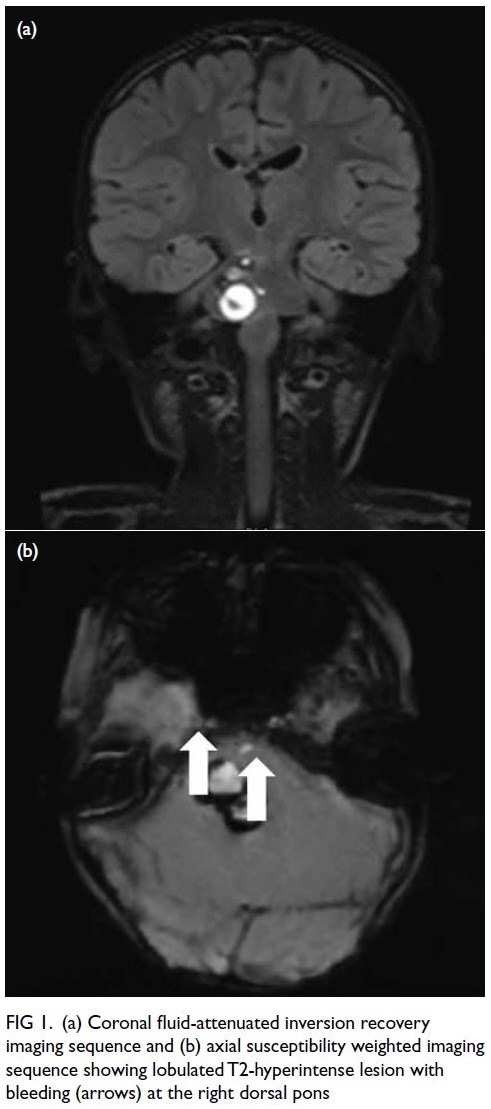
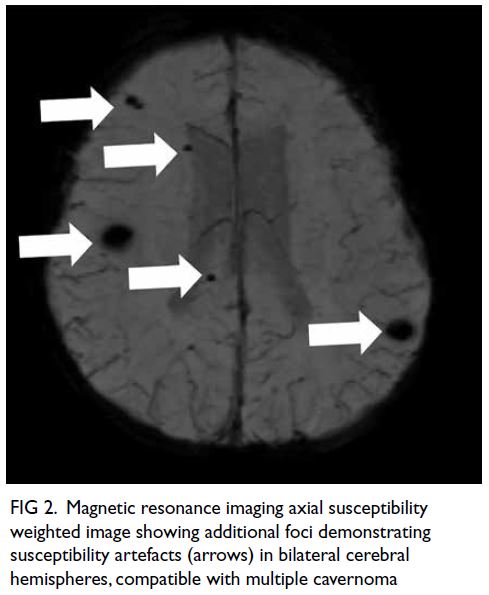
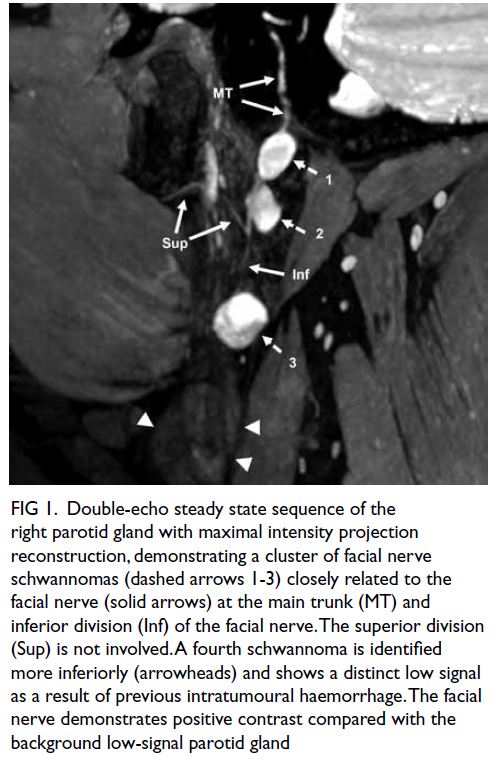
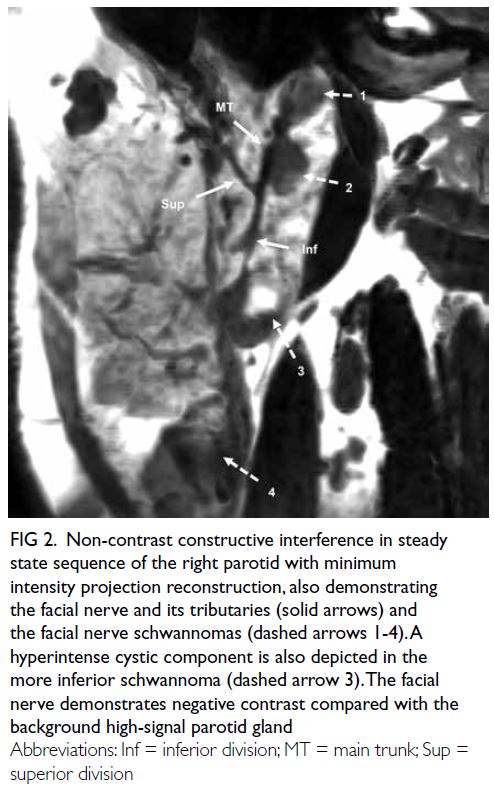
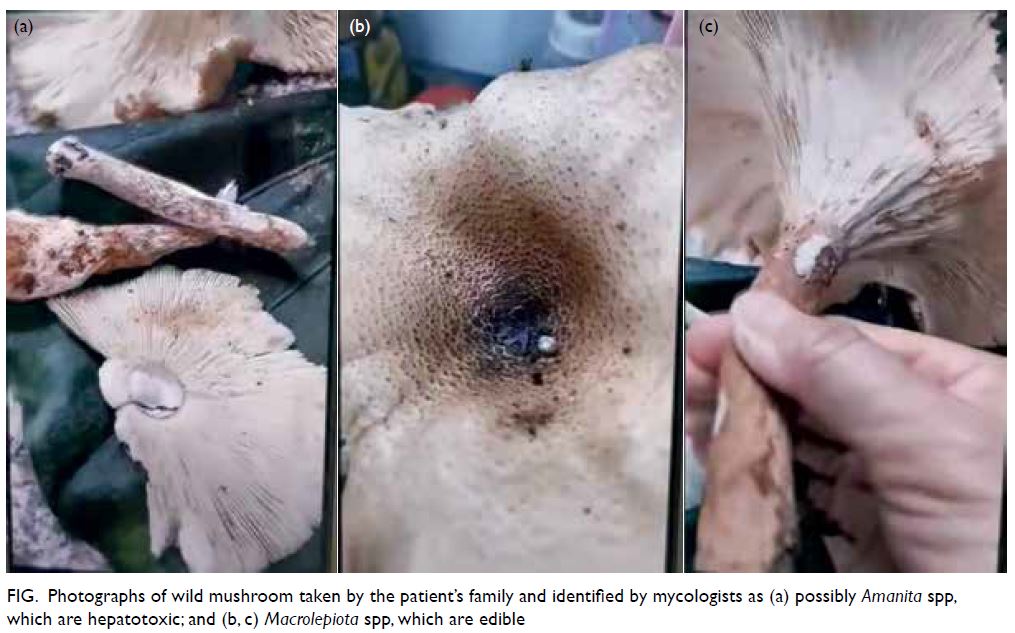
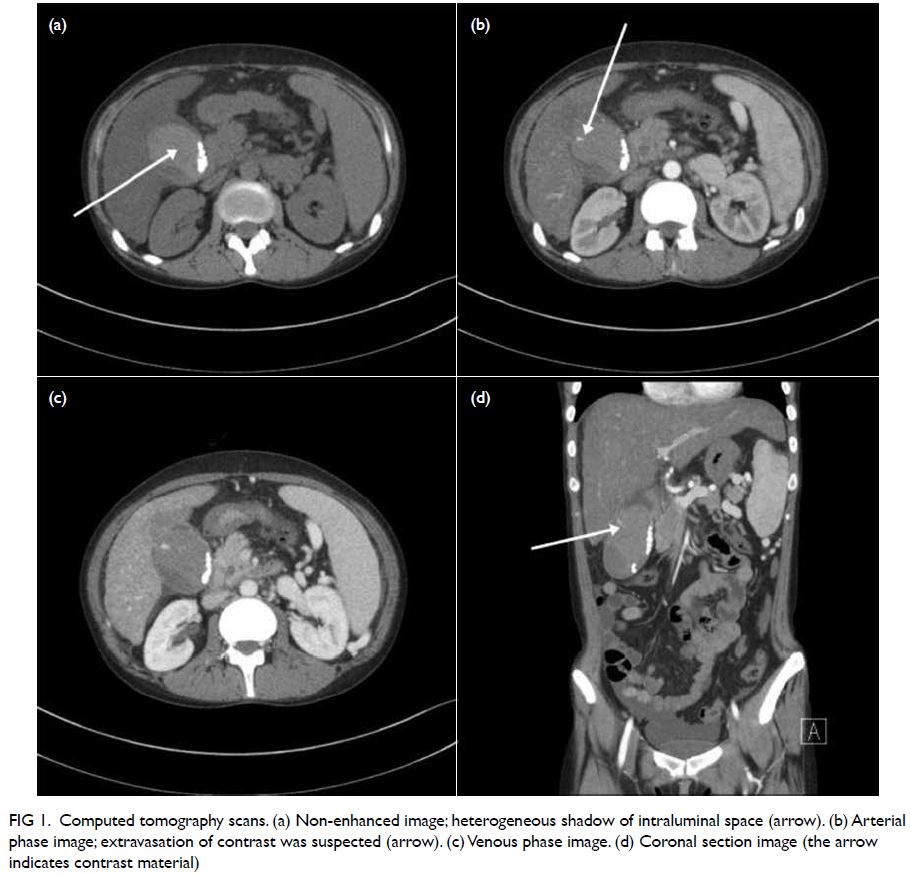
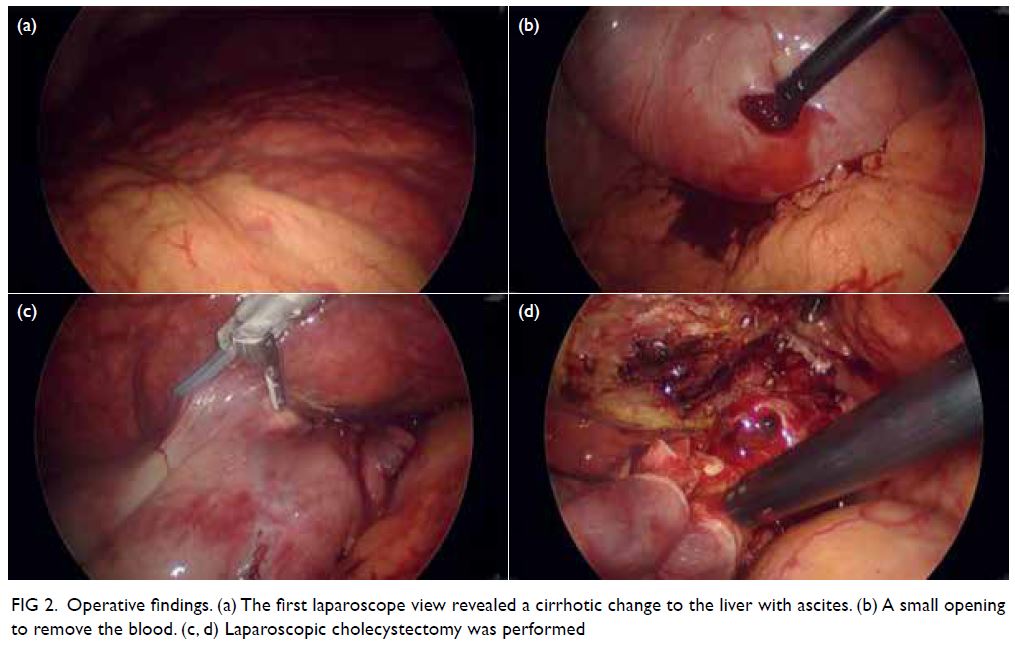
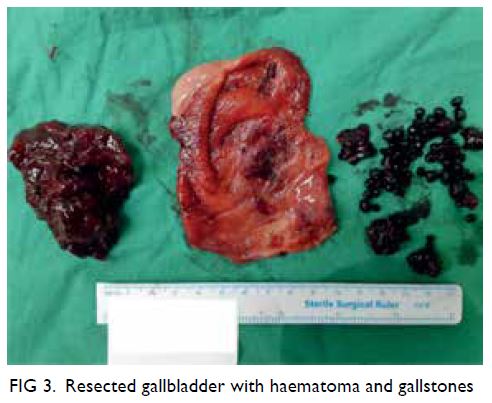
A curious case of small vessel vascular dementia
Hong Kong Med J 2023 Apr;29(2):174.e1–3
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
A curious case of small vessel vascular dementia
Whitney CT Ip, MRCP (UK)1; YF Shea, FHKAM (Medicine)1; HF Tong, FHKCPath2,3; CY Law, FHKAM (Pathology)2; CW Lam, FHKAM (Pathology)2,4; Patrick KC Chiu, FHKAM (Medicine)1
