Hong Kong Med J 2024 Oct;30(5):400–8 | Epub 30 Apr 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
2024 Hong Kong College of Obstetricians and Gynaecologists guideline on antenatal screening and management of hepatitis B for prevention of mother-to-child transmission
KW Cheung, MD1; PL So, MB, BS2; Loey LY Mak, MD3; Florrie NY Yu, MB, ChB4; WL Cheung, MB, BS5; SL Mok, MB, BS5; TY Leung, MD7; Mimi TY Seto, MB, BS1
1 Department of Obstetrics and Gynaecology, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, Tuen Mun Hospital, Hong Kong SAR, China
3 Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
4 Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China
5 Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
6 Department of Obstetrics and Gynaecology, Princess Margaret Hospital, Hong Kong SAR, China
7 Department of Obstetrics and Gynaecology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr KW Cheung (kawang@hku.hk)
Abstract
Hepatitis B virus (HBV) infection remains a global
threat and causes a substantial disease burden. The
World Health Organization has set a goal to eliminate
viral hepatitis as a public health threat by 2030.
Mother-to-child transmission (MTCT) is the main
route of HBV transmission. Most MTCT cases can be
prevented by timely active and passive immunisation
at birth, but failed immunoprophylaxis in infants
continues to occur among women with a high viral
load during pregnancy. Hepatitis B virus disease
activity in infected mothers should be assessed
during early pregnancy, and multidisciplinary
management with antiviral medication should be
offered to women with a high viral load. In these
guidelines, we present management strategies for
HBV-infected pregnant women that are intended to
reduce the risk of MTCT in Hong Kong.
Introduction
Hepatitis B virus (HBV) infection is a key cause of
liver diseases associated with high rates of morbidity
and mortality. The World Health Organization
(WHO) estimated that in 2019, 296 million people
had chronic HBV infections and there were
approximately 820 000 HBV-related deaths, mostly
due to cirrhosis and hepatocellular carcinoma.1 2 The
estimated global prevalence of HBV infection in 2019
was 3.8%.1 2 To eliminate viral hepatitis as a public
health threat by 2030, the WHO has outlined a set of
global impact and service coverage targets, including
a prevalence of ≤0.1% for hepatitis B surface antigen
(HBsAg) among children 5 years of age; this target
will help eliminate mother-to-child transmission
(MTCT) of HBV.3 4
Prevention is the main strategy for HBV
elimination because there is currently no complete
cure for HBV. In high endemic regions, MTCT,
also referred to as vertical transmission, remains the primary route of HBV transmission; the risk
of chronicity after HBV infection is 90% in the
perinatal period, compared with 5% in adulthood.5
Hepatitis B virus can also be spread through
horizontal transmission, especially during early
childhood. Pregnancy offers an ideal occasion to
eliminate HBV through proper screening and timely
treatment for asymptomatic HBV-infected mothers
and their infants. Universal timely neonatal hepatitis
B vaccination is the most important intervention
for reducing MTCT of HBV. The administration
of hepatitis B immunoglobulin (HBIG) for infants
born to HBV-infected mothers, as well as maternal
peripartum prophylaxis with antivirals, would provide
additional protection against MTCT of HBV.
In these guidelines, we focus on the
management of HBV-infected pregnant women for
reduction of MTCT risk, which is a core strategy in
the Hong Kong Viral Hepatitis Action Plan 2020-2024.6
Prevention of mother-to-child transmission by active and passive immunisation
Evidence to support active and passive immunisation
Neonatal hepatitis B vaccination remains the most
effective measure for prevention of MTCT; it can
reduce the rate of MTCT from 90% to 21% in hepatitis
B e antigen (HBeAg)–positive women and from 30%
to 2.6% in HBeAg-negative women.7 The addition
of a birth dose of HBIG can further reduce the risk
to 6% in HBeAg-positive women and 1% in HBeAg-negative
women.7 A delayed birth dose of hepatitis
B vaccination and failed administration of HBIG at
birth have been associated with immunoprophylaxis
failure (IF) [see below].8
In Hong Kong, infants born to HBV-infected
mothers have received hepatitis B vaccination
and HBIG since 1984. Neonatal vaccination was
extended to all infants, regardless of their mothers’
HBV infection status, in 1988. The implementation
of a universal childhood hepatitis B vaccination
programme has led to a continuous reduction in
HBV prevalence in Hong Kong, achieving coverage
rates of >99% for the birth dose, as well as the second
and third doses of vaccine.9 Thus, HBsAg prevalence
among the antenatal population in Hong Kong
gradually decreased from 10.8% in 1992 to 2.5% in
2022.10
Recommendations of the World Health Organization
The WHO recommends the followings11 12 13:
All pregnant women should undergo HBsAg
testing at least once, as early in their pregnancy
as possible.
All infants should receive their first dose of
hepatitis B vaccine as soon as possible after birth,
preferably within 24 hours after delivery.
