Primary pelvic retroperitoneal ancient schwannoma—a rare diagnosis of pelvic complex cystic lesion
Hong
Kong Med J 2019 Apr;25(2):160–1.e1–3
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Primary pelvic retroperitoneal ancient schwannoma—a
rare diagnosis of pelvic complex cystic lesion
TS Chan, MB, BS, FRCR; T Wong, FRCR, FHKAM
(Radiology); NY Pan, FRCR, FHKAM (Radiology)
Department of Radiology, Princess Margaret
Hospital, Kwai Chung, Hong Kong
Corresponding author: Dr TS Chan (drsunchan@gmail.com)
A 42-year-old woman was admitted through our
emergency department for subacute onset of lower abdominal pain in
September 2014. Bedside ultrasound by a gynaecology specialist showed a
left adnexal cyst with fluid interface. Magnetic resonance imaging of the
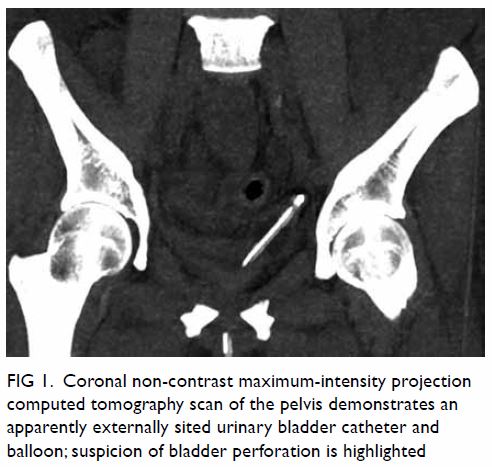
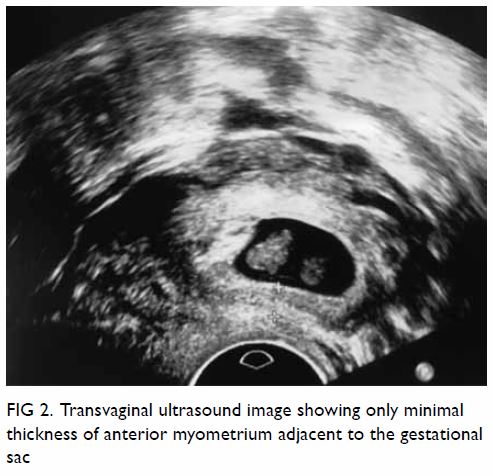
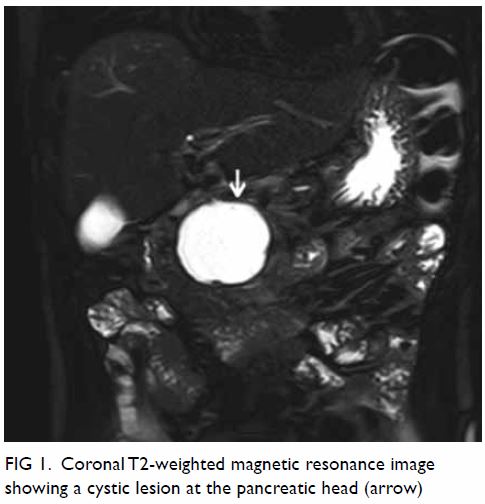
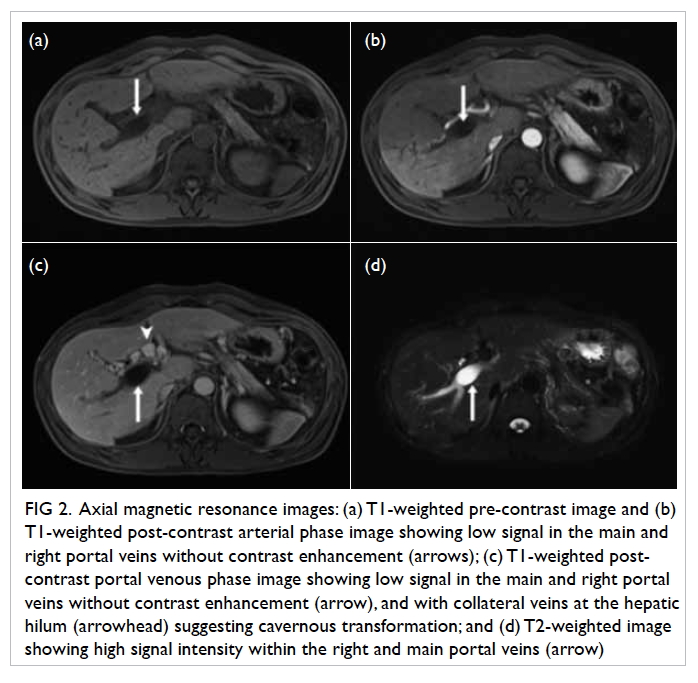
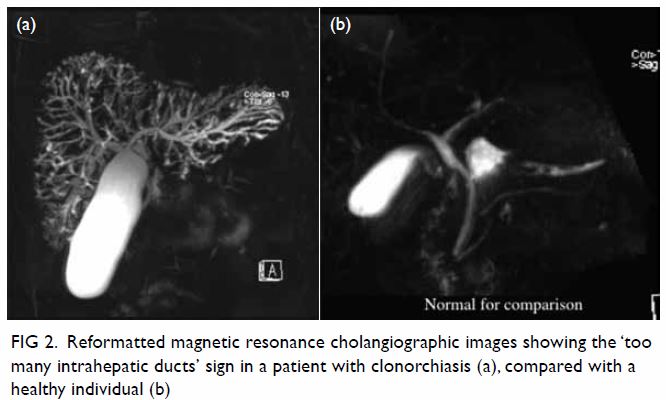
pelvis showed a large well-defined multiloculated cystic lesion measuring
9.2 cm (width) × 8.5 cm (depth) × 8.9 cm (height) with thick T1 and T2
hypointense enhancing wall and septa at the left side of the pelvis (Fig
1). An internal slightly T1 hyperintense component with fluid-fluid
