Hong Kong Med J 2024 Aug;30(4):310–9 | Epub 14 Aug 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Management of overactive bladder: consensus statements from the Hong Kong Urological Association and the Hong Kong Geriatrics Society
William KK Wong, MB, BS, FHKCP1; Raymond WM Kan, MB, BS, FHKAM (Surgery)2; PS Lam, MB, BS, FHKCP3; Phoebe MH Cheung, MB, ChB, FHKAM (Surgery)4; Elaine YL Cheng, LMCHK, FHKCP5; Terrilyn CT Pun, MB, BS, FHKAM (Surgery)6; Maria WS Tang, MB, ChB, FHKCP7; Clarence LH Leung, MB, ChB, FHKAM (Surgery)6; Sandy WS Woo, MB, BS, FHKCP8; TK Lo, MB, BS, FHKAM (Surgery)9; Peggy SK Chu, MB, BS, FCSHK9; Tony NH Chan, MB, BS, FHKCP10; Peter KF Chiu, MB, ChB, FHKAM (Surgery)11
1 Department of Medicine, Alice Ho Miu Ling Nethersole Hospital, Hong Kong SAR, China
2 Division of Urology, Department of Surgery, Queen Elizabeth Hospital, Hong Kong SAR, China
3 Department of Rehabilitation and Extended Care, Wong Tai Sin Hospital, Hong Kong SAR, China
4 Division of Urology, Department of Surgery, Tseung Kwan O Hospital, Hong Kong SAR, China
5 Department of Medicine and Geriatrics, United Christian Hospital, Hong Kong SAR, China
6 Division of Urology, Department of Surgery, Kwong Wah Hospital, Hong Kong SAR, China
7 Department of Medicine and Geriatrics, Shatin Hospital, Hong Kong SAR, China
8 Department of Medicine and Geriatrics, Ruttonjee Hospital, Hong Kong SAR, China
9 Division of Urology, Department of Surgery, Tuen Mun Hospital, Hong Kong SAR, China
10 Department of Medicine and Geriatrics, Pok Oi Hospital, Hong Kong SAR, China
11 SH Ho Urology Centre, Department of Surgery, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Peter KF Chiu (peterchiu@surgery.cuhk.edu.hk)
Abstract
Overactive bladder (OAB) is a common urological
disease with a high prevalence in older adult
populations. Antimuscarinic drugs have been the
most common treatment for OAB for more than a
decade, but their anticholinergic side-effects and
potential impact on cognitive function among older
patients are usually underestimated. This consensus
aimed to provide practical recommendations
concerning OAB management, with a particular
emphasis on older patients. A joint consensus panel
was formed by representatives of the Hong Kong
Urological Association and the Hong Kong Geriatrics
Society. Literature searches regarding OAB and its
management were performed in PubMed and Ovid.
Several working meetings were held to present
and discuss available evidence, develop consensus
statements, and vote for the statements. A modified
Delphi method was used in this consensus process. To
address questions regarding various aspects of OAB,
29 consensus statements were proposed covering the
following areas: diagnosis, initial assessment, non-pharmacological
treatments, considerations before
administration of pharmacological treatments,
various pharmacological treatments, combination
therapy, and surgical treatment. Twenty-five
consensus statements were accepted.
Introduction
Overactive bladder (OAB) is defined by the
International Continence Society as urinary
urgency in the absence of urinary tract infection
or other detectable diseases, usually accompanied
by increased daytime frequency and/or nocturia,
with or without urinary incontinence.1 Its reported
prevalence ranges from 9.6% to 35.6%.2 A survey
in Hong Kong showed that the age-adjusted OAB
prevalence was 15.1%, and age was a significant risk
factor.3
Although many patients can benefit from
lifestyle modifications, medical therapy may
be warranted for patients with persistent and
bothersome OAB symptoms.4 Antimuscarinic
agents have been the most commonly prescribed
class of medications for OAB over the past two
decades.4 However, their anticholinergic effects on
cognitive function have long been both concerning
and underestimated. This is particularly significant
among older individuals because any cognitive
decline they experience could easily be attributed to normal ageing or dementia.5 Such cognitive decline
might be more pronounced when multiple drugs with
anticholinergic side-effects are used concurrently.5
The concept of anticholinergic burden has been
introduced to help clinicians estimate the combined
anticholinergic effects (and potential impact on
cognitive function) of all medications prescribed to
a single patient.5
Beta-3 agonists represent a new class of drugs
approved for the treatment of OAB.6 They do not
have any anticholinergic side-effects and have
therefore become alternatives to antimuscarinics for
the treatment of OAB, particularly among patients
with advanced age, dementia, or polypharmacy.6
To provide recommendations for the
treatment of OAB in Hong Kong, a joint consensus
panel was formed by representatives from the Hong
Kong Geriatrics Society (HKGS) and the Hong
Kong Urological Association (HKUA). Consensus
statements were produced based on the latest
evidence and international guidelines, supplemented
by expert opinions from panel members.
Methods
The joint consensus panel consisted of 13 experts
from Hong Kong: six geriatricians representing
the HKGS and seven urologists representing the
HKUA. Among these experts, seven were female
clinicians and six were male clinicians. In total,
seven meetings were held to discuss the scope of
the consensus, present key evidence, formulate
consensus statements, vote, and have a final
discussion regarding manuscript preparation.