1 Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
2 Division of Chemical Pathology, Department of Pathology, Queen Mary Hospital, Hong Kong SAR, China
3 Department of Pathology, Princess Margaret Hospital, Hong Kong SAR, China
4 Department of Pathology, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr YF Shea (syf321@ha.org.hk)
A 63-year-old man was referred to the memory
clinic of Queen Mary Hospital in December 2021 for
early-onset dementia. He had stepwise deterioration
in cognitive function over the previous 6 months,
especially in short-term memory, poor judgement,
and spatial and temporal disorientation. His home
environment was poor with rotten food. Physical
examination revealed symmetrical parkinsonism.
Montreal Cognitive Assessment Hong Kong version
score was 10/30 (<2nd percentile). Vitamin B12,
folate and thyroid function tests were normal.
A review of his medical history revealed three
episodes of stroke since the age of 50 years. These
episodes presented as left lower limb monoplegia,
left-sided hemiplegia and slurring of speech 12
years, 8 years, and 1 year ago, respectively. Extensive
workup including 24-hour Holter monitoring and
transthoracic echocardiogram was unremarkable.
He had hypertension and hyperlipidaemia and was
prescribed amlodipine 5 mg and rosuvastatin 20 mg
daily. His blood pressure was under control and the
latest low-density lipoprotein was 1.7 mmol/L. A
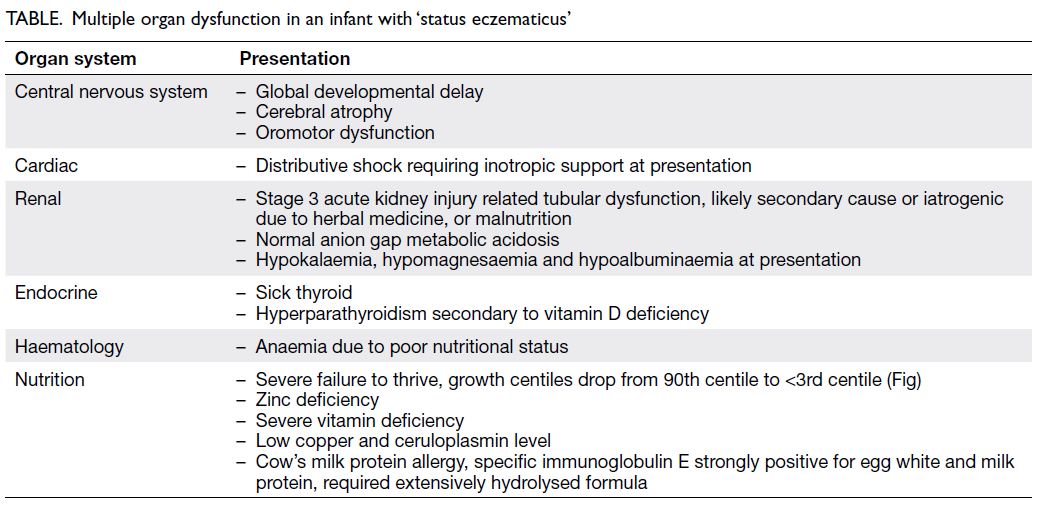
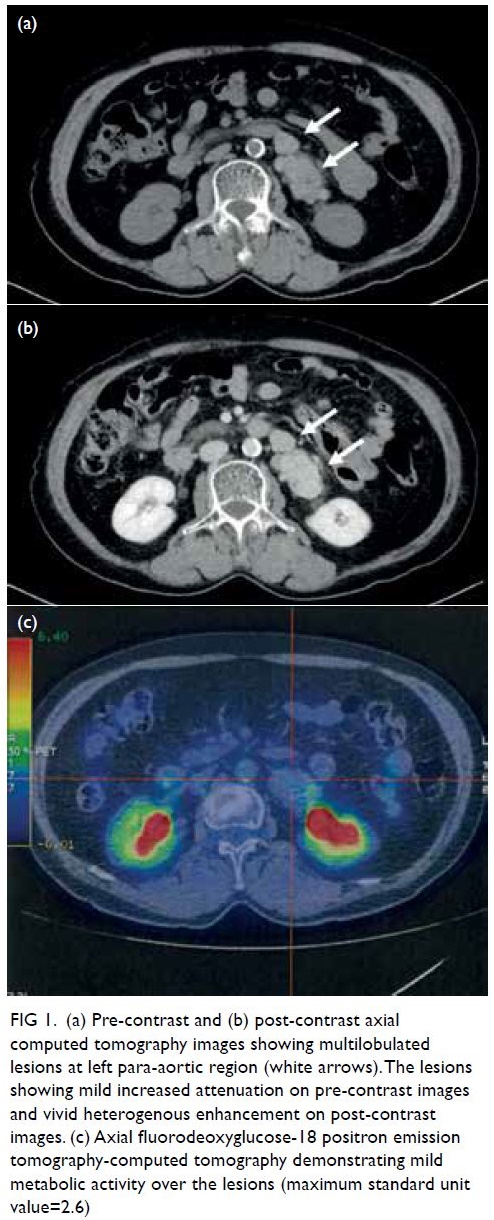
review of his computed tomography of the brain over the last 11 years showed a progressive increase in
periventricular hypodensities (Fig 1). Brain magnetic
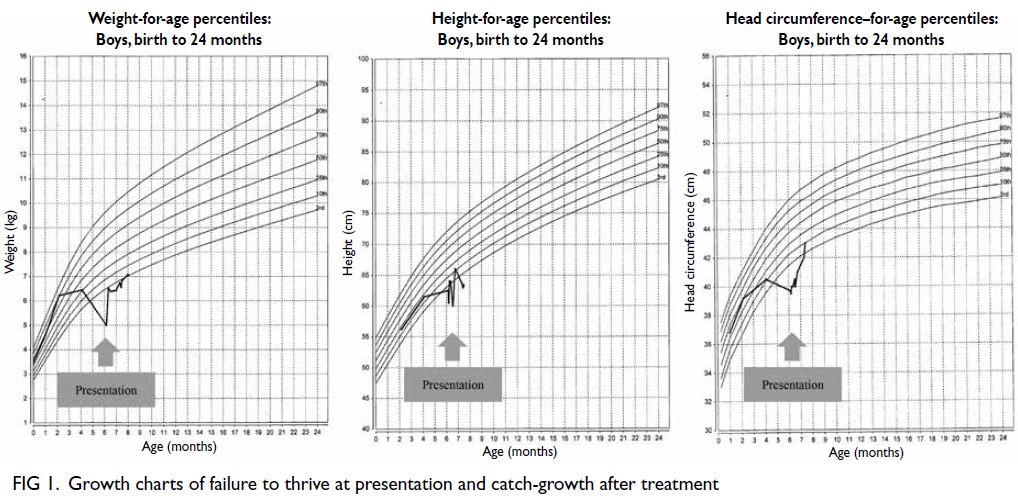
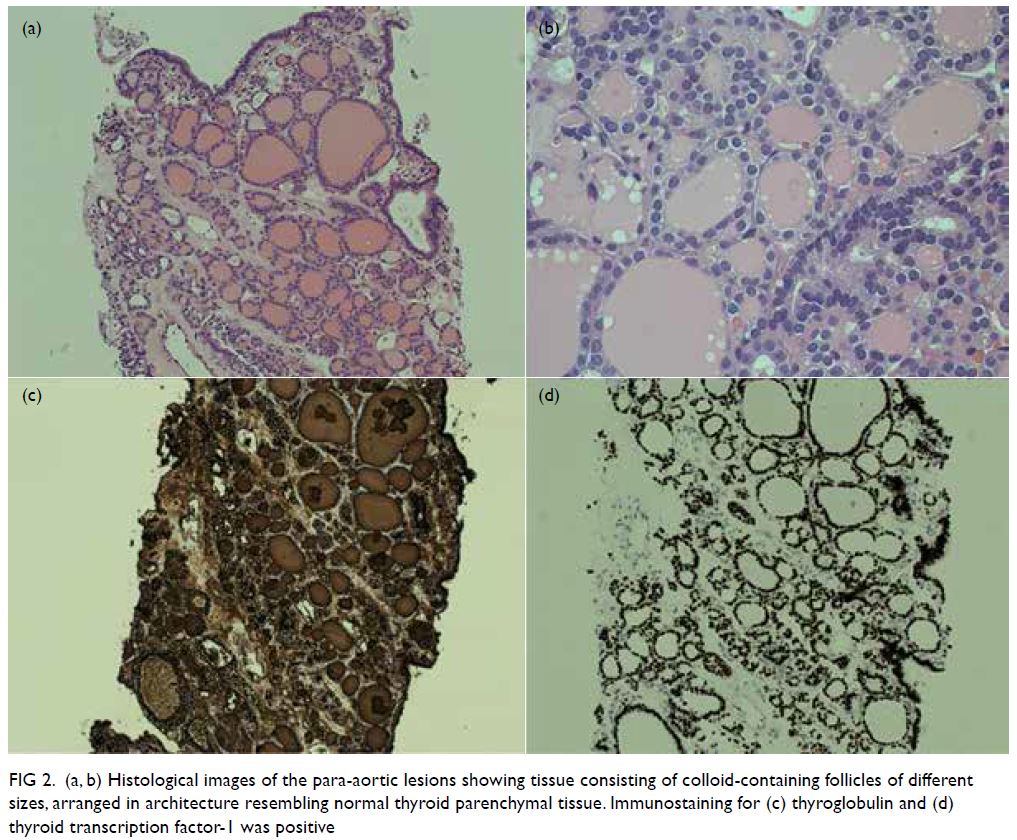
resonance imaging (1 year previously) showed
extensive periventricular hyperintensities and an
