Crossref
7. Ramanathan S, Langguth D, Hardy TA, et al. Clinical
course and treatment of anti-HMGCR antibody–
associated necrotizing autoimmune myopathy. Neurol
Neuroimmunol Neuroinflamm 2015;2:e96. Crossref
8. Allenbach Y, Mammen AL, Benveniste O, Stenzel W;
Immune-Mediated Necrotizing Myopathies Working
Group. 224th ENMC International Workshop: Clinicosero-
pathological classification of immune-mediated
necrotizing myopathies Zandvoort, The Netherlands,
14-16 October 2016. Neuromuscul Disord 2018;28:87-99. Crossref
9. Albayda J, Christopher-Stine L. Identifying statinassociated
autoimmune necrotizing myopathy. Cleve Clin
J Med 2014;81:736-41. Crossref