Literature reviews were performed in PubMed
and Ovid to retrieve relevant articles related to this topic. The key words used included ‘overactive
bladder’, ‘anticholinergic’, ‘antimuscarinic’, ‘beta3-adrenoceptor agonist’, ‘β3-adrenoceptor agonist’,
‘β3 agonist’, ‘guidelines’, ‘behavioural therapy’,
‘behavioural treatment’, ‘combination therapy’,
and ‘surgery’. In total, 34 articles were selected for
presentation and in-depth discussion, including
four major guidelines, 15 meta-analyses/systematic
reviews, and 15 randomised controlled trials. When
discussing areas with inadequate evidence, the
modified Delphi method was used. Panel discussions
were carried out in a structured manner using
appropriate content; each panel member contributed
to the discussion in a fair and equal manner. The
discussion was divided into seven parts, namely,
introduction and overview of OAB, assessment
and diagnostic approaches, non-pharmacological
treatment, antimuscarinic agents, beta-3 agonists,
combination therapy, and surgical treatment for
OAB.
Panel members were divided into small
working subgroups to review the existing literature,
present their findings to other panel members, draft
consensus statements, and finalise the consensus
statements during panel meetings. In the last
meeting, panellists voted anonymously on the
practicability of recommendation in Hong Kong
for each statement, based on predefined judgement
criteria (online supplementary Table 1). If ≥75%
of panellists chose ‘accept completely’ (option A)
or ‘accept with some reservations’ (option B), a
consensus statement was regarded as accepted. A
total of 29 consensus statements were proposed,
and 25 of them were accepted. The complete voting
record and all consensus statements are listed in
online supplementary Table 2.
The AGREE (Appraisal of Guidelines,
Research and Evaluation) reporting guideline
was used to ensure the methodological quality,
comprehensiveness, completeness, and transparency
of this consensus document.
History and physical examination
The initial assessment is intended to diagnose OAB, rule out other pathologies, assess symptom severity, and formulate an individualised management plan.
Statement 1: The clinician should begin the diagnostic process with careful history taking and physical examination.
Statement 2: Storage lower urinary tract symptoms may be a sign of more serious underlying conditions, and their management can be complicated by co-morbidities and polypharmacy. The clinician should seek a specialist’s opinion if red flag features are detected.
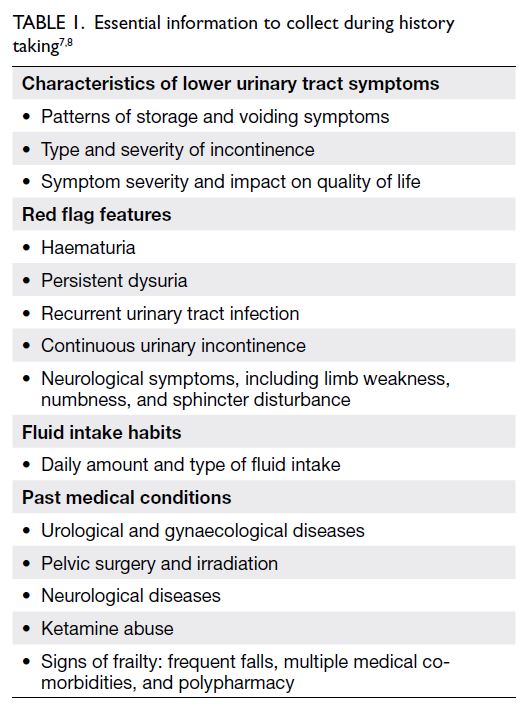
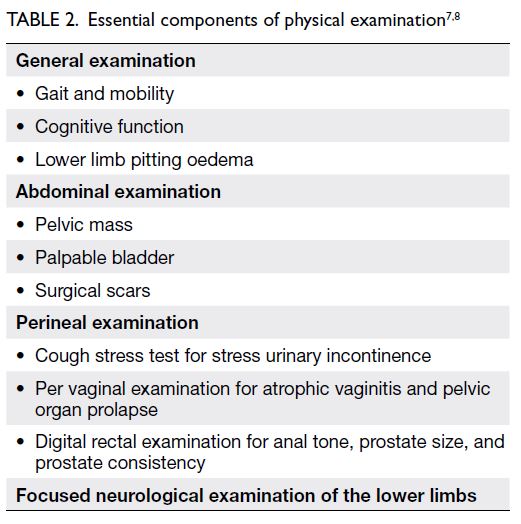
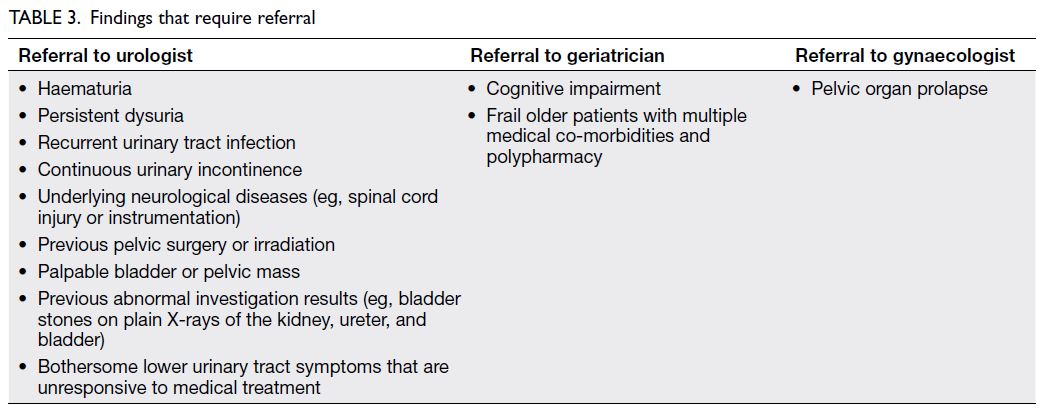
History taking and focused physical examination are important in the assessment of storage lower urinary tract symptoms7 8 (Tables 1 and 2). Patients with alarming symptoms should be
referred to relevant specialists (Table 3). Past health
also provides clues to the aetiology of the problem.
