Hong
Kong Med J 2018 Dec;24(6):634–5.e1–4
DOI: 10.12809/hkmj176964
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Magnetic resonance imaging monitoring of post-treatment
changes to Crohn’s disease–related anal fistula in patients prescribed
infliximab
KY Man, MB, ChB, FRCR1; Esther MF Wong,
MB, BS, FHKCR1; Francis KY Cho, MB, BS, FHKCR1; CM
Leung, FHKAM (Medicine)2
1 Department of Radiology, Pamela Youde
Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Medicine, Pamela Youde
Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr KY Man (dsgundam@hotmail.com)
The incidence of inflammatory bowel disease,
particularly Crohn’s disease, is rising in Hong Kong.1 The age-adjusted incidence of Crohn’s disease increased
from 0.01 per 100 000 population in 1985 to 1.46 per 100 000 population in
2014.1 Crohn’s disease is a
multisystem disorder with specific radiological features such as
transmural inflammation, fistulation, and skip lesions. Perianal fistulas
often complicate Crohn’s disease, affecting up to 36% of patients.2
Infliximab, a monoclonal antibody against tumour
necrosis factor-α, has revolutionised the treatment of Crohn’s
disease–related anal fistula. Current evidence shows encouraging results
for closure of perianal fistulas. According to a local consensus
statement, biologics are advocated in patients with active fistulising
Crohn’s disease, particularly those with complex perianal fistulising
disease.3
Response to monoclonal antibody therapy needs to be
monitored. This pictorial review illustrates the post-treatment changes on
magnetic resonance imaging (MRI) of anal fistula in patients prescribed
infliximab.
Patients with a known history of Crohn’s disease
complicated by perianal fistula and prescribed infliximab between 2012 and
2016 were reviewed. The treatment regimen at our centre comprises an
intravenous loading dose of infliximab 5 mg/kg, followed by the same dose
at week 2 and week 6. Thereafter a maintenance dose of 5 mg/kg is given
every 8 weeks.
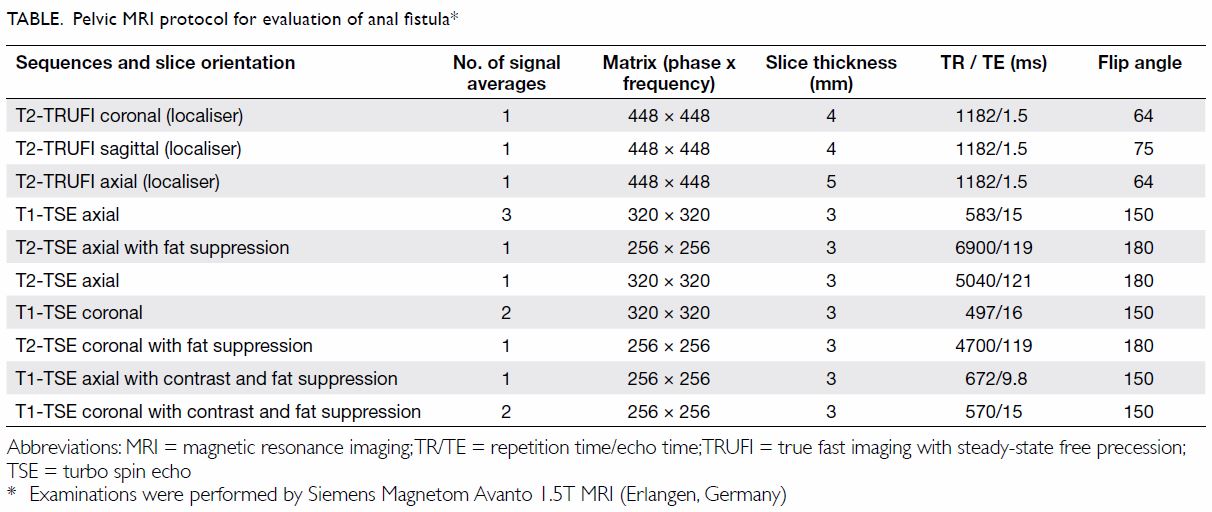
Magnetic resonance images were acquired with the
1.5T Siemens Magnetom Avanto system (Erlangen, Germany). The pelvic MRI
protocol for perianal fistula evaluation consists of T1-weighted and
high-spatial-resolution T2-weighted imaging sequences without fat
saturation to delineate the muscle groups, fat planes, and fistula tract.
