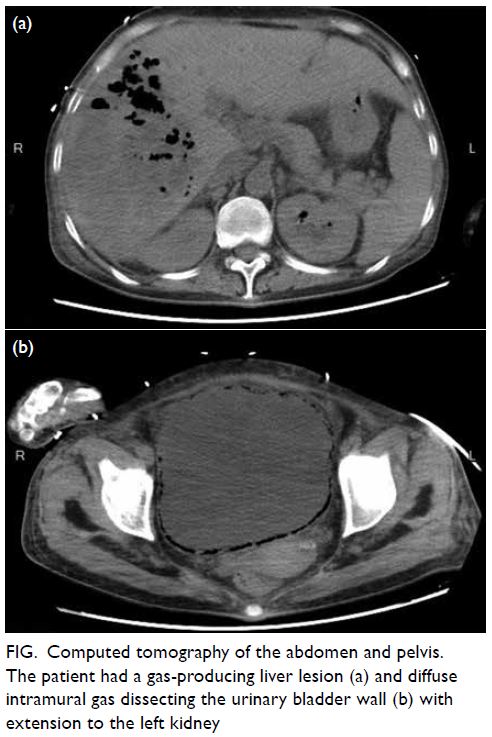
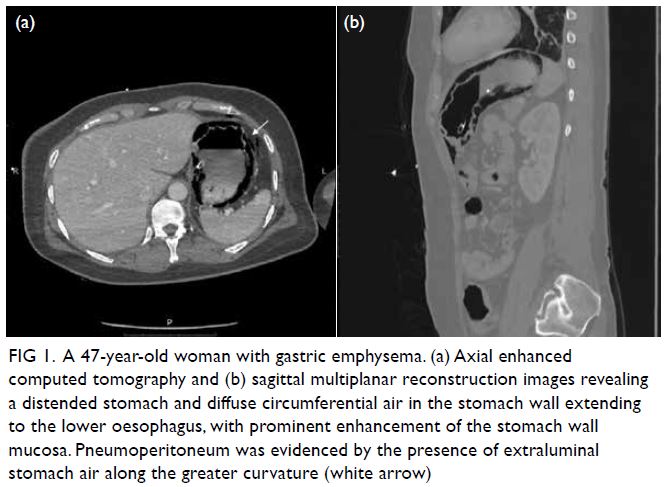
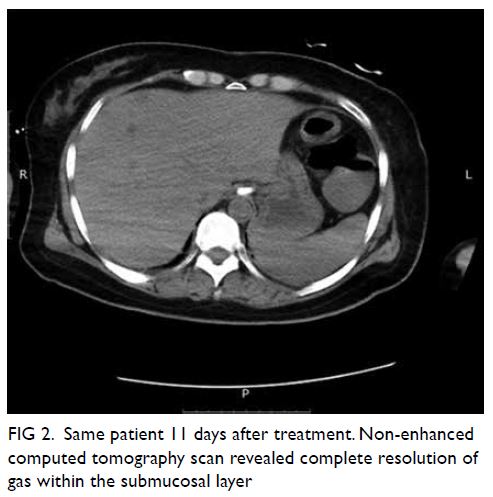
Indocyanine green angiography and lymphography in microsurgical subinguinal varicocelectomy with evolving video microsurgery and fluorescence imaging platforms
Hong Kong Med J 2022 Apr;28(2):181.e1–2
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Indocyanine green angiography and
lymphography in microsurgical subinguinal
varicocelectomy with evolving video
microsurgery and fluorescence imaging platforms
CL Cho, FRCSEd (Urol), FHKAM (Surgery)1; Ringo WH Chu, FRCSEd (Urol), FHKAM (Surgery)2
1 SH Ho Urology Centre, Department of Surgery, The Chinese University of Hong Kong, Hong Kong
2 Private Practice
Corresponding author: Dr CL Cho (chochaklam@yahoo.com.hk)
Intra-operative use of indocyanine green (ICG)
angiography and lymphography has been reported as
a valuable adjunct during microsurgical subinguinal
varicocelectomy (MSV).1 The development of a
video microsurgery platform and fluorescence
imaging technology further facilitates identification
of testicular arteries and lymphatics. We report
the intra-operative imaging of two patients who
underwent varicocele repair for grade 3 left
varicoceles in February and March 2021 using the
new platform.
The operations were performed under three-dimensional
(3D) optical magnification images on
the television monitors using the video microsurgery
platform with VITOM® 3D system (KARL STORZ
SE, Tuttlingen, Germany) as previously described.2
A pack of 25 mg ICG (Diagnogreen; Daiichi Sankyo
Co, Tokyo, Japan) was dissolved in 10 mL water.