In particular, medical diseases including diabetes
mellitus, fluid status, obstructive sleep apnoea,
as well as their treatments, can contribute to such
symptoms.
Symptom severity can be assessed by the
number of pads used per day, degree of restriction
in daily activities, and presence or absence of
psychological stress.7 8 Assessments of frailty,
cognitive function, and anticholinergic burden are
especially relevant in older individuals, considering
their implications for subsequent management.
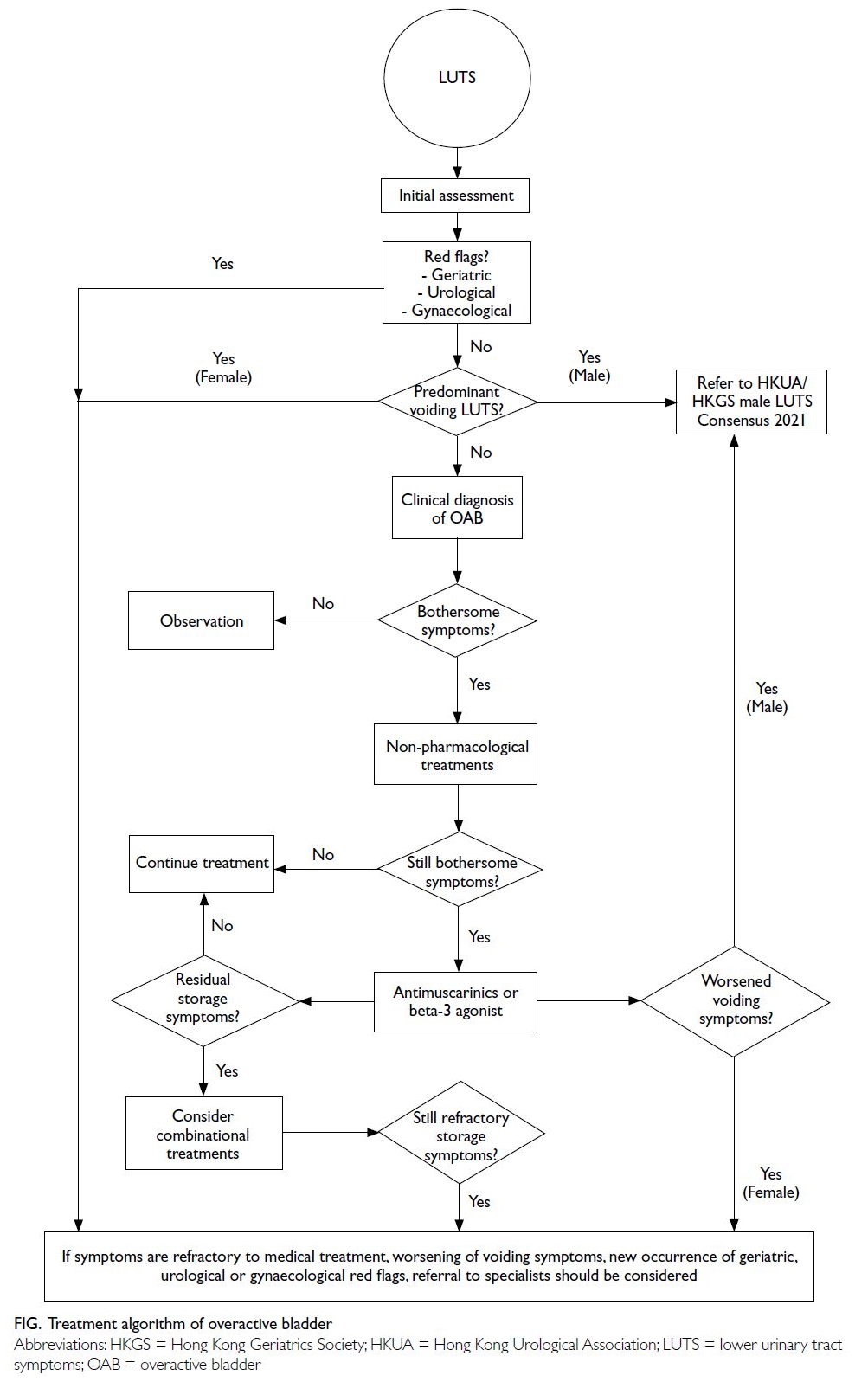
The treatment algorithm for OAB is illustrated in the Figure.
Investigations
Statement 3: Urinalysis should be considered during the initial assessment of overactive bladder syndrome.
Urinalysis is recommended as an initial
assessment for patients with OAB in most guidelines,
including the European Association of Urology 2023
guidelines on lower urinary tract symptoms7 8; the
results can rule out urinary tract infection, diabetes
mellitus, and proteinuria.7 8
Statement 4: A bladder diary should be considered during the assessment of overactive bladder syndrome.