old ischaemic insult over bilateral external capsules
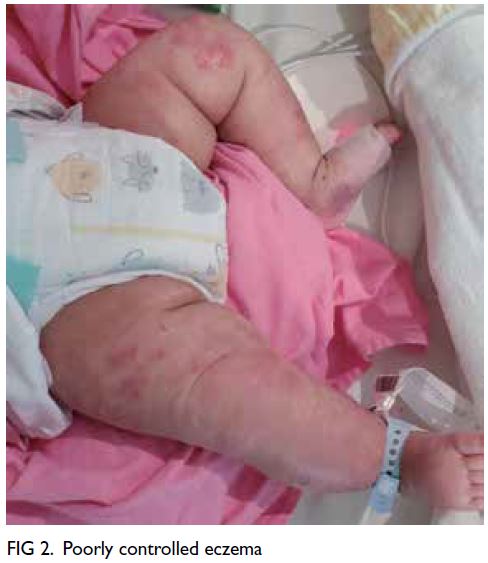
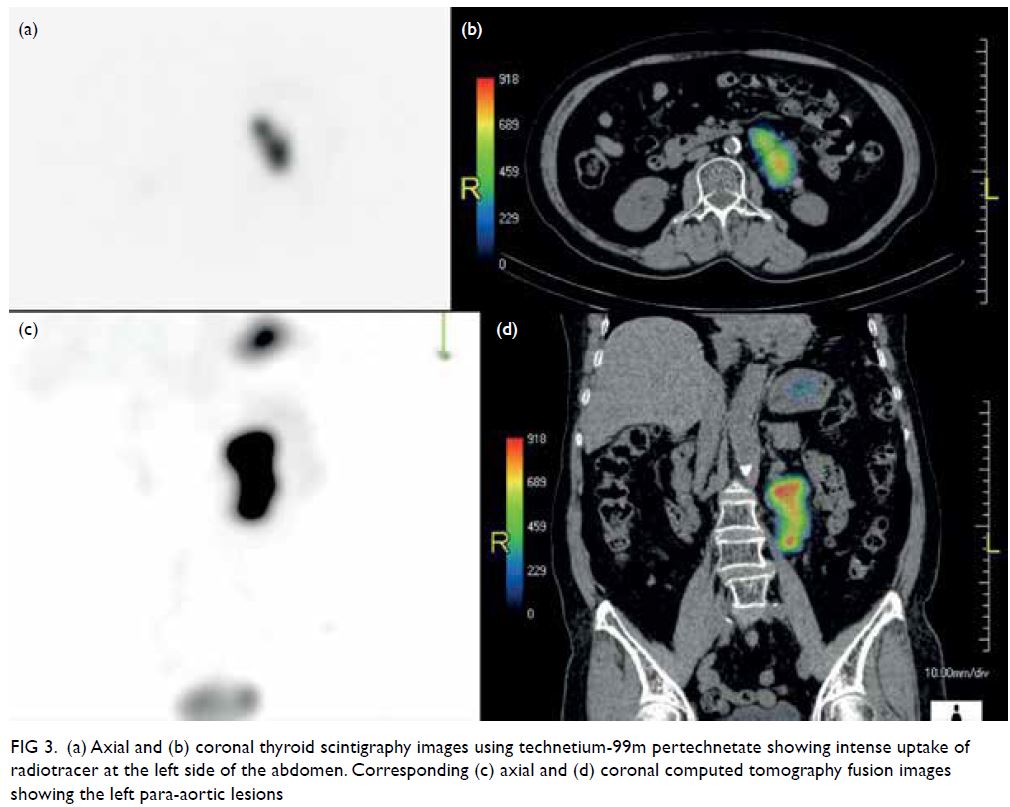
(Fig 2). Family history was notable for multiple
first-degree relatives with young-onset stroke in
their fifties and a suspected autosomal dominant
inheritance pattern (Fig 3). Genetic testing of
the neurogenic locus notch homolog protein 3
(NOTCH3) gene revealed a heterozygous mutation
with a pathogenic variant (c.1630C>T, p.Arg544Cys),
confirming the diagnosis of cerebral autosomal
dominant arteriopathy with subcortical infarcts and
leukoencephalopathy (CADASIL). The family was
referred for genetic counselling.
