Simultaneous pancreas and kidney transplantation as the standard surgical treatment for diabetes mellitus patients with end-stage renal disease
Hong Kong Med J 2016 Feb;22(1):62–9 | Epub 8 Jan 2016
DOI: 10.12809/hkmj154613
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Simultaneous pancreas and kidney transplantation as the standard surgical treatment for diabetes mellitus patients with end-stage renal disease
CM Chan;
Thomas MY Chim;
KC Leung;
CH Tong;
TF Wong;
Gilberto KK Leung, MB, BS, FHKAM (Surgery)
Centre of Education and Training, Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong
Corresponding author: Dr Gilberto KK Leung (gilberto@hku.hk)
Abstract
Objectives: To review the outcome following
simultaneous pancreas and kidney transplantation
in patients with type 1 diabetes mellitus and end-stage
renal disease, as well as those with type 2
diabetes mellitus, and to discuss the applicability of
this treatment in this locality.
Methods: A systematic literature review was performed by searching the PubMed and Elsevier databases. The search terms used were “simultaneous pancreas and kidney transplantation”,
“diabetes”, “pancreas transplant” and “SPK”. Original and major review articles related to simultaneous pancreas and kidney transplantation were reviewed. Papers published in English after 1985 were included. Clinical outcomes following transplantation were extracted for comparison between different treatment methods. Outcomes of simultaneous pancreas and kidney transplant and other transplantation
methods were identified and categorised into patient survival, graft survival, diabetic complications, and
quality of life. Patient survivals and graft survivals were also compared.
Results: Currently available clinical evidence
shows good outcomes for type 1 diabetes mellitus
in terms of patient survival, graft survival, diabetic complications, and quality of life. For type 2
diabetes mellitus, the efficacy and application of the
procedure remain controversial but the outcomes
are possibly comparable with those in type 1
diabetes mellitus.
Conclusions: Simultaneous pancreas and kidney
transplantation is a technically demanding procedure
that is associated with significant complications, and
it should be regarded as a ‘last resort’ treatment in
patients whose diabetic complications have become
life-threatening or severely burdensome despite best
efforts in maintaining good diabetic control through
lifestyle modifications and medications.
Introduction
Simultaneous pancreas and kidney transplantation
(SPK) has emerged as the worldwide standard for
treatment of patients with end-stage renal disease
(ESRD) resulting from type 1 diabetes mellitus
(T1DM). Multiple studies have shown that SPK can
significantly improve both their quality of life (QOL)
and long-term survival. With the advances in surgical
techniques, immunosuppression, management of
graft rejection, and other related complications, SPK
can now be performed successfully in the majority
of patients, with the pancreatic graft survival
rate comparable with those of kidney and liver
transplants.1 It is currently the predominant type of
pancreas transplantation for diabetic patients with
ESRD.2 According to the International Pancreas
Transplant Registry, among over 35 000 pancreas
transplantations reported by the end of 2010,
approximately 75% were SPK, 18% were pancreas
after kidney transplantation (PAK), and 7% were
pancreas transplantation alone.2 The vast majority of
SPK performed in various countries used grafts from
cadavers, while living donor pancreas and/or kidney
grafts were used only in a minority of cases.
In this review, we first discuss the effectiveness
of SPK in improving the outcome for diabetic patients
with ESRD in comparison with other transplant
options including kidney transplant alone (KTA). In
addition, we address the controversy about whether
patients with type 2 diabetes mellitus (T2DM)–associated ESRD, as with T1DM patients, should
also receive SPK. This is followed by a discussion on
the surgical risks, operative complications, future
directions, and the application of this treatment
approach in this locality. We reviewed original
and review articles related to SPK. PubMed and
Elsevier databases were searched using the keywords
“simultaneous pancreas and kidney transplantation”,
“diabetes”, “pancreas transplant”, and “SPK”. Articles
published in English since 1985 were included.
Outcomes of type 1 diabetes with end-stage renal disease following simultaneous pancreas and kidney transplantation versus kidney transplant alone (living or deceased donor)
The outcomes of SPK can be assessed in terms of
patient survival, graft survival, control of diabetic
complications, and improvement in QOL. In the
following, comparisons with KTA are made.