level was suspected to be proteinaceous or haemorrhagic content. A 1.6-cm
non-enhancing mural nodule was noted at the posterior aspect. Partial
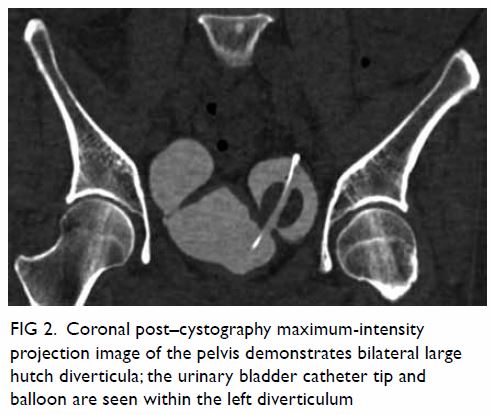
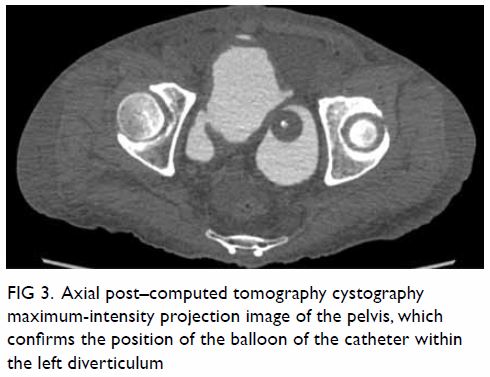
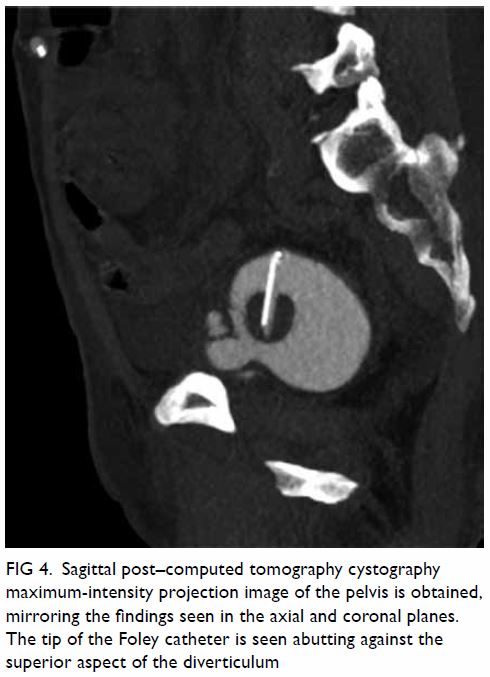
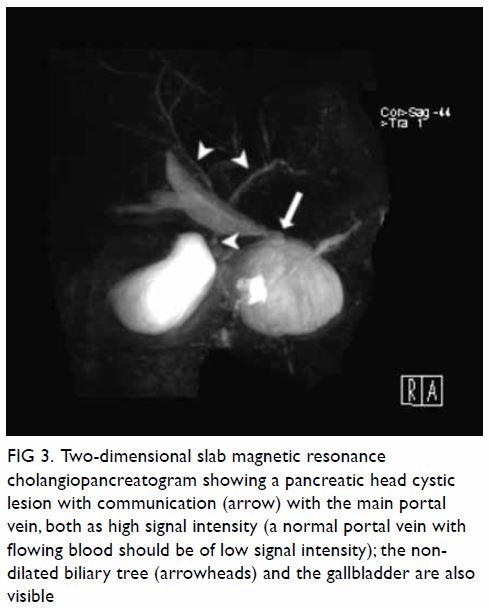
bicornuate uterus was noted. Bilateral ovaries were normal in size with
small cysts (Fig 2). Laparoscopy was done, with
excision of the lesion. Intra-operative frozen section showed a benign
spindle cell tumour. Final histopathological evaluation revealed a
retroperitoneal schwannoma.