Identification of testicular arteries was assisted by
ICG angiography with intravenous injection of 2 mL
(5 mg) ICG solution. After preservation of the
testicular arteries, the differentiation between
lymphatics and small veins was facilitated by
ICG lymphography performed following intra-parenchymal
testicular injection of 0.5 mL (1.25 mg)
ICG solution followed by gentle testicular massage.3 The setting of ICG fluorescence imaging consisted
of an IMAGE1 S™ 4U RUBINA™ with 4K 3D
monitor system (KARL STORZ SE, Tuttlingen,
Germany) and a HOPKINS™ Straight Forward
Telescope 0° (10-mm diameter/20-cm length)
[KARL STORZ SE, Tuttlingen, Germany]. We
demonstrate intra-operative ICG angiography of
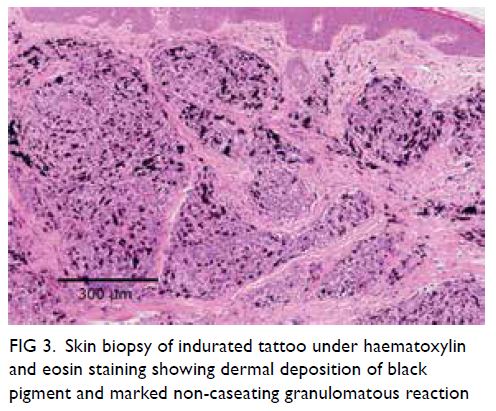
patient 1, a 35-year-old man with primary infertility
and oligoasthenoteratozoospermia. The testicular
artery appeared green on the overlay image mode
with clear simultaneous visualisation of white light
microscopy images in the background (Fig 1a and b).
In addition to the testicular artery, the small
(<1 mm) cremasteric artery could also be identified
in the monochromatic mode that further enhanced
the contrast (Fig 1c). After successful preservation
of the testicular arteries in patient 2, a 28-year-old
man with left scrotal pain, two probable lymphatics
were identified under white light microscopy
(Fig 2a). The strong green colouration seen in the
overlay mode after ICG injection unambiguously
confirmed the successful preservation of lymphatics
in MSV (Fig 2b).
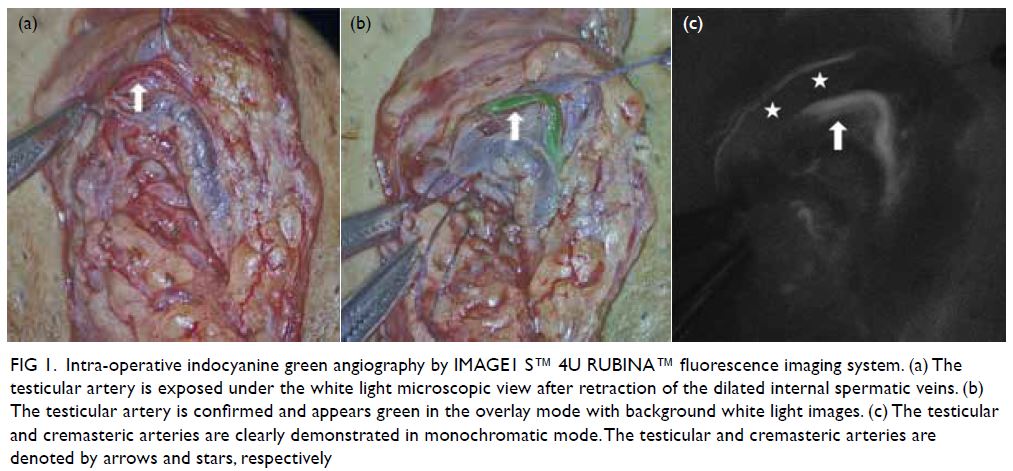
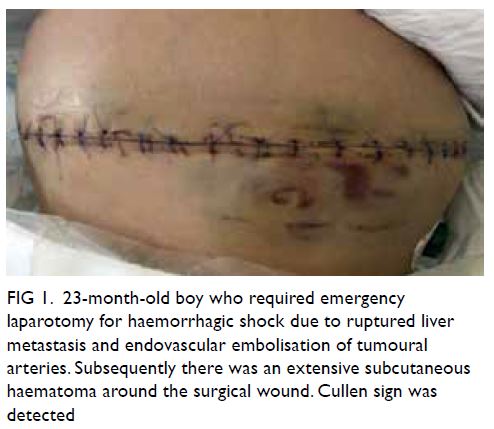