The birth dose should be followed by two or three
doses to complete the primary vaccination series.
Recommendations of the Hong Kong College of Obstetricians and Gynaecologists
The Hong Kong College of Obstetricians and Gynaecologists (HKCOG) recommends the followings:
All pregnant women should undergo HBsAg
screening in early pregnancy.
All infants should receive the birth dose of
hepatitis B vaccine as soon as possible (within
24 hours after delivery), followed by the second
and third doses at 1 month and 6 months of age,
respectively.
Infants born to HBV-infected mothers should
receive the birth dose of HBIG when they receive
the birth dose of hepatitis B vaccine.
Immunoprophylaxis failure
Definition and prevalence
Among infants who undergo active and passive
immunisation for hepatitis B, some do not develop
adequate antibodies. Immunoprophylaxis failure is
defined as persistent HBsAg seropositivity in infants
when tested at the ages of 9 to 12 months, or 1 to 2
months after completion of the vaccination series.14
The reported rates of IF range from 1% to 9% in the
literature.15 In Hong Kong, a single-centre study
revealed an IF rate of 2.5% (3/121) in 2001.16 Another
prospective study involving five maternity units in
Hong Kong showed that the IF rate was 1.1% (7/641)
between 2014 and 2016.17
Possible mechanisms
The possible mechanisms of IF are outlined in Figure 1.18 Germline infection is a possibility because HBV
DNA has been detected in sperm and ova of people
with HBV infection, as well as embryos from male
HBsAg-positive/female HBsAg-negative couples
or male HBsAg-negative/female HBsAg-positive
couples.19 20 21 Transplacental infection is another
possible mechanism of IF. The gradual decreases
in the detection rates of HBV markers and layers of
affected placental cells from the maternal side to the
fetal side support the hypothesis that progressive
HBV placental infection could lead to in utero
infection.22 23 Additionally, invasive prenatal tests
can cause HBV inoculation from maternal blood,
especially in HBsAg-positive pregnant women
with a high viral load. A retrospective cohort study
showed that the risk of IF after amniocentesis was
higher in women with an HBV DNA level of ≥7
log10IU/mL than in women with an HBV DNA level
of <7 log10IU/mL (10.8% vs 0%; P=0.004).24 During
vaginal delivery, contact with vaginal secretions
harbouring HBV may also increase the risk of vertical transmission. Thus, a delay in vaccination to
infants at birth increases the risk of IF.
Risk factors
The risk of IF mainly depends on the degree of
viral replication, any invasive prenatal procedures,
and the availability and timing of the birth dose
vaccine and HBIG. Immunoprophylaxis failure is
strongly correlated with the viral load (ie, DNA
level) in maternal blood during the antenatal and
perinatal periods.21 25 Although non-infectious
HBeAg is produced during viral replication, it is
associated with high HBV DNA levels. Thus, both
maternal HBeAg-positive status and a high maternal
HBV DNA level are risk factors for IF.17 26 27 In a
multicentre study involving 641 women and 654
infants in Hong Kong, all seven cases of IF were born
to HBeAg-positive mothers with an HBV DNA level
of >7.2 log10IU/mL.17 To reduce the maternal viral
load and the risk of IF, maternal use of antivirals as
peripartum prophylaxis should be considered.
Antenatal antiviral prophylaxis to prevent immunoprophylaxis failure
Evidence of the effectiveness of antenatal antiviral treatment
Immunoprophylaxis failure occurs in infants of
highly viraemic mothers despite timely birth doses
of hepatitis B vaccine and HBIG. Similar to human
immunodeficiency virus and herpes simplex virus,
antenatal antiviral treatment can suppress the viral
load and reduce the risk of MTCT.28 29
A randomised controlled trial involving 200 HBsAg-positive pregnant women showed that
daily oral intake of 300-mg tenofovir disoproxil
