Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Importance of allergological evaluation and skin
testing for severe cutaneous adverse reactions:
a case report
Philip H Li, MRCP (UK), FHKCP, Jane CY Wong, MB, BS, MRCP (UK), CS Lau, MD, FRCP
Division of Rheumatology and Clinical Immunology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Philip H Li (liphilip@hku.hk)
Case report
This is the first case of acute generalised
exanthematous pustulosis (AGEP) due to amoxicillin
reported in Hong Kong, confirmed by complete in
vivo and in vitro allergological investigations. It is
vital to highlight the importance of an appropriate
and thorough drug allergy evaluation for patients
with a suspected causative agent.
A 24-year-old man was admitted in January 2016
to his local hospital with knee pain. Arthrocentesis
was performed and empirical intravenous
amoxicillin-clavulanate prescribed for suspected
septic arthritis. He was also prescribed Hartmann’s
solution, paracetamol, tramadol, chlorpheniramine,
metoclopramide, and zopiclone during his
in-patient stay. A few hours later he developed fever
and generalised pustulosis. There was no mucosal
involvement or skin necrosis. Culture of the pustules
was negative and he declined skin biopsy. However,
his fever persisted and pustulosis began to worsen
despite continuation of amoxicillin-clavulanate.
After almost 1 week, the patient was discharged
against medical advice and no other investigations
were ordered. His fever subsided and rash improved
without treatment. He was referred to our division
2 weeks after discharge for persistent knee pain.
Upon examination, there were residual pustules
with desquamation and plaques over the trunk and
limbs. A diagnosis of AGEP likely to amoxicillin
and/or clavulanate was suspected, agreed on review
by our dermatologist. However, other possible
culprits could not be excluded as he was prescribed
multiple medications at the time.
He was reviewed 2 months later after
improvement of his skin condition. He consented
to patch testing (PT) and intradermal testing (IDT)
on his back. The PT was performed using a Finn
Chamber (SmartPractice, Phoenix [AZ], United
States) with amoxicillin at 10% (2 mg/mL) and 1%
(0.2 mg/mL) dilutions in water. The IDT was
performed with amoxicillin 20 mg/mL. Immediate
PT and IDT readings were negative after 20 minutes.
A delayed IDT reading at 48 hours was positive with
pustule formation (Fig 1). The patient declined skin
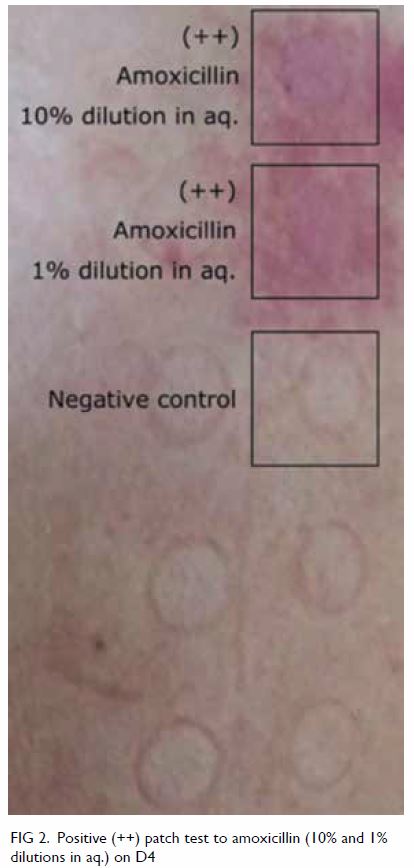
biopsy and culture of the pustule was negative. The PT was strongly positive (++) at D2 and D4 with a
crescendo effect for amoxicillin at both 10% and 1%
dilutions (Fig 2). Lymphocyte transformation test
showed consistent findings, with strong positive
results for amoxicillin and amoxicillin-clavulanate.
The patient was advised to avoid penicillins prior
to further allergological testing. He was reassured
that other concomitant medications were safe. He
tolerated paracetamol, tramadol, chlorpheniramine,
and metoclopramide thereafter on separate
occasions with no adverse effects.