Patient survival
The most important parameter is patient survival.
Lindahl et al3 reviewed 15 studies that compared the
survival outcomes of SPK, living donor kidney alone
(LDKA), and deceased donor kidney alone (DDKA).
The authors included nine studies with short-term
(up to 10 years) and six studies with long-term
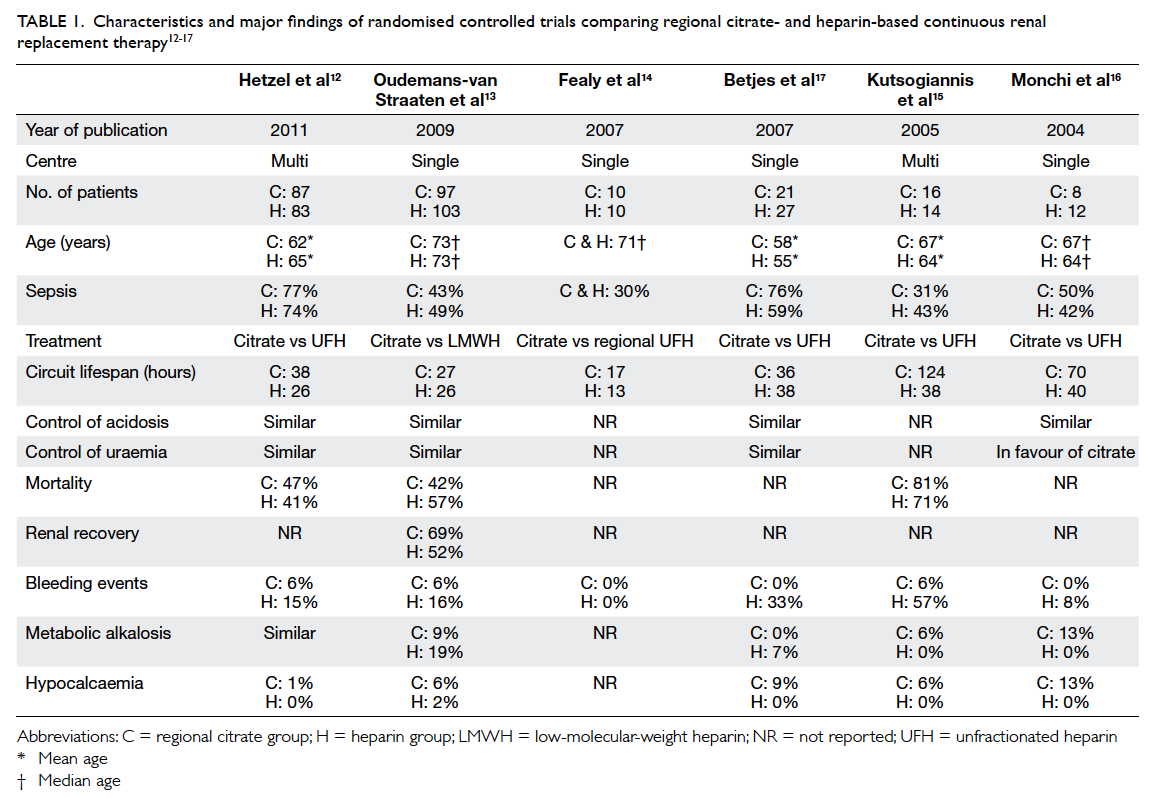
(beyond 10 years) follow-up (Table 14 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19; one more latest short-term study in 2015 was included19).