T2-weighted imaging with fat suppression is used to assess oedema and
fluid-containing tracts and cavities, whereas fat-suppressed T1-weighted
unenhanced and contrast-enhanced sequences are used to assess the presence
and degree of inflammation (Table). Diffusion-weighted imaging is not routinely
performed in view of the need for an extended examination time.
Information about the presence of fluid, oedema, cavities, and
inflammation can be obtained through these sequences. Anal fistulae are
classified according to the Parks’ classification system (Fig
1).4
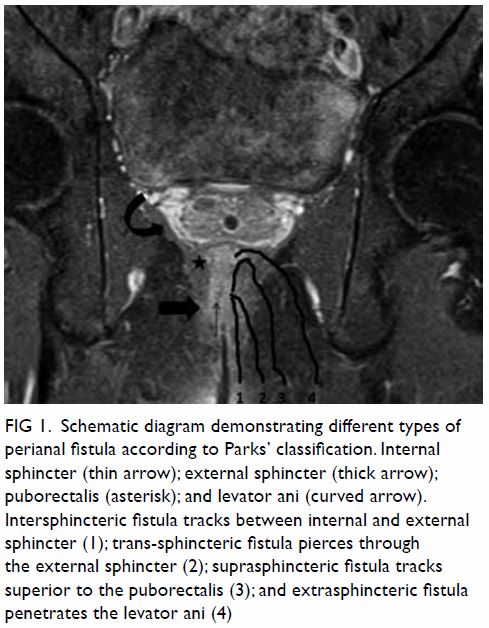
Figure 1. Schematic diagram demonstrating different types of perianal fistula according to Parks’ classification. Internal sphincter (thin arrow); external sphincter (thick arrow); puborectalis (asterisk); and levator ani (curved arrow). Intersphincteric fistula tracks between internal and external sphincter (1); trans-sphincteric fistula pierces through the external sphincter (2); suprasphincteric fistula tracks superior to the puborectalis (3); and extrasphincteric fistula penetrates the levator ani (4)
Three patients (two men, one woman) were reviewed
and all received infliximab. At least one pre-treatment and one
post-treatment MRI were performed.
Patient A was a 34-year-old woman with a history of
systemic lupus erythematosus, retinitis, and neuropsychiatric lupus. She
had had recurrent ischiorectal abscess and perianal fistula since 2002.
Rectal biopsy confirmed Crohn’s disease. Despite treatment with
azathioprine, the perianal fistula failed to close. She was scheduled to
initially receive three doses of infliximab. Close monitoring was
essential in view of the potential to develop lupus-like disease. Progress
MRI after the third dose of infliximab showed slight interval improvement
in her perianal fistula. Biologics were continued in view of the residual
disease. After the seventh dose of infliximab, progress MRI revealed a
largely quiescent perianal fistula (Fig 2). In view of the radiologically healed
fistula, clinical improvement and potential risk of lupus-like disease,
the decision was taken to stop the infliximab infusion but continue close
clinical and radiological monitoring.