fumarate (TDF) from 30 to 32 weeks of gestation
could significantly lower maternal HBV DNA at
delivery and thus reduce the rates of IF (intention-to-treat analysis: 5% vs 18%, P=0.007; per-protocol
analysis: 0% vs 7%, P=0.01).30 Although a subsequent
multicentre, double-blinded, randomised controlled
trial involving 331 women did not show any
significant difference in the rate of IF between
women receiving TDF and placebo beginning at 28
weeks of gestation (0 vs 2%; P=0.12), the zero MTCT
rate in the TDF group confirmed the efficacy of TDF
intake.31 The inclusion of women with lower viral
loads and the timely administration of hepatitis B
vaccine and HBIG (median time: approximately 1.3
hours after birth) might explain the comparable IF
rates between the TDF and placebo groups.32
In a meta-analysis, the pooled odds ratios
derived from randomised controlled trials of the
efficacy of peripartum antiviral prophylaxis for
reducing MTCT risk were 0.10 for TDF (95%
confidence interval [CI]=0.03-0.35), 0.16 for
lamivudine (95% CI=0.10-0.29), and 0.14 for
telbivudine (95% CI=0.09-0.21).33 Although these
three antivirals are highly effective in preventing
MTCT and can be safely used during pregnancy
without maternal or infant safety concerns, TDF is
recommended because it has a high threshold for
drug resistance.11 33 34
World Health Organization and international recommendations
According to the WHO, pregnant women who test
positive for HBV infection (ie, HBsAg-positive) and have an HBV DNA level of ≥5.3 log10U/mL
(≥200 000 IU/mL) should receive TDF prophylaxis
from the 28th week of pregnancy until birth or later
to prevent MTCT of HBV. This prophylaxis should
be provided along with the three-dose hepatitis
B vaccination for infants, including a timely birth
dose.11 This recommendation is consistent with
clinical guidelines from other international bodies
including the American Association for the Study of
Liver Diseases (AASLD),35 the European Association
for the Study of the Liver,36 and the Asian Pacific
Association for the Study of the Liver (Table).37
Some experts have also suggested initiation of TDF
early in the second trimester for individuals with a
high risk of preterm birth or an HBV DNA level of
≥8 log10IU/mL (≥100 000 000 IU/mL).15
Use of antenatal antiviral treatment to prevent immunoprophylaxis failure in Hong Kong
To further reduce the risk of MTCT of HBV, since
August 2020, all birthing hospitals under the
Hospital Authority have been referring HBV-infected
pregnant women with a high HBV viral load (ie, an
HBV DNA level of >200 000 IU/mL) to hepatology
clinics and hepatitis nurse clinics for assessment and
consideration of initiating antiviral prophylaxis by
the third trimester. Other HBV-infected pregnant
women are also referred to the appropriate level of care for routine HBV management.
Recommendations of the Hong Kong College of Obstetricians and Gynaecologists
The HKCOG recommends the followings (Fig 2):
Hepatitis B virus–infected pregnant women
should undergo assessments of HBV DNA level,
HBeAg status, and baseline liver function in early
pregnancy to determine the need for antiviral
prophylaxis to prevent MTCT of HBV and the
need for antiviral treatment to manage maternal
indications.38 39 40
There is no need for repeat HBV DNA
quantification in later stages of pregnancy.
Hepatitis B virus DNA levels typically remain
stable during pregnancy and similar cut-offs
could be used to predict the risk of IF.40
For women with an HBV DNA level of >5.3
log10IU/mL (>200 000 IU/mL), multidisciplinary
care involving hepatologists is advised to discuss
the indications and safety of antenatal TDF to
reduce the risk of IF.11
For women with an HBV DNA level of ≤5.3
log10IU/mL (≤200 000 IU/mL), reminders should
be established for long-term regular monitoring
and follow-up after delivery, in accordance with
established protocols for patients with chronic
HBV infection.34 41