Overall, most large-scale studies agree that DDKA
is inferior to SPK and LDKA. Nonetheless, whether
SPK or LDKA achieves superior patient survival
remains controversial. For short-term outcomes,
the overall survival rate of SPK recipients has been
shown to be almost equivalent to that of LDKA
recipients, except in one large-scale study by Young
et al4 which yielded a better survival rate in LDKA
patients after adjustment for high-risk characteristics
in this group of patients. Consistent with other studies, the
unadjusted overall patient survival was equivalent
for SPK and LDKA. For long-term outcomes, SPK
recipients had a higher survival rate than LDKA
recipients. This may be because the additional
beneficial effects of pancreas transplantation on
glycaemic control need time to manifest. Morath
et al5 postulated that, over time, SPK would provide
greater survival benefits since the initially higher
associated operational mortalities would later
be compensated by improved glycaemic control
that reduced death from diabetic complications,
particularly in terms of cardiovascular death. This
view is further supported by the fact that the major
cause of death in all these patients is primarily
cardiovascular disease (62%), followed by infection
(16%), malignancy (8%), and other causes (14%).6 It
also potentially explained why in Young et al’s study,4
despite initial superior patient survival following
LDKA compared with SPK (1-year survival of
LDKA, SPK, and DDKA was 97%, 95%, and 93%,
respectively), the results began to favour SPK by the
end of the 72-month study period.
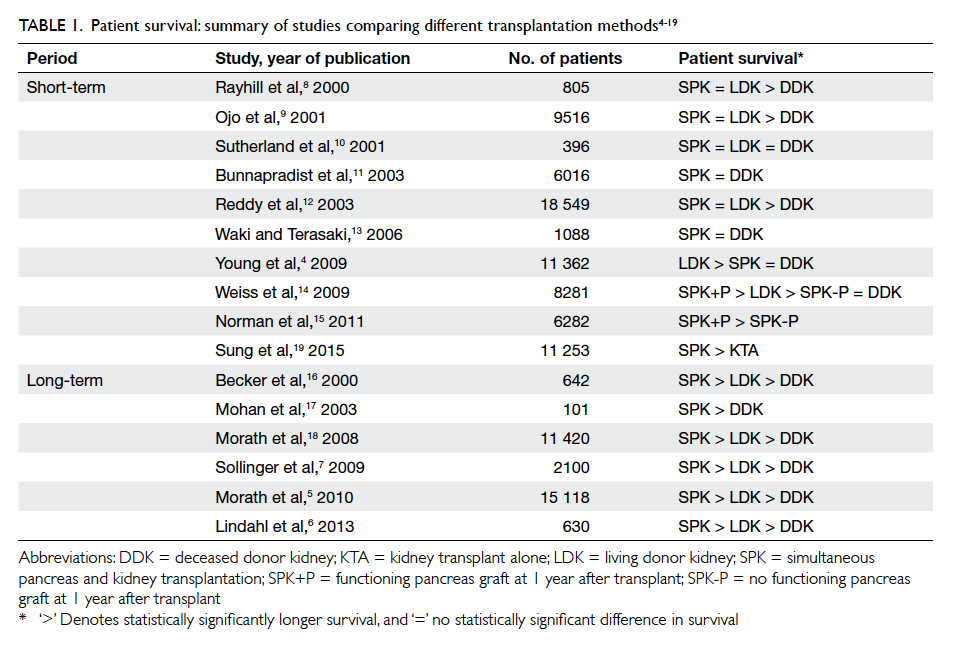
Table 1. Patient survival: summary of studies comparing different transplantation methods4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
Graft survival
The assessment of graft survival in SPK includes
that of the kidney and the pancreas. For kidney graft
survival, the review by Lindahl et al3 found similar
short-term (up to 10 years) kidney graft survival rates
following SPK and LDKA; graft survival after DDKA
was inferior. Long-term results (>10 years) showed
that DDKA was inferior to both SPK and LDKA,
and most long-term studies agreed that SPK was
equivalent to LDKA, with the exception of a study
by Morath et al5 that showed SPK to be superior to
LDKA. As such, the current view is that SPK is at
least non-inferior to LDKA in terms of long-term
kidney graft survival, even though most SPK in the
literature reviewed were from cadaver donors (Table 24 5 6 8 10 11 12 13 14 15 16 17 18 19 20).