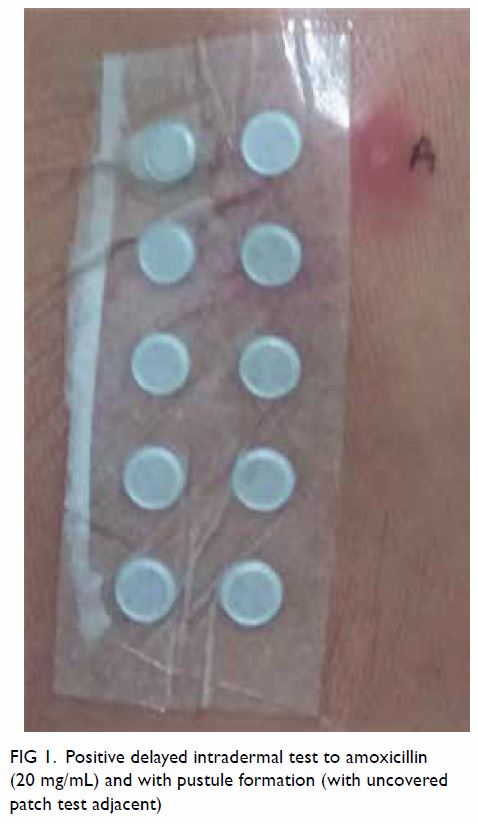
Figure 1. Positive delayed intradermal test to amoxicillin (20 mg/mL) and with pustule formation (with uncovered patch test adjacent)
Discussion
Acute generalised exanthematous pustulosis is a
severe cutaneous adverse reaction (SCAR) that
manifests with generalised sterile pustules, often
mistaken for infection with subsequent inappropriate
treatment. Symptoms classically appear within
hours, especially in antibiotic-triggered reactions.1
Differential diagnoses of pustular skin eruptions
should be considered including pustular psoriasis,
hypersensitivity syndrome reaction with pustulation,
and subcorneal pustular dermatosis. However, given
the clear chronological administration timeline of
a suspected drug, AGEP should remain the prime differential diagnosis. Once a diagnosis of SCAR is
suspected, possible causative medications should
be immediately withheld. After acute management,
allergological evaluation is required to identify the
causative drug and prevent unnecessary avoidance
of other (often multiple) medications or inadvertent
re-exposure (to misidentified culprits) in future. As
a type IV hypersensitivity reaction, PT and/or IDT
can confirm suspected drugs as the cause of AGEP
and other SCARs.2 A lymphocyte transformation
test may be useful but is generally available only in
research institutes.3 Lymphocyte transformation
testing is highly specific, with near 100% specificity
for beta-lactams.4 Physicians are reminded of
the importance of comprehensive allergological
evaluation to confirm suspected aetiologies in cases
of SCAR.
Author contributions
Concept or design: PH Li.
Acquisition of data: PH Li.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: PH Li.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors would like to thank Ms Mei-shan Lui (RN) for her aid with patch testing and outstanding service to patient care.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient consented to this publication.
References
1. Alvarado SA, Muñoz-Mendoza D, Bahna SL. High-risk
drug rashes. Ann Allergy Asthma Immunol 2018;121:552-
60. Crossref
2. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A, European
Society of Contact Dermatitis. Guidelines for performing
skin tests with drugs in the investigation of cutaneous
adverse drug reactions. Contact Dermatitis 2001;45:321-8. Crossref
3. Mayorga C, Celik G, Rouzaire P, et al. In vitro tests for drug
hypersensitivity reactions: an ENDA/EAACI Drug Allergy
Interest Group position paper. Allergy 2016;71:1103-34. Crossref
4. Doña I, Torres MJ, Montañez MI, Fernández TD. In vitro
diagnostic testing for antibiotic allergy. Allergy Asthma
Immunol Res 2017;9:288-98. Crossref