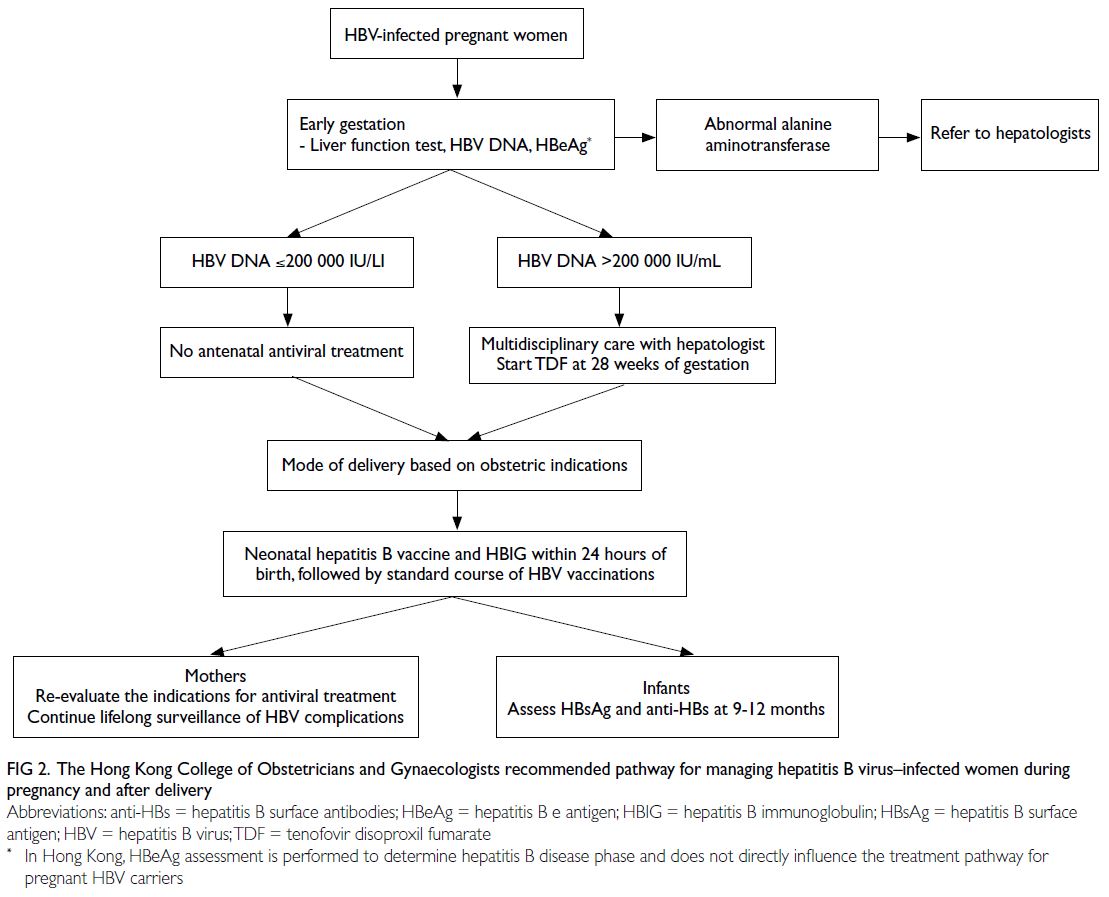
Figure 2. The Hong Kong College of Obstetricians and Gynaecologists recommended pathway for managing hepatitis B virus–infected women during pregnancy and after delivery
Antenatal management and the mode of delivery
There is conflicting evidence regarding the
associations of HBV infection with adverse pregnancy
outcomes. The results of some studies have suggested
an increased risk of gestational diabetes and preterm
birth,42 43 44 45 46 47 whereas the results of other studies have not
supported this association.48 49 50 More data are needed
to evaluate the impacts of viral load on pregnancy
complications, but the available evidence does not
warrant additional antenatal surveillance. Although
there is a theoretical risk of intrauterine HBV
exposure after chorionic villus sampling, fetal scalp
blood sampling, and the use of fetal scalp electrodes,
data concerning the risk of vertical transmission
after these procedures are scarce. The effects of
viral load on these procedures are also unknown,
but the risk of vertical transmission is likely to be
smaller in women with a lower viral load. Women should be counselled about this limited evidence,
and these procedures generally should be avoided.
For amniocentesis, the risk of IF is low when the viral
load is <7 log10IU/mL. In one study, transplacental
amniocentesis did not increase the rate of IF, but
this finding was based on a small number of cases.51
A reasonable approach comprises implementing
transamniotic amniocentesis while avoiding
transplacental puncture. Caesarean delivery should
not be offered solely to prevent MTCT of HBV, and
the mode of delivery should be based on obstetric
indications.
Breastfeeding, maternal follow-up, and neonatal follow-up after delivery
Breastfeeding is not contraindicated for mothers
who continue to receive TDF. Although a low
level of TDF can be detected in breast milk, there is no evidence that this low level leads to adverse
outcomes.35 Infants should receive a routine three-dose
course of hepatitis B vaccination at birth, 1
month of age, and 6 months of age.
The WHO emphasises the need for postvaccination
serological testing (PVST) of infants
born to HBsAg-positive mothers.12 This testing
includes testing of antibodies to HBsAg, as well
as HBsAg itself, at 9 to 12 months of age (or 1 to
2 months after the final dose of the vaccine series,
if the series is delayed).14 It allows vaccine non-responders
to receive a booster dose of vaccine
to reduce the risk of horizontal transmission.
Additionally, infants with IF should be monitored by
healthcare professionals to identify liver conditions
and potential complications (eg, cirrhosis and
hepatocellular carcinoma).52 Finally, it can provide
valuable information concerning the effectiveness of
MTCT prevention strategies.
The Department of Health and the Hospital
Authority established a collaboration to provide a
PVST service, initiated in January 2022, for infants
born to HBV-infected mothers in April 2021 or
later; covered infants must attend the Maternal
and Child Health Centres of the Department of
Health. Beginning in June 2022, mop-up PVST was
arranged for infants born in or after October 2020;
testing completion was required before the age of 24
months. Blood collection for PVST is conducted in
Hong Kong Children’s Hospital at 9 to 12 months of
age, and up to the age of 24 months (or 1 to 2 months
after the final dose of hepatitis B vaccine), to identify
HBV-infected infants and infants with an inadequate
immune response to the primary series of hepatitis B
vaccine; these infants are eligible for re-vaccination.
Hepatitis B virus–infected infants are referred to
paediatric units within the Hospital Authority for
management. Infants who have an inadequate or
absent immune response after the second course of
hepatitis B vaccine are referred to the Hong Kong
Children’s Hospital for further assessment.