Figure 1. Intra-operative indocyanine green angiography by IMAGE1 S™ 4U RUBINA™ fluorescence imaging system. (a) The testicular artery is exposed under the white light microscopic view after retraction of the dilated internal spermatic veins. (b) The testicular artery is confirmed and appears green in the overlay mode with background white light images. (c) The testicular and cremasteric arteries are clearly demonstrated in monochromatic mode. The testicular and cremasteric arteries are denoted by arrows and stars, respectively
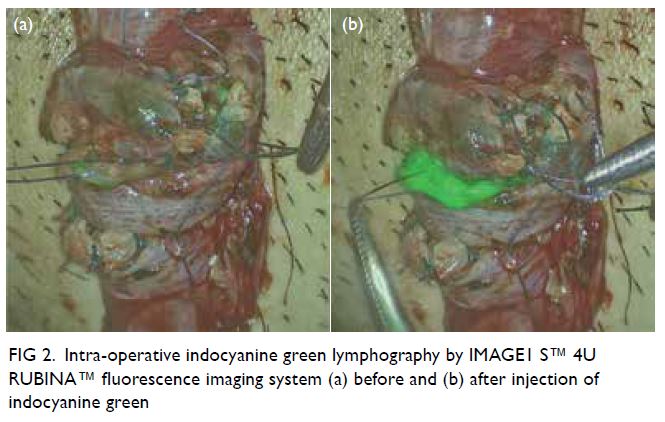
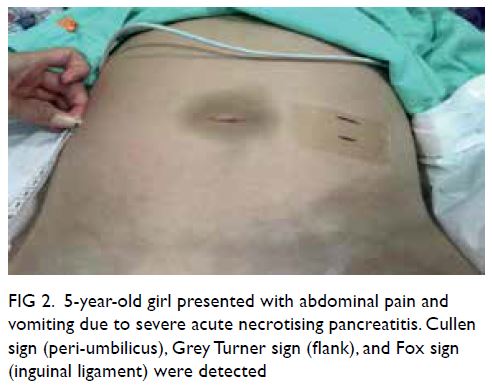
Figure 2. Intra-operative indocyanine green lymphography by IMAGE1 S™ 4U RUBINA™ fluorescence imaging system (a) before and (b) after injection of indocyanine green
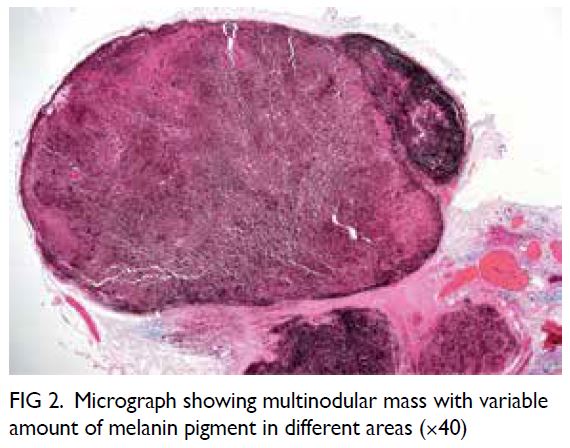
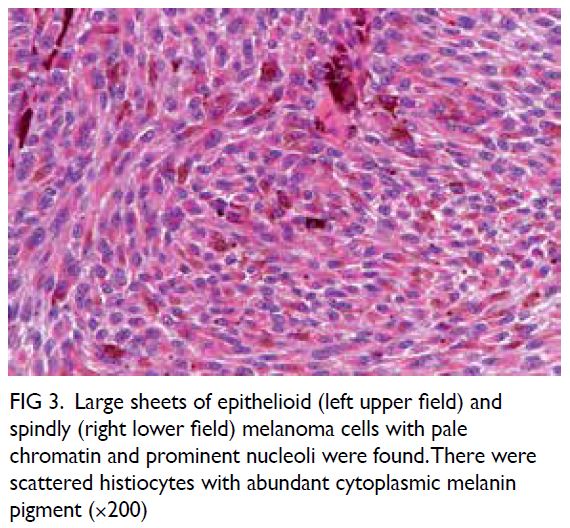
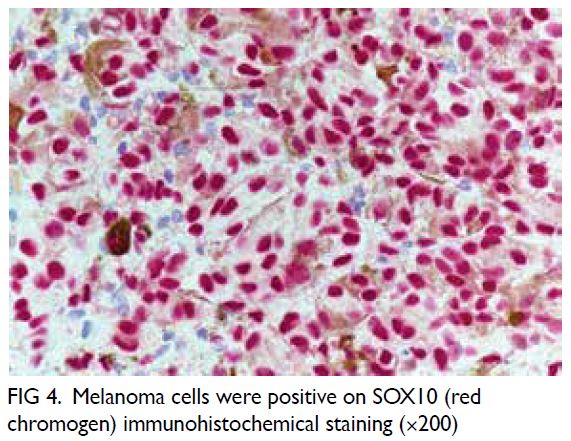
Microsurgical subinguinal varicocelectomy
is the standard for varicocele repair with excellent
surgical outcomes reported.4 Nonetheless identification of testicular arteries and lymphatics
under white light microscopy alone is operator-dependent
and remains challenging for novice
surgeons. Several adjuncts have been introduced to
facilitate artery- and lymphatic-sparing procedures
during MSV. One such adjunct is ICG fluorescence
imaging.1 In our opinion, the new overlay mode
provided by the latest platform is particularly useful
for MSV. Without the need to switch between
different modes, the combined regular white light
image and near-infrared/ICG data allow accurate
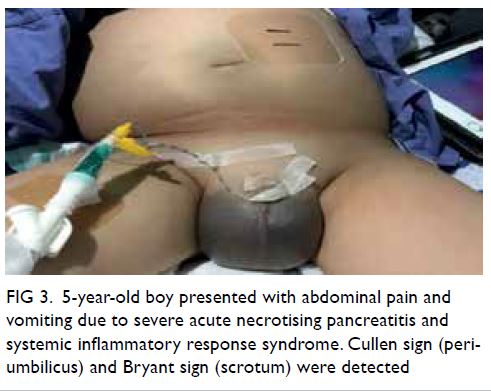
localisation of even the smallest vessel without ambiguity (Fig 3). Moreover, the display of ICG
signal alone in white on a black background in the
monochromatic mode maximises contrast and
further improves identification of target vessels
(Fig 3). We believe the advances of this surgical
platform and imaging technology play a role in
enhancing patient safety by increasing the success of
arterial and lymphatic preservation in MSV.