A bladder diary serves as documentation
of the patient’s drinking habits, voiding patterns,
and incontinence episodes. It is useful for OAB
diagnosis, baseline symptom quantification,
treatment response monitoring, and bladder training
programme planning.
Statement 5: Urine culture, post-void residual urine, plain X-rays of the kidney, ureter, and bladder, and patient questionnaires may be performed during the initial assessment of overactive bladder syndrome at the clinician’s discretion.
Statement 6: If questionnaires are used for assessment of overactive bladder syndrome, appropriate questionnaires validated in the patient’s language should be used.
Statement 7: Cystoscopy, urodynamics, ultrasonography of the urinary system, and pad tests should not be routinely included in the initial assessment of overactive bladder syndrome.
Urine culture, post-void residual urine,
plain X-rays of the kidney, ureter, and bladder, and
symptom/quality of life questionnaires are not
considered routine tests in the initial assessment.
Questionnaires including the OAB Symptom Score,
Urogenital Distress Inventory-6, and Incontinence
Impact Questionnaire, Short Form have been
validated in Cantonese within Hong Kong. The
OAB Symptom Score is a screening tool used to
measure symptom severity in both male and female
patients, whereas Urogenital Distress Inventory-6
and Incontinence Impact Questionnaire, Short Form are used to measure symptom distress and health-related
quality of life in female patients. Cystoscopy,
urodynamics, ultrasonography of the urinary system,
and pad tests are not recommended for the initial
assessment of OAB in most guidelines.
Treatment
Non-pharmacological treatments
Statement 8: Non-pharmacological treatments, including fluid management, bladder training, and pelvic floor exercises, should be offered to patients with overactive bladder, regardless of drug treatment initiation.
Statement 9: Clinicians should identify medications and substances that may contribute to overactive bladder and consider modifications or alternatives.
Fluid management comprising a 25% decrease
in fluid intake can reduce urination frequency
and urgency.9 Reduced liquid consumption after
dinner or within several hours before bedtime is
a reasonable management approach for nocturia.
Caffeine irritates the bladder and can induce urinary
urgency; therefore, caffeine intake should be avoided.
Some drugs (eg, diuretics and acetylcholinesterase inhibitors) may worsen OAB
symptoms; the use of these drugs should be identified,
and the patient should be switched to a suitable
alternative. Additionally, angiotensin-converting
enzyme inhibitors can induce coughing, thereby
exacerbating urinary incontinence. Sodium-glucose
cotransporter-2 (SGLT2) inhibitors cause increased
urine output and higher urinary frequency; thus,
they should be avoided.
Statement 10: Weight reduction should be advised for obese individuals with overactive bladder.
Among obese women, weight loss of 8.0%
reduces the overall amounts of weekly incontinence
episodes by 47% (vs 28% in the control group) and urgency urinary incontinence episodes by 42% (vs 26% in the control group) within 6 months.10
Statement 11: For patients who have difficulty performing pelvic floor exercises and bladder
training, early pharmacological therapy should be considered.
Behavioural therapy and bladder training are
recommended as conventional approaches before
considering drug treatment; the success of these
approaches requires active participation by patients
and their caregivers. Upfront pharmacological
therapy may be considered for patients (especially
frail older individuals) who have difficulty complying
with these approaches.
Statement 12: Before initiating treatment, clinicians should educate patients and caregivers about the symptoms and natural course of overactive bladder, and the benefits and risks of currently available treatments.
Before drug treatment is initiated, the natural
course of the disease and the safety profiles of
various treatment options should be explained to
increase patient compliance. Clinicians should
establish feasible treatment goals with patients and
their caregivers.
Statement 13: Antimuscarinics should be used cautiously in patients with cognitive impairment or a high anticholinergic burden.
Statement 14: Because polypharmacy is very common, a detailed review of drug history is recommended to avoid creating a clinically significant anticholinergic burden in patients.
Cognitive impairment (eg, delirium
and dementia) has been linked to the use of
antimuscarinic agents, particularly in older
patients.11 It is important to review drug history before prescribing antimuscarinics to this group of
patients. The concurrent use of medications with
anticholinergic effects, such as antihistamines and
anti-Parkinson’s drugs, may potentiate the side-effects
of antimuscarinic agents and should be
modified accordingly.
Pharmacological treatment
Antimuscarinic agents
Randomised controlled trials have revealed
improvements in symptom control and differences
in the cure rates of urgency incontinence when using
antimuscarinic agents. However, no single agent has
demonstrated superiority over the others in terms of
efficacy.12 13
Statement 15: Antimuscarinics should be offered to patients who have been unsuccessful with non-pharmacological approaches.
Statement 16: Extended-release antimuscarinics are preferred over immediate-release antimuscarinics because of better tolerability, particularly regarding
dry mouth.
Statement 17: Antimuscarinics should not be used in patients with narrow-angle glaucoma unless approved by an ophthalmologist.
The overall withdrawal rates of antimuscarinic
agents due to side-effects range from 3% to 10%.14
Adverse events include dry mouth, pruritus, blurred
vision, and dizziness (29.6%, 15.4%, 3.8%, and
3.5%, respectively).14 The results of a meta-analysis
suggested that extended-release formulations
have lower rates of adverse events, particularly dry
mouth.14 However, the rates of constipation and
withdrawal due to side-effects are not significantly
different between immediate-release and extended-release
formulations. Notably, antimuscarinics can
cause pupil dilation and precipitate closed-angle
glaucoma, particularly among patients with narrow-angle
glaucoma.15
Statement 18: Antimuscarinics are effective in treating overactive bladder but regular monitoring of voiding symptoms is recommended, especially among older individuals.