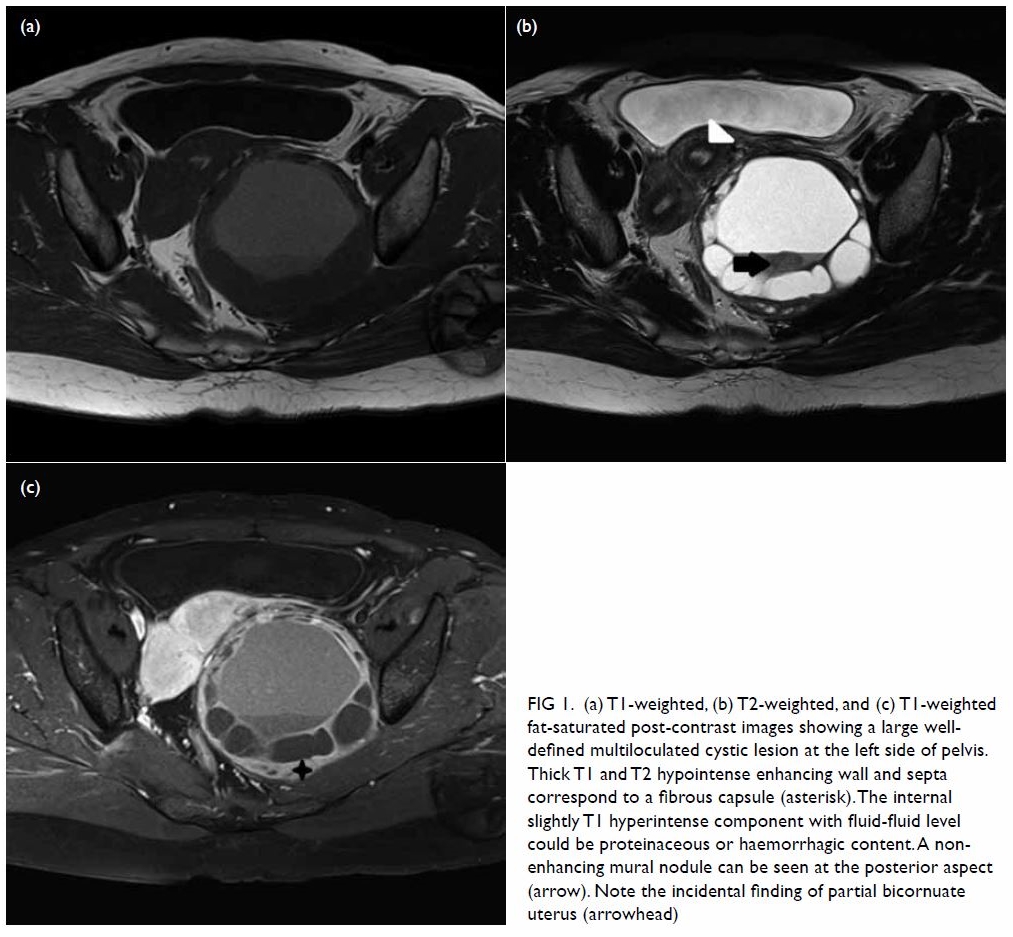
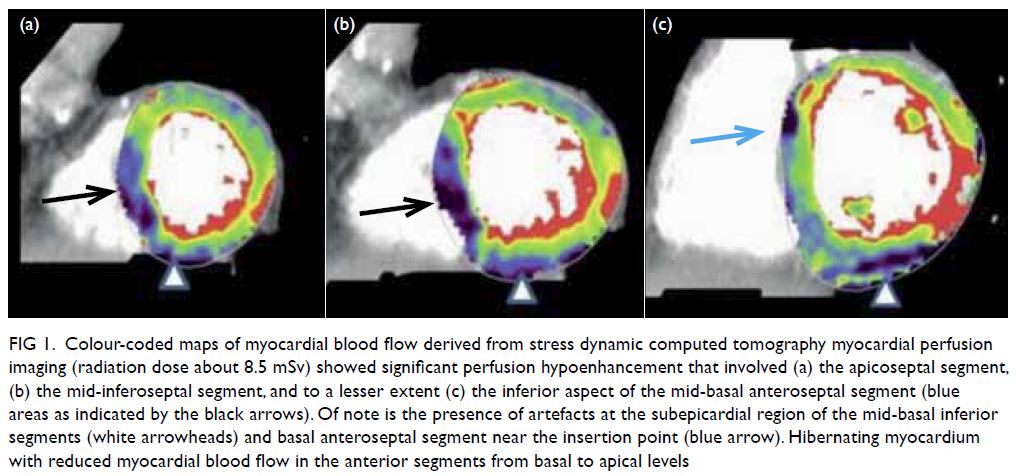
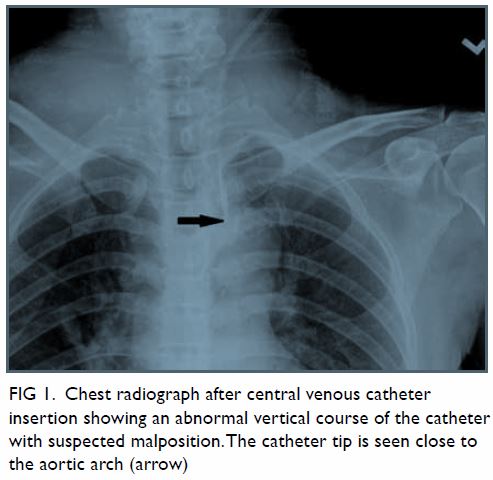
Figure 1. (a) T1-weighted, (b) T2-weighted, and (c) T1-weighted fat-saturated post-contrast images showing a large welldefined multiloculated cystic lesion at the left side of pelvis. Thick T1 and T2 hypointense enhancing wall and septa correspond to a fibrous capsule (asterisk). The internal slightly T1 hyperintense component with fluid-fluid level could be proteinaceous or haemorrhagic content. A nonenhancing mural nodule can be seen at the posterior aspect (arrow). Note the incidental finding of partial bicornuate uterus (arrowhead)