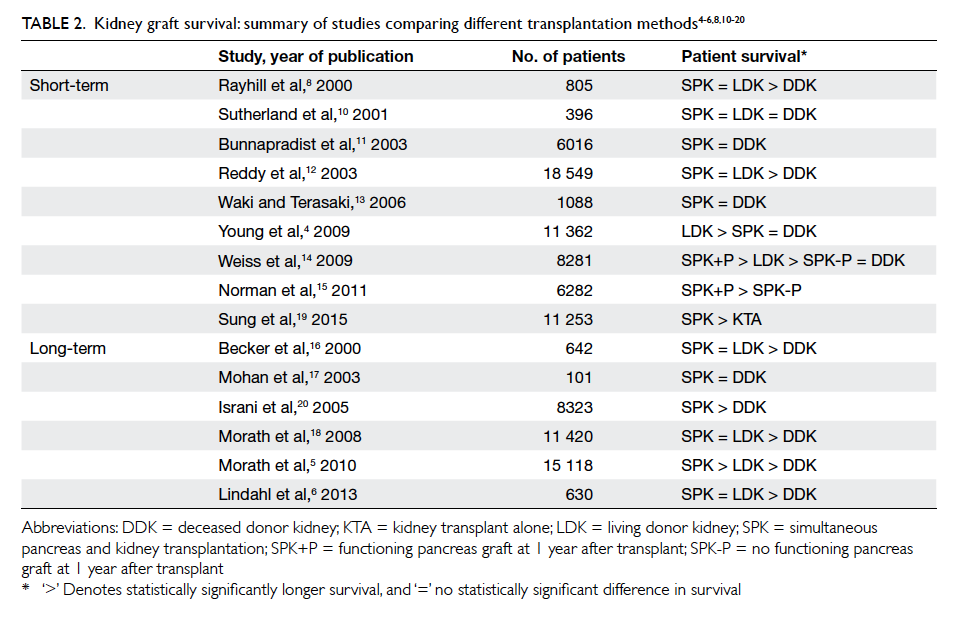
Table 2. Kidney graft survival: summary of studies comparing different transplantation methods4 5 6 8 10 11 12 13 14 15 16 17 18 19 20
For pancreas graft survival, the results from
SPK have improved significantly over the past
years due to improved surgical techniques and
immunosuppressive regimens.3 Surgical techniques
have evolved from the use of pancreatic duct
occlusion (1983-1987) to that of exocrine drainage
into the urinary bladder (1988-1999), and later to
that of direct drainage into the proximal jejunum
(2000 onwards). With the latter, the mean pancreas
graft survival rates after 1 and 5 years have been
reported to be 87% and 75%, respectively.3
Diabetic complications
In the present context of transplantation therapy,
the important complications of T1DM include
diabetic nephropathy, diabetic neuropathy, and
an increased risk of cardiovascular diseases. For
diabetic nephropathy, several studies have included
kidney biopsy in graft assessment following SPK, so
as to capture the early diabetes-related changes that
might otherwise take time to manifest clinically. The
common diabetes-related changes under electron
microscopy include thickening of the glomerular
basement membrane (GBM) and an increase in
mesangial volume. The study by Bohman et al21 was the first to
perform kidney biopsy in two SPK patients and six
KTA patients. Diabetes-related changes were seen in
five of the six KTA recipients but not in any of the SPK
recipients. Wilczek et al22 included a larger sample
size (20 SPK vs 30 KTA) with a mean postoperative
biopsy time of 1 to 6.8 years. The associated
changes under light and electron microscopy were
significantly fewer in the SPK group than the KTA
group. Bilous et al23 biopsied 12 PAK patients before
and at least 1.9 years after the pancreas transplant,
and found no glomerular disease progression. They also compared the
12 PAK with 13 KTA patients, and found lower
mesangial volume in the PAK group. Although most
studies showed that the outcomes would be better
in patients with SPK relative to those with KTA,
whether the pancreas graft can halt nephropathy
progression remains controversial. Nyberg et al24
biopsied 11 SPK patients 2 to 4 years postoperatively,
and found a mean increase in GBM thickness when
compared with normal controls. While recurrence
of diabetic nephropathy is still possible in the long
run, current evidence nonetheless supports that
SPK can at least delay the progression of diabetic
nephropathy in comparison with KTA.
Apart from its effect on the kidney, a pancreas
transplant in SPK may potentially improve other
organ systems that can be affected by diabetes.