Lack of continuity of care has been common
in Hong Kong; for example, 52.6% of HBV-infected
individuals did not receive any medical
care within 1 year after delivery.53 Mothers with
chronic HBV infection should undergo regular
monitoring of disease activity and surveillance of
HBV complications, in accordance with established
protocols for patients with chronic HBV infection.34 41
Indications for and duration of continued antiviral treatment after delivery
Limited evidence to support postnatal antiviral treatment
Among antiviral-treated pregnant women with HBV
infection, alanine aminotransferase (ALT) flares can occur during pregnancy (10.9%), although most occur
in the postpartum period (45.7%), as demonstrated
by a prospective study of 303 Chinese pregnant
women.54 After cessation of prophylactic antivirals,
the HBV DNA level rebounded in nearly all of the
women, but only 73% of the women developed ALT
flares and 21% of the women required retreatment.55
In a study of 91 highly viraemic HBV-infected
mothers, the incidences of postpartum flares were
similar regardless of whether antivirals were stopped
at 2 weeks (50%, n=22/44) or 12 weeks (40%,
n=17/43) after delivery. Additionally, there were no
significant differences between the two groups in
terms of the timing of flare onset (8.2 vs 10.2 weeks),
peak ALT level (229 U/L vs 209 U/L), proportion
of severe flares (ie, ALT rise ≥20 times the upper
limit of normal of 19 U/L) [14% vs 12%], and rate of
spontaneous resolution of ALT flares (75% vs 53%).56
Another multicentre study showed that the rates of
postpartum ALT flares were similar between women
who stopped treatment at delivery (33%, n=3/9) and
those who continued treatment for a longer duration
(22%, n=4/18).57
Overall, there is no evidence that prolonging
the duration of prophylactic antiviral treatment
after delivery would reduce the rate or severity of
postpartum ALT flares.
Other international recommendations concerning postnatal antiviral treatment
For HBV-infected pregnant women receiving
antiviral prophylaxis during pregnancy solely to
prevent MTCT (ie, without maternal indications),
the recommended duration of antiviral therapy is
not well-defined and varies among guidelines. The
European Association for the Study of the Liver
guidelines recommend continuing prophylactic
antiviral treatment until 12 weeks after delivery.36
In contrast, the AASLD guidelines recommend
stopping prophylactic antiviral treatment at delivery
or continuing until 12 weeks postpartum. Notably,
the AASLD guidelines are the only international
guidelines that emphasise close monitoring of
serum ALT every 3 months for up to 6 months
after delivery.35 The Asian Pacific Association for
the Study of the Liver guidelines also recommend
stopping prophylactic antiviral treatment at delivery
or continuing until 12 weeks after delivery (Table).37
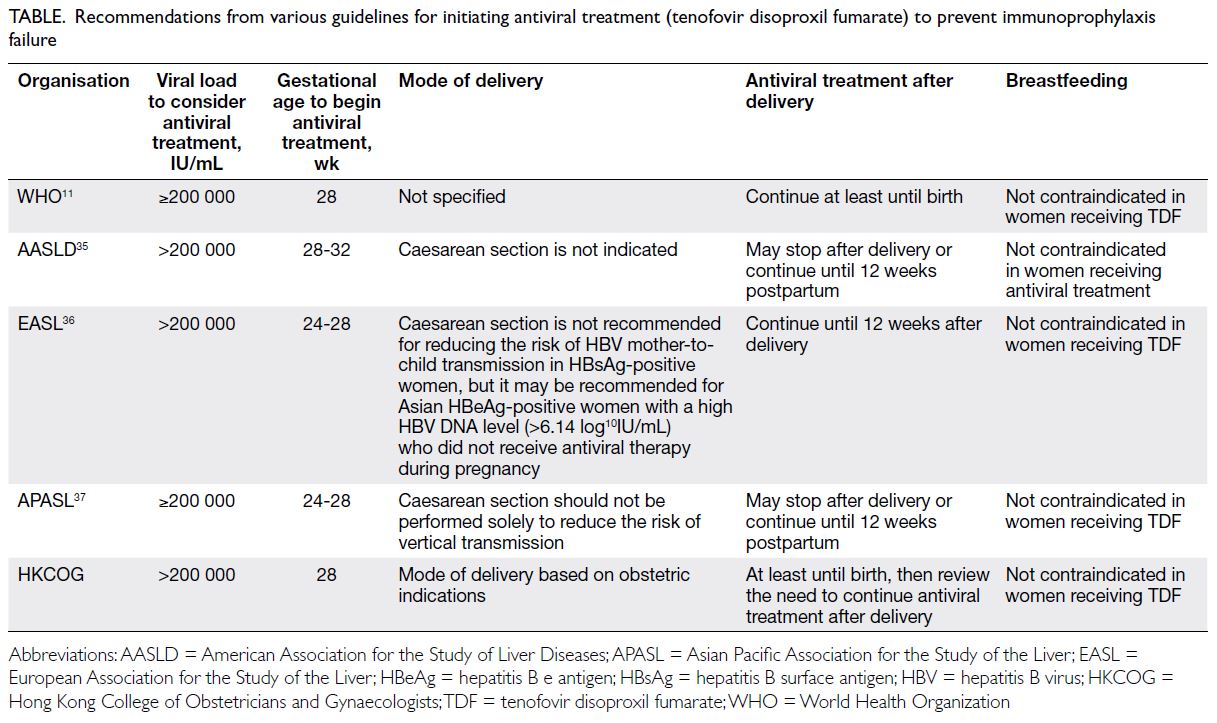
Table. Recommendations from various guidelines for initiating antiviral treatment (tenofovir disoproxil fumarate) to prevent immunoprophylaxis failure
Recommendations of the Hong Kong College of Obstetricians and Gynaecologists
The HKCOG recommends the followings:
Multidisciplinary care is essential to ensure
that women are counselled about the existing
evidence and allowed to engage in thorough
discussion with the treating physician regarding
the risks and benefits of the timing for cessation
of prophylactic treatment.