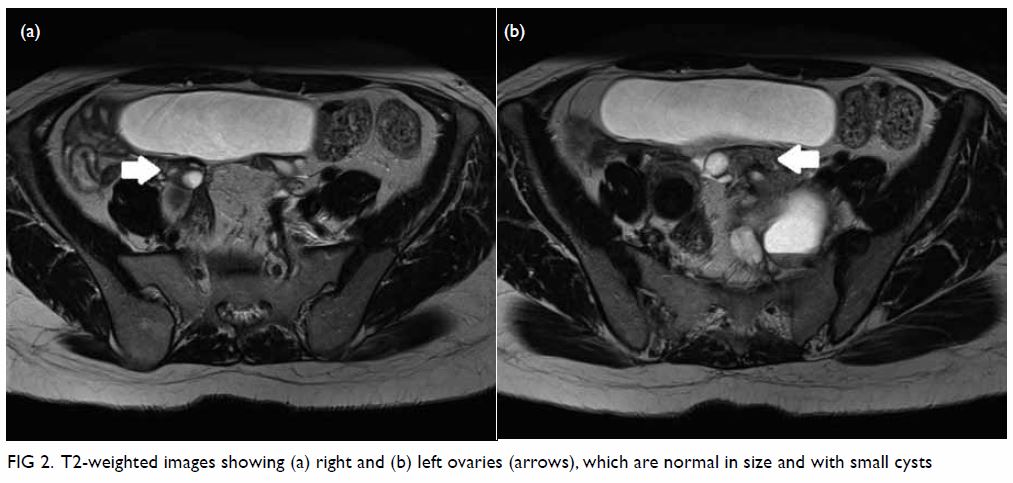
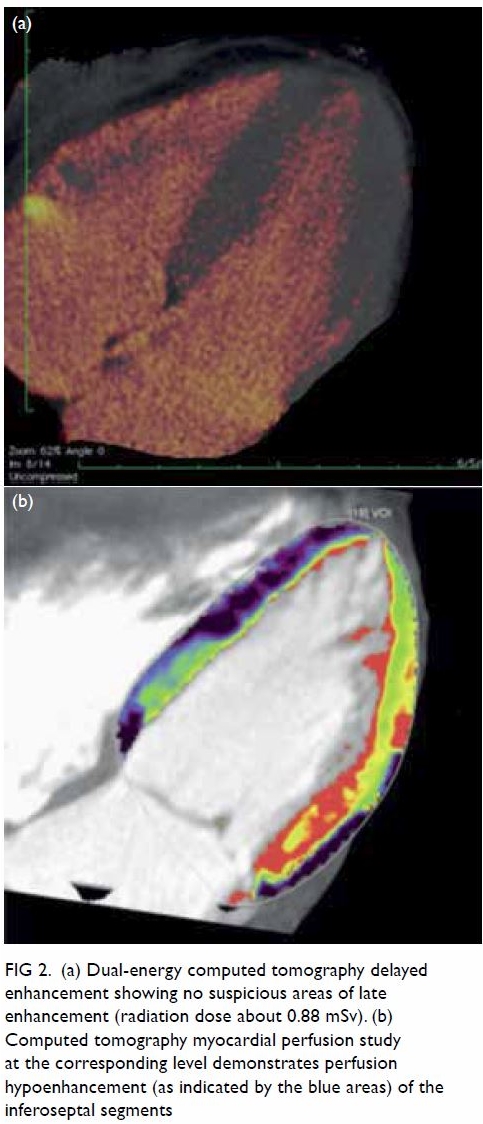
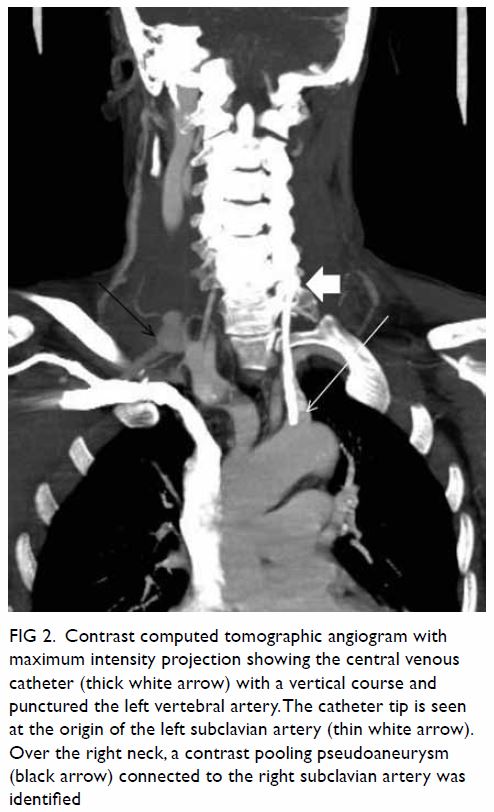
Figure 2. T2-weighted images showing (a) right and (b) left ovaries (arrows), which are normal in size and with small cysts
The majority of cystic pelvic masses originate from
the ovary. Mimics of ovarian cystic masses have a wide variety of
diagnoses. It is important to understand the relationship of a mass with
its anatomic location, identify normal ovaries at imaging, and correlate
imaging findings with the patient’s clinical history to avoid
misdiagnosis.