Diabetic neuropathy is an example. Navarro et al25
compared 115 pancreas recipients with 92 patients
prescribed standard insulin therapy. Neurological
status was assessed by clinical examination, nerve
conduction studies, and autonomic function
test. Results up to 10 years showed significant
improvement in nerve conduction studies and slight
improvement on clinical examination and autonomic
index in the SPK group. We proposed that
pancreas transplantation could potentially halt the
progression of diabetic neuropathy and may even
lead to a degree of neurological improvement.
Besides microvascular complications,
macrovascular complications are a major concern
in diabetic patients. Cardiovascular diseases, in
particular coronary artery disease, contribute to a
significant portion of mortality in T1DM patients.
Whether SPK can provide benefit in this respect has
been studied. Jukema et al26 observed 32 SPK patients
with 26 functioning pancreas grafts and six non-functioning
pancreas grafts. Glycaemic control was
measured by blood glucose level, and the progression
of diffuse and focal coronary atherosclerosis was
assessed by coronary angiography. It was found
that in the presence of a functioning pancreas graft,
glycaemic control was better and progression of
coronary atherosclerosis was slower. This might also
correlate with a lower risk of cardiovascular death
and explain why long-term survival in SPK patients
is superior to that of KTA patients.
Quality of life
In addition to survival benefit and reduced co-morbidity,
improvement in QOL may also represent
an important consideration. A functioning pancreas
graft can potentially free a patient from the need
for self-administered insulin and achieve more
stable blood glucose levels.27 28 29 These outcomes
may be associated with improvement in QOL. To directly quantify such improvement, some studies
have used validated health-related quality of life (HRQOL) questionnaires to evaluate treatment
outcomes. The latest cohort study by Martins et al30 compared the HRQOL scores of 126 patients before and after SPK with a follow-up duration of around 5 years. There were improvements in
all domains under the Gastrointestinal Quality of Life Index post-transplantation, with a significant
visual analogue scale health state improvement from 38% to 84%.30 Assessment by another tool, the EuroQol-5 Dimension questionnaire, also showed improvement in physical function, psychological
status, social function, gastro-intestinal complaints, burden of medical treatment as well as the rate
of unemployment.30 The majority of available
studies have focused mainly on comparison of pre-transplant and post-transplant scores, or on
transplanted and non-transplanted patients. Few studies have compared QOL outcomes between
patients undergoing SPK and KTA. Sureshkumar et al31 conducted a case-control study involving 27 SPK patients and 27 KTA patients. The authors concluded
that SPK patients had a significantly better diabetes-related QOL.
Considerations in type 1 versus type 2 diabetes mellitus
Indications (in type 1 versus type 2 diabetes mellitus)
The predominant indication for SPK is T1DM
patients with ESRD and adequate cardiac reserve
who have no opportunity for a living donor kidney
transplantation.32 Currently, most centres will
perform SPK mainly for T1DM, less commonly
for T2DM patients. Nonetheless there has been
pervasive controversy on whether the long-term
outcomes of SPK in the two groups actually differ.
This has important implications in organ allocation
as T2DM is much more prevalent than T1DM.
Several factors have to be considered. First, there
are differences in pathogenesis between the two
conditions—T2DM is attributed to insulin resistance
in addition to insulin secretion defect; in T1DM,
the pathogenesis involves the auto-destruction of
islet cells, causing absolute insulin deficiency. This
would theoretically render pancreas transplantation
less efficacious in T2DM. Second, there is as yet
no consensus on the distinction between the two
conditions. For example, C-peptide, which is used
in patient selection for SPK between T1DM and
T2DM patients, has been shown by some studies to
be unreliable in determining the outcomes of SPK.33
Third, it should be noted that there exists major
differences between the two groups of diabetic
patients that make meaningful comparison difficult.
These include disproportionate sample sizes; the
presence of confounders such as age, obesity, co-morbidities;
and duration and treatment of the underlying diabetes.