Importantly, although most flares are mild and
spontaneously resolve, liver function tests should
be performed every 3 months for 6 months after
cessation of prophylactic antiviral treatment.35
Hepatitis B virus–infected pregnant women
receiving antiviral treatment for maternal
indications should continue therapy, even after
delivery, and be managed in accordance with
standard protocols.35 36 37 41
Author contributions
All authors contributed to the concept or design, acquisition
of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
LLY Mak has served as an advisor for Gilead Sciences. Other authors have disclosed no conflicts of interest.
Acknowledgement
The guidelines were produced by the Hong Kong College of
Obstetricians and Gynaecologists as an educational aid and
reference for obstetricians and gynaecologists practising
in Hong Kong. These guidelines do not define a standard of
care, nor are they intended to dictate an exclusive course of
management. They present recognised clinical methods and
techniques for practitioners to consider incorporating into
their practices. It is understood that clinical management
may vary and must always be responsive to the needs of
individual patients, as well as the resources and limitations
unique to each institution or type of practice. These guidelines
also highlight areas of clinical uncertainty in which further
research may be warranted.
The authors thank members of the Department of Health and the Hospital Authority for providing expert comments on the guidelines.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. World Health Organization. Hepatitis B. Available from:
https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 1 Oct 2023.
2. World Health Organization. Global progress report on
HIV, viral hepatitis and sexually transmitted infections,
2021. Accountability for the global health sector strategies
2016–2021: actions for impact. Web Annex 1: Key data
at a glance. 2021. Available from: https://iris.who.int/bitstream/handle/10665/342808/9789240030985-eng.pdf. Accessed 1 Oct 2023.
3. World Health Organization. Global Health Sector
Strategy on viral hepatitis 2016-2021: towards ending viral
hepatitis. 2016. Available from: http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1. Accessed 1 Oct 2023.
4. World Health Organization. Global Hepatitis Report 2017.
2017. Available from: http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng;jsessionid=B32461CA5A37D9C9D7A90BCE32C43F92?sequence=1.
Accessed 1 Oct 2023.
5. Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC.
The influence of age on the development of the hepatitis B
carrier state. Proc Biol Sci 1993;253:197-201. Crossref
6. Department of Health, Hong Kong SAR Government.
Hong Kong Viral Hepatitis Action Plan 2020-2024.
2020. Available from: https://www.hepatitis.gov.hk/doc/action_plan/Action%20Plan_Full%20Version_PDF_en.pdf. Accessed 1 Oct 2023.
7. Isaacs D, Kilham HA, Alexander S, Wood N, Buckmaster A,
Royle J. Ethical issues in preventing mother-to-child
transmission of hepatitis B by immunisation. Vaccine
2011;29:6159-62. Crossref
8. Kang W, Ding Z, Shen L, et al. Risk factors associated
with immunoprophylaxis failure against mother to child
transmission of hepatitis B virus and hepatitis B vaccination
status in Yunnan province, China. Vaccine 2014;32:3362-6. Crossref
9. Department of Health, Hong Kong SAR Government
Surveillance of Viral Hepatitis in Hong Kong: 2021
Report. 2022. Available from: https://www.hepatitis.gov.hk/english/health_professionals/files/hepsurv21.pdf. Accessed 1 Oct 2023.
10. Viral Hepatitis Control Office, Department of Health, Hong
Kong SAR Government. Mother-to-child transmission
of hepatitis B. 2023. Available from: https://www.hepatitis.gov.hk/english/mtct/maternal_transmission_of_hepatitis_b.html. Accessed 1 Oct 2023.
11. World Health Organization. Prevention of mother-to-child
transmission of hepatitis B virus: guidelines on antiviral
prophylaxis in pregnancy. 2020. Available from: https://www.who.int/publications/i/item/978-92-4-000270-8. Accessed 1 Oct 2023.
12. World Health Organization. Hepatitis B vaccines: WHO
position paper–July 2017. 2017. Available from: https://www.who.int/publications/i/item/WER9227 . Accessed 1
Oct 2023.