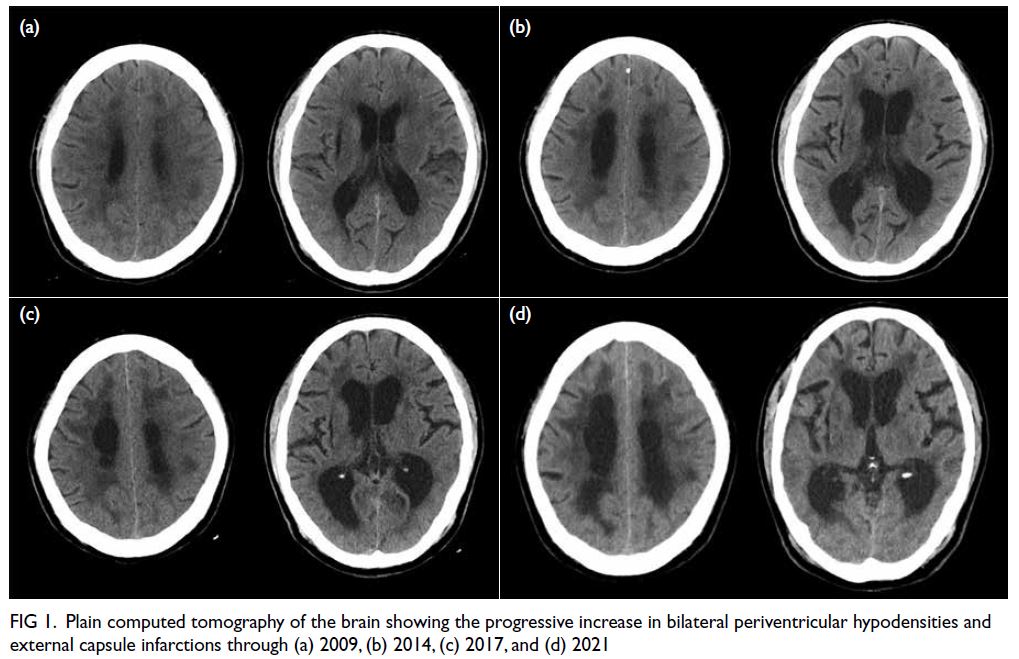
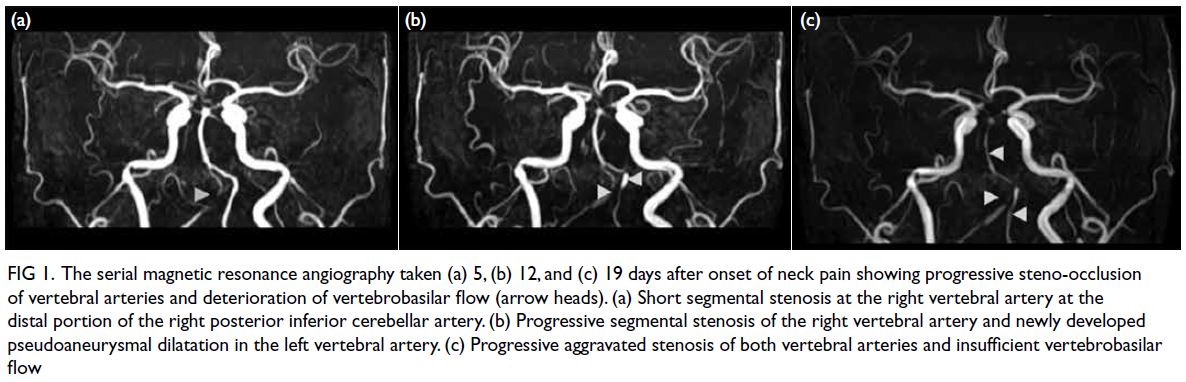
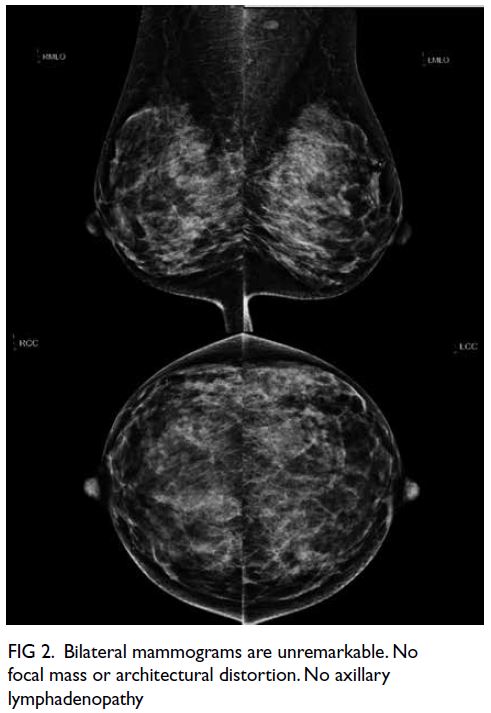
Figure 1. Plain computed tomography of the brain showing the progressive increase in bilateral periventricular hypodensities and external capsule infarctions through (a) 2009, (b) 2014, (c) 2017, and (d) 2021
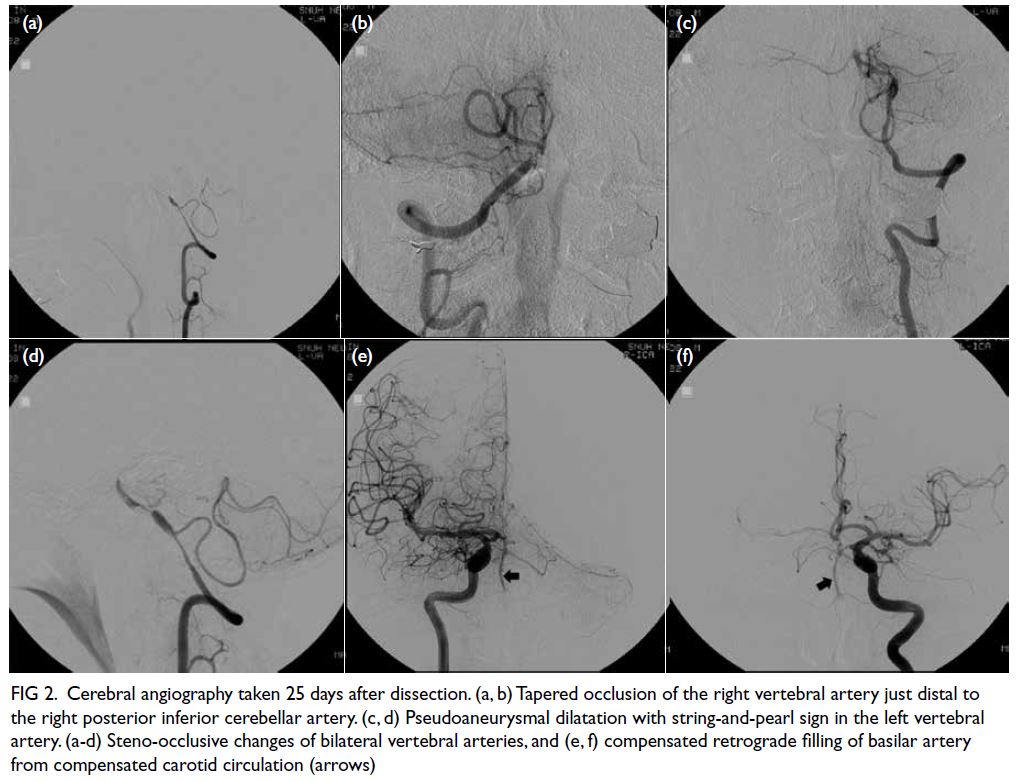
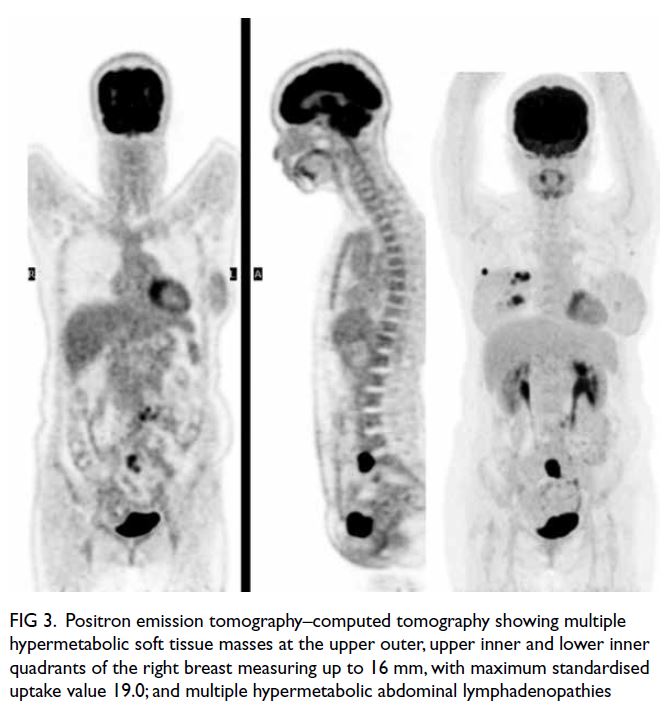
Figure 2. Brain magnetic resonance imaging (MRI) revealed extensive white matter abnormalities compatible with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). (a) Coronal fluid-attenuated inversion recovery sequence MRI showing periventricular hyperintensities. Temporal lobes had focal subcortical white matter hyperintensities, which are common findings in CADASIL. (b) Axial T2-weighted MRI showing extensive white matter hyperintensities. (c) Axial T2-weighted MRI showing the infarcts located at bilateral basal ganglia and external capsules. (d) T2 gradient echo sequence showed hemosiderin deposition over the bilateral external capsules suggestive of previous haemorrhage over the infarcted areas
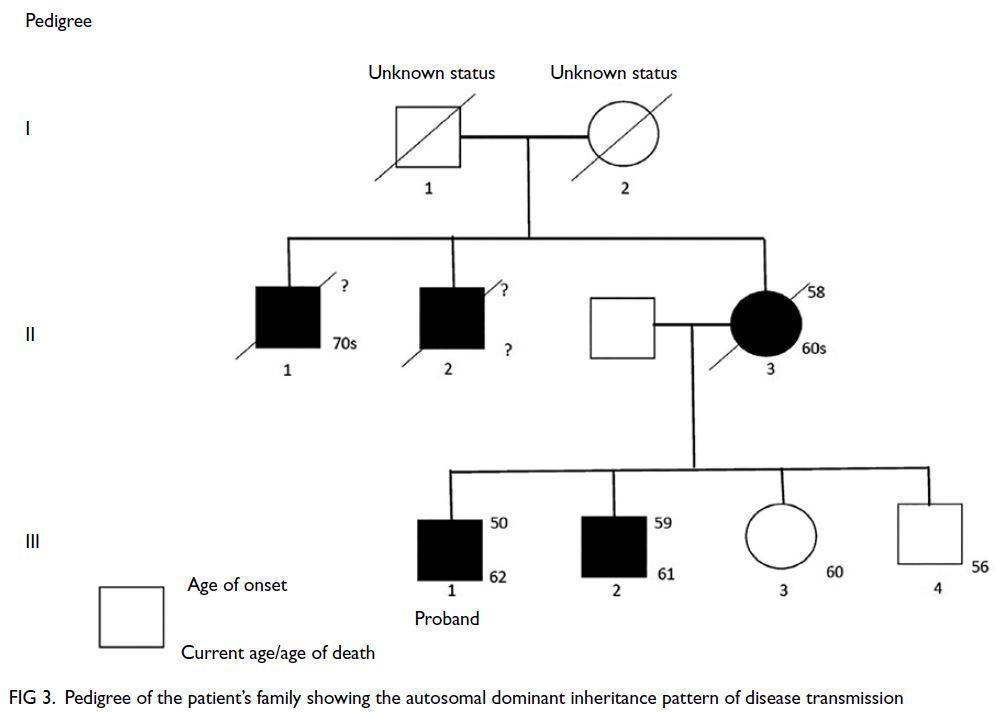
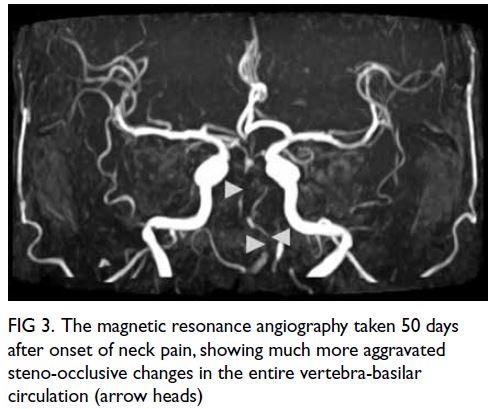
Figure 3. Pedigree of the patient’s family showing the autosomal dominant inheritance pattern of disease transmission
Cerebral autosomal dominant arteriopathy
with subcortical infarcts and leukoencephalopathy
is caused by cysteine-altering pathogenic variants in
the NOTCH3 gene, with consequent vasculopathic