Notwithstanding, there has been an increasing
amount of evidence showing comparable results
of SPK in selected T2DM and T1DM patients.34
In a large study using the data obtained from the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) between 2000 and 2007, Sampaio et al35 showed no
significant difference in 5-year survival rate between T1DM and T2DM recipients despite the fact that
the latter group had a higher risk of death due to older age and longer pre-transplant dialysis time. In
the same study, the 5-year pancreas graft survival in T2DM patients (69.8%) was comparable with that
in T1DM patients (72.4%); an inferior 5-year kidney graft survival was found (77.8% vs 73.5%; P=0.007).35 After adjusting for other potential risk factors (eg time on dialysis, obesity), however, diabetes type was not identified as an independent prognostic factor.35
Concerning the role of C-peptide in defining
T1DM and T2DM during patient selection, Stratta
et al36 stratified 162 SPK recipients according to pre-transplant
C-peptide levels into C-peptide ‘positive’ (≥2.0 ng/mL; n=30) and C-peptide ‘negative’ (<2.0 ng/mL; n=132) groups. With a mean follow-up duration of 5.6 years, the two groups showed no
statistically significant differences in pancreas graft, kidney graft, or patient survival.36 In a similar study involving 80 SPK recipients, 10 were classified as
T2DM and 70 as T1DM.37 On Cox regression survival
analyses, no statistically significant difference in graft and patient survival was found between the
two groups.37 The authors concluded that selected
T2DM patients with ESRD should be considered potential candidates for SPK, and that the use of C-peptide
as the predominant marker of the diabetes type was unreliable and potentially misleading.37
Simultaneous pancreas and kidney transplantation versus other transplant options in type 2 diabetes mellitus patients
At present, there is insufficient evidence to support
the use of SPK over other kidney transplant options in patients with T2DM. In an early study that
compared the outcomes of SPK, DDKA, and LDKA in patients with T2DM, Wiseman and Gralla38 concluded that both patient and graft survival rates were superior with LDKA transplantation,
whereas patient but not graft survival rate was higher in SPK versus DDKA transplantation. After
multivariable analysis, the survival advantage of SPK over DDKA was related not so much to the
pancreas transplantation but other variables such as younger donor and recipient ages in the SPK
cohort (Table 338). These findings, however, should not completely dismiss the consideration of SPK for selected T2DM patients who have little prospect for LDKA since other outcome measures such as the benefits of euglycaemia in terms of better QOL and secondary complications of diabetes will also have to be considered. Nonetheless, given the superior long-term outcome of SPK over LDKA in T1DM patients
and that the same has yet to be demonstrated in T2DM patients, T1DM patients should still be given
a stronger allocation priority of SPK grafts.3
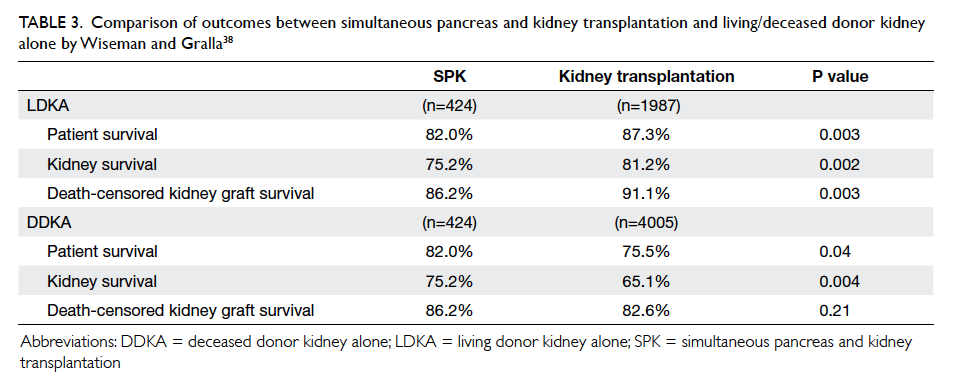
Table 3. Comparison of outcomes between simultaneous pancreas and kidney transplantation and living/deceased donor kidney alone by Wiseman and Gralla38
Complications of simultaneous pancreas and kidney transplantation
Most complications of SPK are related to the
transplanted pancreas.39 Repeated laparotomy
may be required in up to 50% of patients.40 41 The pancreatic graft survival at 1 year may range from 74% to 88%, with the sharpest drop during the first year.7 39 42 The reported 10-year and 20-year survival rates were 63% and 36%, respectively.7 Most complications occur within the first 60 days of operation and include graft pancreatitis (3%-12%), infection/abscess (1%-5%), focal/diffuse necrosis (12%), graft-vessel thrombosis (6%-17%), anastomotic leak (0.5%-2%), and intra-abdominal haemorrhage (0%-0.5%).39 40 41 42 43 Venous thrombosis has been reported to be the most common cause of graft failure; graft pancreatitis and focal/diffuse necrosis were the most common causes of mortality among graft-specific complications.39 40