The use of antimuscarinic agents is associated
with a minimal increase in post-void residual urine
volume among male patients. In a study of men with
proven bladder outlet obstruction, this increase in
post-void residual urine volume did not lead to acute
urinary retention.16 Nevertheless, changes in voiding
symptoms after the initiation of antimuscarinic
agents, particularly among older patients, should be
monitored.
As a quaternary amine compound with
hydrophilic properties, trospium has a theoretical advantage in that it does not cross the blood-brain
barrier and therefore may result in less cognitive
impairment. There is evidence to support the claim
that trospium does not worsen cognitive function in
patients with Alzheimer’s disease.17 A pooled analysis
indicated that trospium has a lower treatment
withdrawal rate due to side-effects compared with
other anticholinergics.18 However, there remains
a lack of strong evidence concerning the degree
of cognitive decline from various anticholinergic
agents.
Antimuscarinic agents registered for the
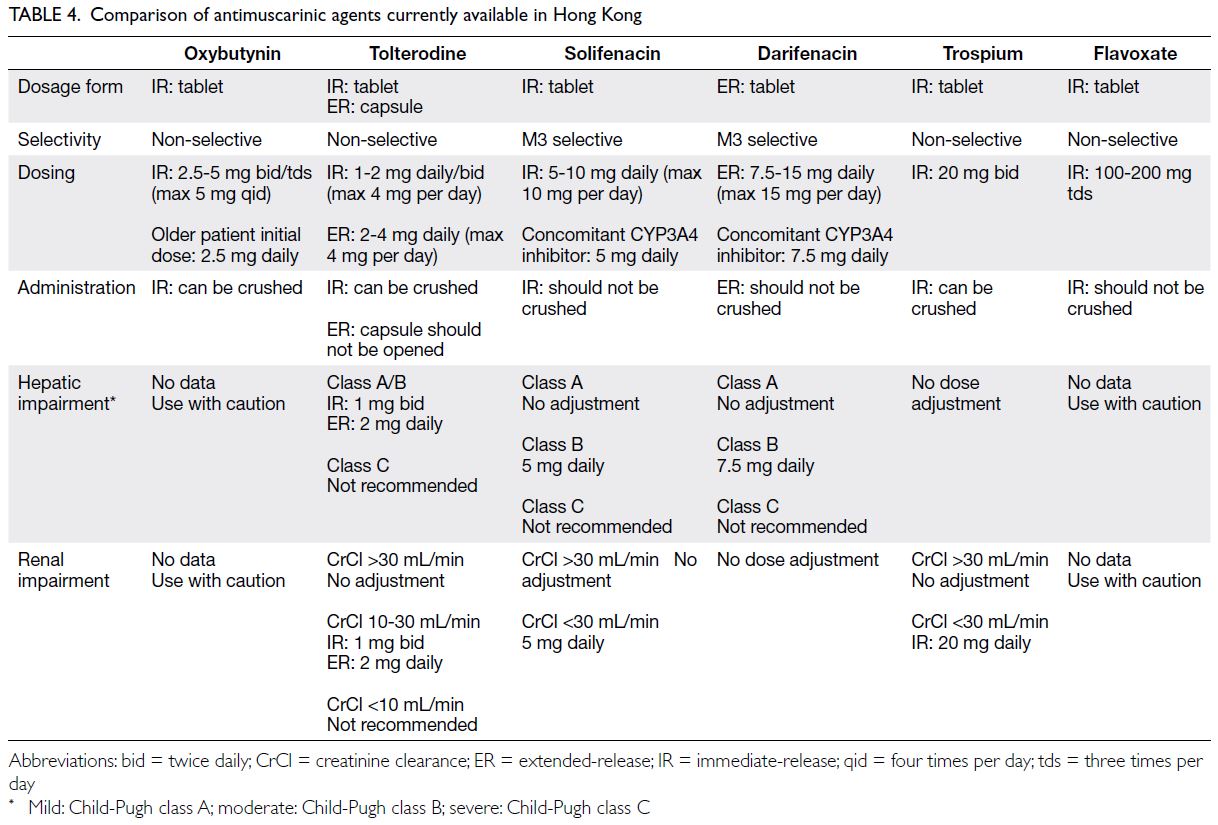
treatment of OAB in Hong Kong are listed in Table 4.
Beta-3 agonists
Statement 19: Beta-3 agonists provide overall efficacy similar to that of commonly used antimuscarinic monotherapies.
Beta-3 adrenoceptor agonists (ie, mirabegron
and vibegron) relax detrusor muscles in the urinary
bladder wall, allowing the bladder to remain
distended during the storage phase. Mirabegron
significantly improved incontinence episodes and
micturition frequency compared with placebo in a
phase 3 trial.19 The efficacy of mirabegron 50 mg is
similar to that of most antimuscarinic monotherapies
regarding micturition frequency, urgency urinary
incontinence, dry rate, and 50% reduction in
incontinence episodes.20 Mirabegron is efficacious in
improving OAB symptoms and quality of life.21 22
Statement 20: Beta-3 agonists appear to be better tolerated than antimuscarinics (eg, in terms of dry mouth, constipation, and urinary retention).