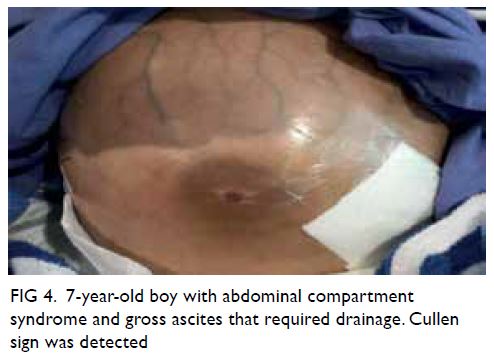
Figure 3. Intra-operative indocyanine green (ICG) angiography with the previous fluorescence imaging platform utilising VITOM II ICG system with SPIES camera (KARL STORZ SE, Tuttlingen, Germany). Without the overlay mode, the images of (a) white light microscopy view and (b) Chroma mode can only be analysed separately. Correlation between the testicular arteries identified on ICG angiography and white light microscopy can be difficult
Author contributions
Concept or design: CL Cho.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have disclosed no conflict of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Patients were treated in accordance with the Declaration of Helsinki. Patients provided written informed consent for the procedures, and verbal consent for publication.
References
1. Cho CL. Is there any role for indocyanine green
angiography in testicular artery preservation during
microsurgical subinguinal varicocelectomy? In: Esteves
SC, Cho CL, Majzoub A, Agarwal A, editors. Varicocele
and Male Infertility: a Complete Guide. Switzerland AG:
Springer Nature; 2019: 603-14. Crossref
2. Cho CL, Chu RW. Use of video microsurgery platform
in microsurgical subinguinal varicocelectomy with
indocyanine green angiography. Surg Prac 2019;23:20-4. Crossref
3. Cho CL, Chiu PK, Chu RW. Preliminary experience with
indocyanine green lymphography during microsurgical
subinguinal varicocelectomy. Surg Prac 2021;25:207-10. Crossref
4. Zini A. Varicocelectomy: microsurgical subinguinal technique is the treatment of choice. Can Urol Assoc J
2007;1:273-6.


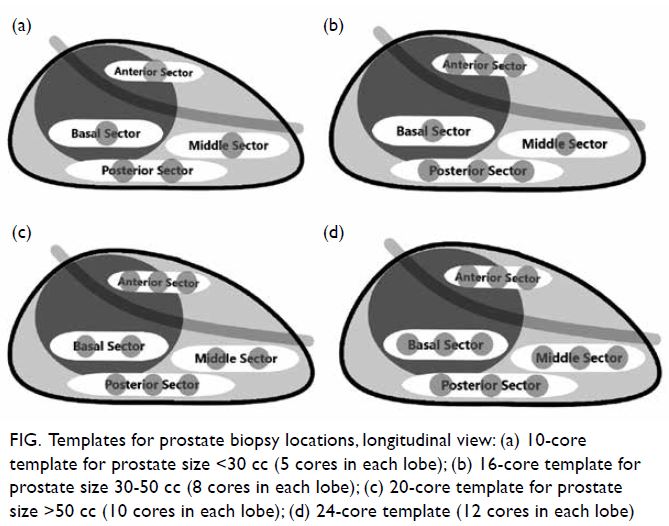
 A video clip demonstrating systematic transperineal prostate biopsy is avaialble at
A video clip demonstrating systematic transperineal prostate biopsy is avaialble at