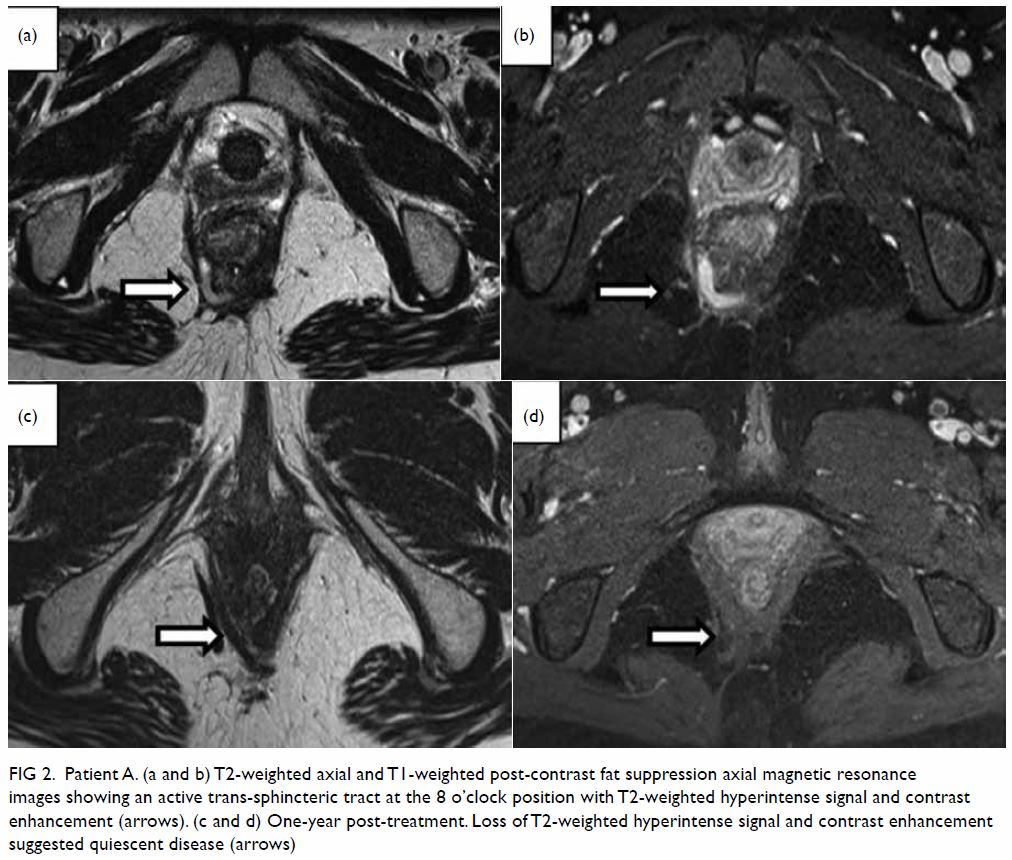
Figure 2. Patient A. (a and b) T2-weighted axial and T1-weighted post-contrast fat suppression axial magnetic resonance images showing an active trans-sphincteric tract at the 8 o’clock position with T2-weighted hyperintense signal and contrast enhancement (arrows). (c and d) One-year post-treatment. Loss of T2-weighted hyperintense signal and contrast enhancement suggested quiescent disease (arrows)
Patient B was a 24-year-old man with a history of
perianal fistula since 2015 and an episode of perianal abscess that
required incision and drainage. Crohn’s disease was confirmed on rectal
biopsy. He had previously developed azathioprine-induced pancytopenia.
Subsequent infusion of infliximab infusion resulted in responsive disease,
evident on MRI (Fig 3). Clinical and radiological monitoring
(progress MRI) at 6-month intervals was carried out to determine progress
of the perianal fistula. Infliximab would be stopped when there was
evidence of healed tract and clinical improvement.
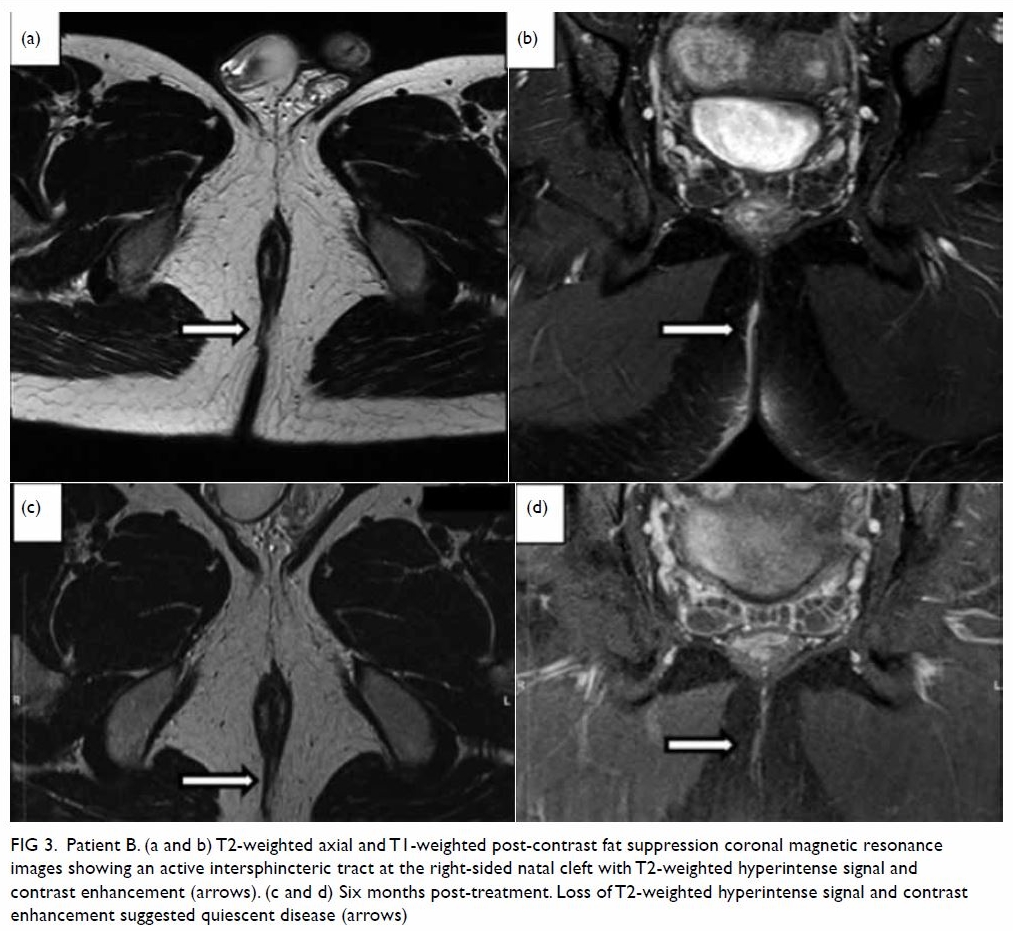
Figure 3. Patient B. (a and b) T2-weighted axial and T1-weighted post-contrast fat suppression coronal magnetic resonance images showing an active intersphincteric tract at the right-sided natal cleft with T2-weighted hyperintense signal and contrast enhancement (arrows). (c and d) Six months post-treatment. Loss of T2-weighted hyperintense signal and contrast enhancement suggested quiescent disease (arrows)
Patient C, a 42-year-old man had a history of
ileocolic Crohn’s disease since 2008, with episodes of perianal abscess
and fistula refractory to steroid and azathioprine treatment. Magnetic
resonance imaging showed progressive perianal fistula (Fig
4) after the second maintenance dose of infliximab. Previous