Compared with antimuscarinic monotherapy,
mirabegron is better tolerated and has significantly
lower risks of dry mouth, constipation, and urinary
retention. This safety profile remains consistent for
up to 1 year of treatment.22 Mirabegron also has
better treatment persistence and adherence rates
at 12 months.22 Therefore, mirabegron can serve as
an alternative pharmacological treatment for older
patients with OAB.23 24
Statement 21: Mirabegron should not be used in patients with severely uncontrolled hypertension.
According to recommendations from
the United Kingdom25 and European26 health
authorities, mirabegron is contraindicated in
patients with severely uncontrolled hypertension
(ie, systolic blood pressure ≥180 mm Hg and/or
diastolic blood pressure ≥110 mm Hg) due to the
lack of studies concerning mirabegron effects in this
group of patients. However, in a phase 3 randomised
controlled trial comparing mirabegron 25 mg,
mirabegron 50 mg, and placebo, the incidence
of hypertension was similar across all subgroups (12%, 11%, and 8.5%, respectively).19 Additionally,
the adjusted mean changes in systolic and diastolic
blood pressures from baseline to the final visit were
comparable between the mirabegron 25 mg and
placebo groups. Patients in the mirabegron 50 mg
group experienced a clinically insignificant increase
in blood pressure (ie, 1.0-1.5 mm Hg) compared with
the placebo group.19
Combination therapy
Statement 22: Combination drug treatment (a beta-3 agonist and an antimuscarinic agent) may be considered for overactive bladder that is unresponsive to monotherapy with either antimuscarinics or beta-3 agonists.
Several trials have investigated the use
of combination drug therapy comprising an
antimuscarinic agent and a beta-3 agonist,
especially solifenacin and mirabegron, in patients
with OAB.20 21 In the SYMPHONY study, three
combination groups (solifenacin 10 mg/mirabegron
25 mg, solifenacin 5 mg/mirabegron 50 mg, and
solifenacin 10 mg/mirabegron 50 mg) displayed
significant improvements in mean volume voided per micturition, micturition frequency, and
urgency episodes compared with solifenacin 5
mg monotherapy.27 Despite a slight increase in
the incidence of constipation among combination
groups using solifenacin 10 mg, combination drug
treatments were well tolerated compared with
monotherapy or placebo.
In the SYNERGY study, the combination of
solifenacin 5 mg/mirabegron 50 mg was superior to
solifenacin or mirabegron monotherapy in terms of
reducing incontinence episodes, urgency episodes,
and nocturia.28 In two other studies (BESIDE29 and
MILAI30), mirabegron was used as an add-on therapy
for patients with OAB who remained symptomatic
on solifenacin alone. Both studies showed that the
combination of solifenacin 5 mg/mirabegron 50 mg
produced greater improvements in incontinence
episodes and micturition frequency.29 30 The
incidence and frequency of treatment-emergent
adverse events were similar in the combination and
monotherapy groups. The withdrawal rate related to
treatment-emergent adverse events was low (ie, 1.1%
to 1.5%).29 30
The long-term safety and efficacy of solifenacin
and mirabegron combination treatment over 12 months were demonstrated in the SYNERGY II31 and
MILAI II studies,32 and the use of antimuscarinics
other than solifenacin in combination therapy was
assessed in the MILAI II study.32 Similar efficacy
and adverse events were observed with various
combinations of antimuscarinics (eg, imidafenacin,
propiverine, and tolterodine) and mirabegron.
Statement 23: Combination treatment using a beta-3 agonist and an antimuscarinic is preferred over the use of two antimuscarinics due to fewer side-effects and a lower anticholinergic burden.
There is a lack of large-scale randomised
controlled trials evaluating combination therapy
with two antimuscarinic agents. In a retrospective
study, the long-term persistence rate for combination
therapy with two antimuscarinics was poor due
to adverse events.20 In contrast, the combination
of an antimuscarinic agent and a beta-3 agonist
exhibited a better persistence rate compared with
monotherapy among patients with OAB who had
moderate to severe symptoms.20 21 Considering the
high anticholinergic burden in older individuals, the
use of two antimuscarinics is not preferable, even if
the response to monotherapy is inadequate. Instead,
combination therapy with an antimuscarinic agent
and a beta-3-agonist is recommended.
Surgical treatment
Statement 24: Posterior tibial nerve stimulation should be considered for patients who have been unsuccessful with pharmacological treatment.
Percutaneous tibial nerve stimulation (PTNS)
is a less invasive procedure among the available
surgical interventions for OAB. A meta-analysis
involving 2461 patients showed that PTNS could
reduce voiding frequency, nocturia frequency,
urgency episodes, and incontinence episodes while
increasing the maximum cystometric capacity.33
The main complication was pain at the puncture
site, but its incidence was low. One trial comparing
the efficacies of PTNS and tolterodine showed that
PTNS had superior results concerning the composite
outcome of cure or symptom improvement (79.5%
vs 54.8%; P=0.01).34 Trials comparing PTNS with
beta-3 agonists are ongoing.
Statement 25: Intravesical botulinum toxin injection or sacral neuromodulation should be considered in carefully selected patients who have been unsuccessful with pharmacological treatment.