changes, predominantly involving small penetrating
arteries, arterioles, and brain capillaries.1 2 The
mutation leads to an odd number of cysteine residues with deposition of osmiophilic material
and progressive degeneration of vascular smooth
muscle cells.1 2 The key to diagnosis includes a strong
family history of young-onset stroke, an absence
of strong vascular risk factors, and salient findings on brain magnetic resonance imaging, especially
extensive white matter abnormalities and subcortical
infarcts involving external capsules. Genetic testing
for the NOTCH3 gene can be arranged after
consultation with chemical pathologists in major public hospitals.3 4 The principle of management
for symptomatic patients is similar to that of other
patients with stroke, ie, antiplatelet therapy, lipid-lowering
agents, and blood pressure control. There
is no disease-modifying therapy currently available.
Family members of affected individuals should
be referred for genetic counselling with referral
to tertiary centres for potential pre-implantation
genetic testing to avoid transferring the mutation
to offspring.5 There have been four other reported
families with CADASIL in Hong Kong with different
mutations. The mean age of symptom onset for
index patients of these families was 51 years.3 4 The
mutation in our patient has been commonly found
in Fujian province and Taiwan, accounting for up to
14.5% to 70% of CADASIL cases.1
In summary, clinicians should obtain a
detailed history and be alert to suspicious magnetic