infliximab dose/frequency was continued and progress MRI planned for the
purpose of reassessment and consideration of alternative treatment if
there was persistent progression.
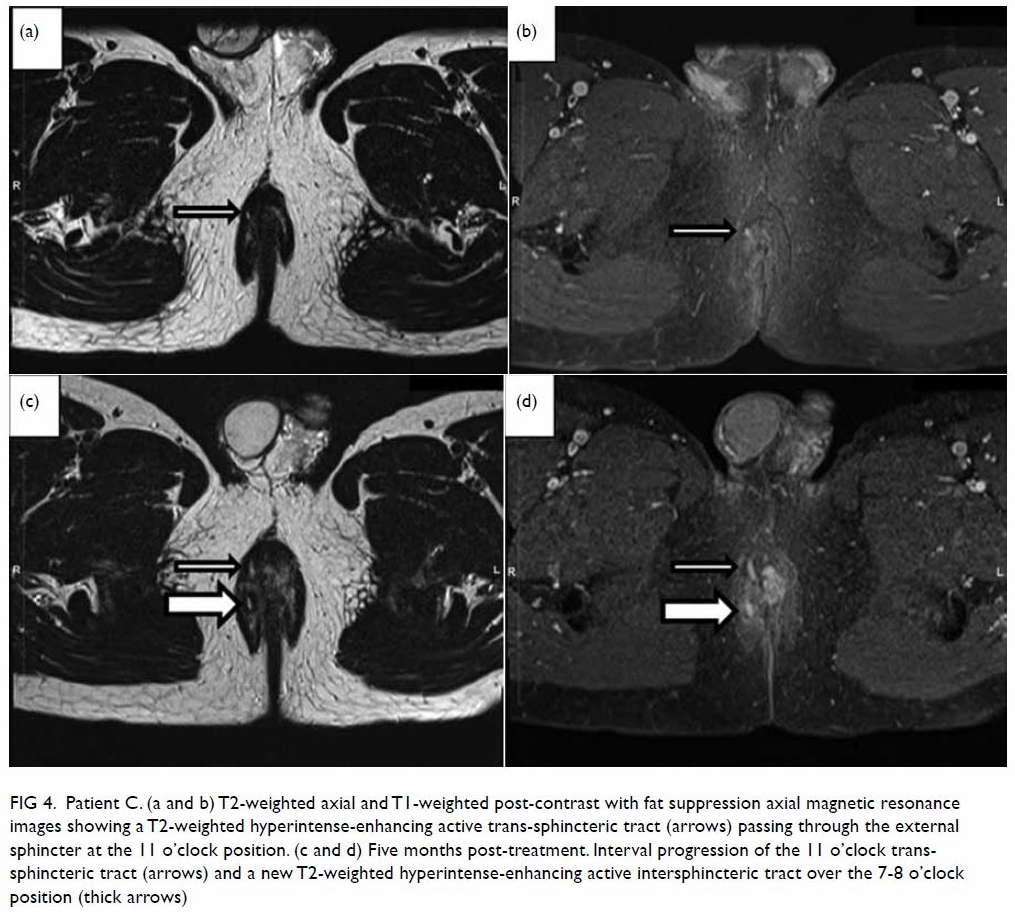
Figure 4. Patient C. (a and b) T2-weighted axial and T1-weighted post-contrast with fat suppression axial magnetic resonance images showing a T2-weighted hyperintense-enhancing active trans-sphincteric tract (arrows) passing through the external sphincter at the 11 o’clock position. (c and d) Five months post-treatment. Interval progression of the 11 o’clock transsphincteric tract (arrows) and a new T2-weighted hyperintense-enhancing active intersphincteric tract over the 7-8 o’clock position (thick arrows)
Crohn’s disease–related anal fistulae are
frequently encountered in radiologic practice due to their complexity and
propensity for incomplete treatment response and relapse.
Magnetic resonance imaging is a well-established
diagnostic tool for anal fistula. Its inherent high spatial and contrast
resolution allows precise anatomical delineation.5
Magnetic resonance imaging plays a critical role in helping determine the
appropriate treatment that should be individualised according to the type
of perianal fistula and the degree of involvement of surrounding pelvic
structures. Clinical examination can often be difficult because of
induration and inflammation in patients with anal sepsis. At MRI,
identification and localisation of the entire fistula, including the
external opening, the primary track, secondary tracks, abscesses, and the
internal opening, are essential for fistula classification and treatment.
Inadequate assessment may result in progression of a simple fistula to a
complex fistula, and failure to recognise secondary extensions can result
in recurrent sepsis. Anti–tumour necrosis factor antibodies (infliximab)