A phase 3, randomised, placebo-controlled
trial demonstrated that intravesical injection of
botulinum toxin A significantly reduced micturition
frequency, increased the rate of complete continence,
and improved OAB symptoms and quality of life
scores compared with placebo.35 The clinical effects of botulinum toxin A usually persisted for 3 months
to 1 year, and additional injections were needed when
the effects diminished. Uncomplicated urinary tract
infection was the most common adverse event, and
urinary retention was observed in 5.4% of patients.35
Sacral neuromodulation (SNM) utilises a
principle similar to PTNS but involves implantation
of electrical leads at the S3 nerve root.36 A systematic
review showed that 15% of patients were completely
cured with SNM, whereas 50% of patients had a >90%
reduction in the number of incontinence episodes.36
The most common complications associated with
SNM were pain at the implant or lead site (25%), lead
migration (16%), and replacement and repositioning
of the implanted pulse generator (15%).36 Pain at
the implant or lead site is similar to sciatica, which
radiates down to the lower back to the hip, thigh,
and toes. A test implant is generally required. If the
pain is intolerable, permanent implantation is not
performed.
Conclusion
Overactive bladder is a common condition with a
substantial impact on quality of life. The number of
patients with increasing OAB complexity is expected
to increase due to population ageing. Representatives
of the HKGS and the HKUA have agreed upon 25
consensus statements regarding the diagnostic
approach, management, and referral mechanism for
OAB in primary care settings. Through collaborations
among primary care practitioners, geriatricians, and
urologists, we hope to provide more holistic care to
patients with OAB in Hong Kong.
Author contributions
Concept or design: PSK Chu, WKK Wong, TNH Chan, RWM Kan.
Acquisition of data: RWM Kan, PS Lam, PMH Cheung, TCT Pun, TK Lo.
Analysis or interpretation of data: EYL Cheng, MWS Tang, SWS Woo, CLH Leung.
Drafting of the manuscript: TCT Pun, TNH Chan, WKK Wong, PKF Chiu.
Critical revision of the manuscript for important intellectual content: PSK Chu, WKK Wong, PKF Chiu.
Acquisition of data: RWM Kan, PS Lam, PMH Cheung, TCT Pun, TK Lo.
Analysis or interpretation of data: EYL Cheng, MWS Tang, SWS Woo, CLH Leung.
Drafting of the manuscript: TCT Pun, TNH Chan, WKK Wong, PKF Chiu.
Critical revision of the manuscript for important intellectual content: PSK Chu, WKK Wong, PKF Chiu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary material
The supplementary material was provided by the authors and some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. D’Ancoa C, Haylen B, Oelke M, et al. The International
Continence Society (ICS) report on the terminology for
adult male lower urinary tract and pelvic floor symptoms
and dysfunction. Neurourol Urodyn 2019;38:433-77. Crossref
2. Eapen RS, Radomski SB. Review of the epidemiology of
overactive bladder. Res Rep Urol 2016;8:71-6. Crossref
3. Yee CH, Chan CK, Teoh JY, et al. Survey on prevalence
of lower urinary tract symptoms in an Asian population.
Hong Kong Med J 2019;25:13-20. Crossref
4. DB Ng, McCart M, Klein C, Campbell C, Schoenhaus R,
Berner T. Evaluating outcomes in patients with overactive
bladder within an integrated healthcare delivery system
using a treatment patterns analyzer. Am Health Drug
Benefits 2016;9:343-53.
5. Kraus SR, Bavendam T, Brake T, Griebling TL. Vulnerable
elderly patients and overactive bladder syndrome. Drugs
Aging 2010;27:697-713. Crossref
6. Bragg R, Hebel D, Vouri SM, Pitlick JM. Mirabegron: a
beta-3 agonist for overactive bladder. Consult Pharm
2014;29:823-37. Crossref
7. European Association of Urology. Guidelines for non-neurogenic
female LUTS. Available from: https://uroweb.org/guidelines/non-neurogenic-female-luts/chapter/introduction. Accessed 1 Jun 2023.
8. European Association of Urology. Guidelines for
management of non-neurogenic male LUTS. Available
from: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts/chapter/introduction. Accessed 1 Jun 2023.
9. Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Intl 2008;102:62-6. Crossref
10. Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Eng J Med 2009;360:481-90. Crossref
11. Painter CE, Suskind AM. Advances in pharmacotherapy
for the treatment of overactive bladder. Curr Bladder
Dysfunct Rep 2019;14:377-84. Crossref
12. Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects
of antimuscarinic treatments in overactive bladder: a
systematic review and meta-analysis. Eur Urol 2005;48:5-26. Crossref
13. Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE,
Weinstein D. The effects of antimuscarinic treatments in
overactive bladder: an update of a systematic review and
meta-analysis. Eur Urol 2008;54:543-62. Crossref
14. Khullar V, Chapple C, Gabriel Z, Dooley JA. The effects
of antimuscarinics on health-related quality of life in
overactive bladder: a systematic review and meta-analysis. Urology 2006;68(2 Suppl):38-48. Crossref
15. Khurana AK, Khurana B, Khurana AK. Drug-induced
angle-closure glaucoma. J Curr Glaucoma Pract 2012;6:6-8. Crossref
16. Abrams P, Kaplan S, de Koning Gans HJ, Millard R.
Safety and tolerability of tolterodine for the treatment of
overactive bladder in men with bladder outlet obstruction.