resonance imaging findings. Referral to chemical
pathologists for genetic testing is key to the diagnosis
of CADASIL.
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Patient consent was obtained for all investigations.
References
1. Chen S, Ni W, Yin XZ, et al. Clinical features and mutation spectrum in Chinese patients with CADASIL: a multicenter retrospective study. CNS Neurosci Ther 2017;23:707-16. Crossref
2. Hu Y, Sun Q, Zhou Y, et al. NOTCH3 variants and genotype-phenotype features in Chinese CADASIL patients. Front Genet 2021;12:705284. Crossref
3. Au KM, Li HL, Sheng B, et al. A novel mutation (C271F) in the Notch3 gene in a Chinese man with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Clin Chim Acta 2007;376:229-32. Crossref
4. Hung LY, Ling TK, Lau NK, et al. Genetic diagnosis of CADASIL in three Hong Kong Chinese patients: a novel mutation within the intracellular domain of NOTCH3. J Clin Neurosci 2018;56:95-100. Crossref
5. Konialis C, Hagnefelt B, Kokkali G, Pantos C, Pangalos C. Pregnancy following pre-implantation genetic diagnosis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Prenat Diagn 2007;27:1079-83. Crossref


 A video clip demonstrating physical examination findings of bilateral fixed left lateral gaze and right lower-motor-neuron seventh cranial nerve palsy is available at
A video clip demonstrating physical examination findings of bilateral fixed left lateral gaze and right lower-motor-neuron seventh cranial nerve palsy is available at