13. World Health Organization. Guidelines on hepatitis B
and C testing. 2017. Available from: https://www.who.int/publications/i/item/9789241549981. Accessed 1 Oct 2023.
14. Schillie S, Murphy TV, Fenlon N, Ko S, Ward JW. Update:
shortened interval for postvaccination serologic testing
of infants born to hepatitis B–infected mothers. MMWR
Morb Mortal Wkly Rep 2015;64:1118-20. Crossref
15. Terrault NA, Levy MT, Cheung KW, Jourdain G. Viral
hepatitis and pregnancy. Nat Rev Gastroenterol Hepatol
2021;18:117-30. Crossref
16. Tse K, Siu SL, Yip KT, et al. Immuno-prophylaxis of babies
borne to hepatitis B carrier mothers. Hong Kong Med J 2006;12:368-74.
17. Cheung KW, Seto MT, Kan AS, et al. Immunoprophylaxis
failure of infants born to hepatitis B carrier mothers
following routine vaccination. Clin Gastroenterol Hepatol
2018;16:144-5. Crossref
18. Cheung KW, Seto MT, Wong SF. Towards complete
eradication of hepatitis B infection from perinatal
transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during
pregnancy. Eur J Obstet Gynecol Reprod Biol 2013;169:17-23. Crossref
19. Huang JM, Huang TH, Qiu HY, et al. Effects of hepatitis
B virus infection on human sperm chromosomes. World J Gastroenterol 2003;9:736-40. Crossref
20. Ye F, Yue Y, Li S, et al. Presence of HBsAg, HBcAg, and
HBVDNA in ovary and ovum of the patients with
chronic hepatitis B virus infection. Am J Obstet Gynecol
2006;194:387-92. Crossref
21. Nie R, Jin L, Zhang H, Xu B, Chen W, Zhu G. Presence of
hepatitis B virus in oocytes and embryos: a risk of hepatitis
B virus transmission during in vitro fertilization. Fertil
Steril 2011;95:1667-71. Crossref
22. Xu DZ, Yan YP, Zou S, et al. Role of placental tissues in the
intrauterine transmission of hepatitis B virus. Am J Obstet
Gynecol 2001;185:981-7. Crossref
23. Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism
of intrauterine infection of hepatitis B virus. World J
Gastroenterol 2004;10:437-8. Crossref
24. Han Z, Zhang Y, Bai X, Yin Y, Xu C, Hou H. Mother-to-child
transmission of hepatitis B virus after amniocentesis:
a retrospective matched cohort study. Prenat Diagn
2019;39:431-40. Crossref
25. Hu XL, Zhou XP, Qian YL, Wu GY, Ye YH, Zhu YM. The
presence and expression of the hepatitis B virus in human
oocytes and embryos. Hum Reprod 2011;26:1860-7. Crossref
26. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic
factors associated with failure to passive-active
immunoprophylaxis in infants born to HBsAg-positive
mothers. J Viral Hepat 2012;19:e18-25. Crossref
27. Song YM, Sung J, Yang S, Choe YH, Chang YS, Park WS.
Factors associated with immunoprophylaxis failure against
vertical transmission of hepatitis B virus. Eur J Pediatr
2007;166:813-8. Crossref
28. Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al.
Lamivudine-zidovudine combination for prevention
of maternal-infant transmission of HIV-1. JAMA
2001;285:2083-93. Crossref
29. Foley E, Clarke E, Beckett VA, et al. Management of
genital herpes in pregnancy. 2014. Available from: https://
www.rcog.org.uk/guidance/browse-all-guidance/other-guidelines-and-reports/management-of-genital-herpes-in-pregnancy/. Accessed 1 Oct 2023.
30. Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016;374:2324-34. Crossref
31. Jourdain G, Ngo-Giang-Huong N, Harrison L, et al.
Tenofovir versus placebo to prevent perinatal transmission
of hepatitis B. N Engl J Med 2018;378:911-23. Crossref
32. Cheung KW, Seto MT, Ng EH. Tenofovir to prevent
perinatal transmission of hepatitis B. N Engl J Med
2018;378:2349-50. Crossref
33. Funk AL, Lu Y, Yoshida K, et al. Efficacy and safety of
antiviral prophylaxis during pregnancy to prevent mother-to-
child transmission of hepatitis B virus: a systematic
review and meta-analysis. Lancet Infect Dis 2021;21:70-84. Crossref
34. World Health Organization. Guidelines for the prevention,
care and treatment of persons with chronic hepatitis B
infection. 2015. Available from: https://www.who.int/publications/i/item/9789241549059. Accessed 1 Oct 2023.