J Urol 2006;175:999-1004. Crossref
17. Isik AT, Celik T, Bozoglu E, Doruk H. Trospium and
cognition in patients with late onset Alzheimer disease. J
Nutr Health Aging 2009;13:672-6. Crossref
18. Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay-Smith EJ. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev
2012;(1):CD005429. Crossref
19. Herschorn S, Barkin J, Castro-Diaz D, et al. A phase III,
randomized, double-blind, parallel-group, placebo-controlled,
multicentre study to assess the efficacy and
safety of the β3 adrenoceptor agonist, mirabegron, in
patients with symptoms of overactive bladder. Urology
2013;82:313-20. Crossref
20. Wani MM, Sheikh MI, Bhat T, Bhat Z, Bhat A. Comparison
of antimuscarinic drugs to beta adrenergic agonists in
overactive bladder: a literary review. Curr Urol 2021;15:153-
60. Crossref
21. Kelleher C, Hakimi Z, Zur R, et al. Efficacy and
tolerability of mirabegron compared with antimuscarinic
monotherapy or combination therapies for overactive
bladder: a systematic review and network meta-analysis.
Eur Urol 2018;74:324-33. Crossref
22. Chapple CR, Siddiqui E. Mirabegron for the treatment
of overactive bladder: a review of efficacy, safety and
tolerability with a focus on male, elderly and antimuscarinic
poor-responder populations, and patients with OAB in
Asia. Expert Rev Clin Pharmacol 2017;10:131-51. Crossref
23. Nakagomi H, Mitsui T, Shimura H, et al. Mirabegron
for overactive bladder in frail patients 80 years or over
(HOKUTO study). BMC Urol 2022;22:40. Crossref
24. Herschorn S, Staskin D, Schermer CR, Kristy RM, Wagg A.
Safety and tolerability results from the PILLAR study: a
phase IV, double-blind, randomized, placebo-controlled
study of mirabegron in patients ≥ 65 years with overactive
bladder-wet. Drugs Aging 2020;37:665-76. Crossref
25. GOV.UK. Mirabegron (Betmiga▼): risk of severe hypertension and associated cerebrovascular and cardiac events. Available from: https://www.gov.uk/drug-safety-update/mirabegron-betmiga-risk-of-severe-hypertension-and-associated-cerebrovascular-and-cardiac-events. Accessed 1 Jun 2023.
26. European Medicines Agency. Assessment report of Betmiga (mirabegron). 2012. Available from: https://www.ema.europa.eu/en/documents/assessment-report/betmiga-epar-public-assessment-report_en.pdf. Accessed 1 Jun 2023.
27. Abrams P, Kelleher C, Staskin D, et al. Combination
treatment with mirabegron and solifenacin in patients
with overactive bladder: exploratory responder analyses of
efficacy and evaluation of patient-reported outcomes from
a randomized, double-blind, factorial, dose-ranging, phase
II study (SYMPHONY). World J Urol 2017;35:827-38. Crossref
28. Herschorn S, Chapple CR, Abrams P, et al. Efficacy and
safety of combinations of mirabegron and solifenacin
compared with monotherapy and placebo in patients
with overactive bladder (SYNERGY study). BJU Int 2017;120:562-75. Crossref
29. Drake MJ, Chapple C, Esen AA, et al. Efficacy and safety
of mirabegron add-on therapy to solifenacin in incontinent
overactive bladder patients with an inadequate response
to initial 4-week solifenacin monotherapy: a randomised
double-blind multicentre phase 3B study (BESIDE). Eur
Urol 2016;70:136-45. Crossref
30. Yamaguchi O, Kakizaki H, Homma Y, et al. Safety and
efficacy of mirabegron as ‘add-on’ therapy in patients
with overactive bladder treated with solifenacin: a postmarketing,
open-label study in Japan (MILAI study). BJU
Int 2015;116:612-22. Crossref
31. Gratzke C, van Maanen R, Chapple C, et al. Long-term
safety and efficacy of mirabegron and solifenacin in
combination compared with monotherapy in patients with
overactive bladder: a randomised, multicentre phase 3
study (SYNERGY II). Eur Urol 2018;74:501-9. Crossref
32. Yamaguchi O, Kakizaki H, Homma Y, et al. Long-term
safety and efficacy of antimuscarinic add-on therapy in
patients with overactive bladder who had a suboptimal response to mirabegron monotherapy: a multicenter,
randomized study in Japan (MILAI II study). Int J Urol
2019;26:342-52. Crossref
33. Wang M, Jian Z, Ma Y, Jin X, Li H, Wang K. Percutaneous
tibial nerve stimulation for overactive bladder syndrome:
a systematic review and meta-analysis. Int Urogynecol J
2020;31:2457-71. Crossref
34. Peters KM, Macdiarmid SA, Wooldridge LS, et al.
Randomized trial of percutaneous tibial nerve stimulation
versus extended-release tolterodine: results from the
overactive bladder innovative therapy trial. J Urol
2009;182:1055-61. Crossref
35. Nitti VW, Dmochowski R, Herschorn S, et al.
OnabotulinumtoxinA for the treatment of patients with
overactive bladder and urinary incontinence: results of
a phase 3, randomized, placebo controlled trial. J Urol
2017;197(2S):S216-23. Crossref
36. Brazzelli M, Murray A, Fraser C. Efficacy and safety of
sacral nerve stimulation for urinary urge incontinence: a
systematic review. J Urol 2006;175:835-41. Crossref