have been introduced with good clinical results. Magnetic resonance
imaging also plays an important role in evaluation of the response to
medical therapy. Magnetic resonance imaging does not have field of view
limitations and offers excellent views of the supralevator, retrorectal
and anteroanal spaces, where occult sepsis may be missed clinically due to
extensive scarring or a remote location.6
This pictorial review demonstrates the ability of
MRI to monitor the response to therapy of anal fistula in Crohn’s disease
patients receiving infliximab.
Author contributions
KY Man is responsible for the design, acquisition
and interpretation of data, and drafting of the article. EMF Wong, FKY
Cho, and CM Leung are responsible for critical revision for important
intellectual content.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
References
1. Ng SC, Leung WK, Shi HY, et al.
Epidemiology of inflammatory bowel disease from 1981 to 2014: results from
a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis
2016;22:1954-60. Crossref
2. Leong RW, Lau JY, Sung JJ. The
epidemiology and phenotype of Crohn’s disease in the Chinese population.
Inflamm Bowel Dis 2004;10:646-51. Crossref
3. Leung WK, Ng SC, Chow DK, et al. Use of
biologics for inflammatory bowel disease in Hong Kong: consensus
statement. Hong Kong Med J 2013;19:61-8.
4. Parks AG, Gordon PH, Hardcastle JD. A
classification of fistula-in-ano. Br J Surg 1976;63:1-12. Crossref
5. Buchanan G, Halligan S, Williams A, et
al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet
2002;360:1661-2. Crossref
6. Bell SJ, Halligan S, Windsor AC,
Williams AB, Wiesel P, Kamm MA. Response of fistulating Crohn’s disease to
infliximab treatment assessed by magnetic resonance imaging. Aliment
Pharmacol Ther 2003;17:387-93. Crossref