35. Terrault NA, Lok AS, McMahon BJ, et al. Update on
prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology
2018;67:1560-99. Crossref
36. European Association for the Study of the Liver;
Williamson C, Nana M, et al. EASL Clinical Practice
Guidelines on the management of liver diseases in
pregnancy. J Hepatol 2023;79:768-828. Crossref
37. Kumar M, Abbas Z, Azami M, et al. Asian Pacific
Association for the Study of Liver (APASL) guidelines:
hepatitis B virus in pregnancy. Hepatol Int 2022;16:211-53. Crossref
38. Society for Maternal-Fetal Medicine (SMFM); Dionne-Odom J, Tita AT, Silverman NS. #38: Hepatitis B in
pregnancy screening, treatment, and prevention of vertical
transmission. Am J Obstet Gynecol 2016;214:6-14. Crossref
39. Hui PW, Ng C, Cheung KW, Lai CL. Acceptance of
antiviral treatment and enhanced service model for
pregnant patients carrying hepatitis B. Hong Kong Med J
2020;26:318-22. Crossref
40. Cheung KW, Seto MT, So PL, et al. Optimal timing
of hepatitis B virus DNA quantification and clinical
predictors for higher viral load during pregnancy. Acta
Obstet Gynecol Scand 2019;98:1301-6. Crossref
41. Hospital Authority and Department of Health, Hong
Kong SAR Government. Management of adult patients
with chronic hepatitis B in primary care. 2023. Available
from: https://www.hepatitis.gov.hk/english/health_professionals/files/Management_of_Adult_Patients_with_CHB_in_Primary_Care_full_guidance.pdf . Accessed 1 Oct 2023.
42. Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal
pre-pregnancy infection with hepatitis B virus and the risk
of preterm birth: a population-based cohort study. Lancet
Glob Health 2017;5:e624-32. Crossref
43. Zheng S, Zhang H, Chen R, Yan J, Han Q. Pregnancy
complicated with hepatitis B virus infection and preterm
birth: a retrospective cohort study. BMC Pregnancy
Childbirth 2021;21:513. Crossref
44. Zhao Y, Chen YL, Song HQ, et al. Effects of maternal
hepatitis B surface antigen positive status on the pregnancy
outcomes: a retrospective study in Xiamen, China, 2011-2018. PLoS One 2020;15:e0229732. Crossref
45. Ma X, Sun D, Li C, Ying J, Yan Y. Chronic hepatitis B virus
infection and preterm labor (birth) in pregnant women—an updated systematic review and meta-analysis. J Med
Virol 2018;90:93-100. Crossref
46. Lao TT, Chan BC, Leung WC, Ho LF, Tse KY. Maternal
hepatitis B infection and gestational diabetes mellitus. J
Hepatol 2007;47:46-50. Crossref
47. Giles ML, Davey MA, Wallace EM. Chronic hepatitis
B infection and the risk of gestational diabetes: a cross-sectional
study. BJOG 2020;127:1147-52. Crossref
48. Cheung KW, Wang W, So PL, et al. Relationship between
viral load and pregnancy outcomes among hepatitis B
carriers. Taiwan J Obstet Gynecol 2022;61:630-3. Crossref
49. Keramat A, Younesian M, Gholami Fesharaki M, et al.
Inactive hepatitis B carrier and pregnancy outcomes: a
systematic review and meta-analysis. Iran J Public Health
2017;46:468-74.
50. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY.
Maternal hepatitis B surface antigen status and incidence
of pre-eclampsia. J Viral Hepat 2013;20:343-9. Crossref
51. Han Z, Zhang Y, Bai X, Yin Y, Xu C, Hou H. Mother-to-child
transmission of hepatitis B virus after amniocentesis:
a retrospective matched cohort study. Prenat Diagn 2019;39:431-40. Crossref
52. Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis
B virus infection in children and adolescents. Lancet
Gastroenterol Hepatol 2019;4:466-76. Crossref
53. Cheung KW, Seto MT, Wong D, et al. Pattern and predictors
of medical care received by hepatitis B carriers during
pregnancy and after delivery. Public Health 2019;168:36-42. Crossref
54. Wang X, Song A, Lin X, et al. Clinical characteristics of
hepatitis flares during pregnancy and postpartum in
Chinese chronic hepatitis B virus carriers—a prospective
cohort study of 417 cases. Front Immunol 2022;13:1031291. Crossref
55. Samadi Kochaksaraei G, Castillo E, Sadler MD, et al. Real-world clinical and virological outcomes in a retrospective
multiethnic cohort study of 341 untreated and tenofovir
disoproxil fumarate–treated chronic hepatitis B pregnant
patients in North America. Aliment Pharmacol Ther
2020;52:1707-16. Crossref
56. Nguyen V, Tan PK, Greenup AJ, et al. Anti-viral therapy
for prevention of perinatal HBV transmission: extending
therapy beyond birth does not protect against post-partum
flare. Aliment Pharmacol Ther 2014;39:1225-34. Crossref
57. Chang CY, Aziz N, Poongkunran M, et al. Serum
aminotransferase flares in pregnant and postpartum
women with current or prior treatment for chronic
hepatitis B. J Clin Gastroenterol 2018;52:255-61. Crossref