Retroperitoneal schwannoma is a rare tumour and is
difficult to diagnose, accounting for only 6% of retroperitoneal
neoplasms. A retroperitoneal schwannoma is usually located in the
paravertebral space or pre-sacral pelvic retroperitoneum.1 It usually occurs in young to middle-aged adults, and
women are affected twice as often as men.1
The patient is usually asymptomatic, or complains of a wide variety of
non-specific symptoms when the tumour is large in size.2 Malignant transformation is rare.1 On magnetic resonance images, a schwannoma appears as a
well-defined mass with hypo- or iso-intensity on T1-weighted images and
with hyperintensity on T2-weighted images. The nerve of origin is often
difficult to identify. It is not unusual for a schwannoma to display
cystic changes. However, prominent cystic changes are uncommon and point
to ancient schwannoma, a rare variant of schwannoma that is characterised
by degeneration and decreased cellularity.3
On magnetic resonance images, ancient schwannoma appears as a
well-defined, complex cystic mass with a variable enhancement pattern.
Thick T1 and T2 hypointense enhancing wall and septa correspond to a
fibrous capsule, consisting of epineurium and residual nerve fibres.4
Identification of the nerve adjacent to or along
the tumour is useful for differentiating ancient schwannomas from other
complex cystic lesions, such as serous or mucinous cystadenocarcinoma,
abscess, necrotic soft-tissue sarcoma, or necrotic metastatic
lymphadenopathy.5
In the present case, the patient did not complain
of any neurological symptoms at presentation. The presence of normal
ovaries and fibrous capsule indicated a preoperative diagnosis of ancient
schwannoma. This case illustrates the importance of considering this
uncommon diagnosis when a pelvic complex cystic lesion is detected in
imaging, and seeking specific imaging features (such as fibrous capsule
and close relationship to the nerve) to confirm or exclude this diagnosis.
This would facilitate surgical planning and minimise the risk of
complications such as major neurological deficit.
Author contributions
All authors contributed to the concept or design,
acquisition of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision for important intellectual content. All
authors had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
References
1. Rha SE, Byun JY, Jung SE, Chun HJ, Lee
HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging
characteristics. Radiographics 2003;23:29-43. Crossref
2. Kim SH, Choi BI, Han MC, Kim YI.
Retroperitoneal neurilemoma: CT and MR findings. AJR Am J Roentgenol
1992;159:1023-6. Crossref
3. Dahl I. Ancient neurilemmoma
(schwannoma). Acta Pathol Microbiol Scand A 1977;85:812-8. Crossref
4. Takeuchi M, Matsuzaki K, Nishitani H,
Uehara H. Ancient schwannoma of the female pelvis. Abdom Imaging
2008;33:247-52. Crossref
5. Isobe K, Shimizu T, Akahane T, Kato H.
Imaging of ancient schwannoma. AJR Am J Roentgenol 2004;183:331-6. Crossref