Complications related to the transplanted
kidney include acute tubular necrosis (ATN) or graft rejection, urinary complications, infection,
and vascular thrombosis. According to a study of 112 SPK recipients by Grochowiecki et al,44 ATN and rejection were the most frequent (43.4%) causes leading to the loss of kidney graft function. Infections (28.6%) and vascular thrombosis due to atherosclerosis of the iliac arteries (28.6%) were
the most common reasons for graft nephrectomy.44 The most severe complications were due to fungal infection.44 Overall, the 1-year survival rate for the kidney graft was over 90%.7 44 45 The 10-year and 20-year kidney survival rates were 63% and 38%,
respectively.7 In terms of overall mortality, the most
common causes following SPK have been reported to be cardiopulmonary (7.2%), followed by infection
(3.4%), stroke (1.8%), and renal failure (1.5%). Patient survival rates at 1, 10, and 20 years were 97%, 80%,
and 58%, respectively.7
Comparison of the complications of SPK versus
KTA has revealed that SPK has a lower rate of ATN
(8.9%)44 than KTA (15.3%),46 but a slightly higher rate of urological complications (4.5%)44 than KTA (3.7%).47 The incidence of vascular complications was comparable in SPK (1.8%)44 and KTA (0.5%-4%).48 49 50 As mentioned above, SPK has higher long-term
patient and kidney survival rates than LDKA/DDKA.
Future directions and local applicability of simultaneous pancreas and kidney transplantation
Simultaneous pancreas and kidney transplantation
has become an established treatment for patients
with T1DM complicated by ESRD. The results have,
so far, been promising. Furthermore, apart from
SPK, newer techniques including pancreatic islet
cell transplantation, and different combinations of
living and/or deceased donor pancreas, islet cell and
kidney graft transplantation are being evaluated.
Current research aims to extend these techniques,
still predominantly SPK, to the treatment of T2DM,
for which LDKA remains the first-line treatment
option. In situations where LDKA is not available for
T2DM patients, DDKA remains the next best option.
Regarding the ethical issues about graft allocation,
the allocation of DDKA is based on an allocation
system that takes account of patient age and waiting
time as well as the degree of human leukocyte
antigen (HLA) matching between the potential
donor and the recipient.51 Difficulties may arise,
however, when a deceased donor with both kidney
and a pancreas graft has become available. Should
the priority for these grafts be given to a patient on
the SPK waiting list or a non–diabetes-related ESRD
patient on the DDKA waiting list? And should the
priority of SPK be given to a T2DM patient who is
higher up on the allocation system (with a younger
age, a longer waiting time, or lesser degree of HLA
mismatch) or to a T1DM patient who is lower on
the allocation system but is more likely to achieve
a better outcome? The related ethical issues clearly
deserve an informed discourse within the surgical
community.
Simultaneous pancreas and kidney
transplantation has not yet been performed in this
locality. The main obstacle remains the shortage
of cadaver organs. Here, the number of cadaveric
renal transplantations performed in the Hospital
Authority over the last decade ranges from 44 to 87
cases per year. As of 31 December 2014, the number
of patients waiting for transplantation was close to
2000. This translates into significantly prolonged
dialysis time for this specific group of patients, and
hence a potentially suboptimal outcome after SPK.
The implementation of SPK would not be readily
feasible unless there is a significant improvement in
organ availability.
Conclusions
Simultaneous pancreas and kidney transplantation
has become a standard treatment worldwide
for patients with T1DM and ESRD. There is a
large volume of clinical evidence supporting
good outcomes in patient survival, graft survival,
diabetic complications, and QOL. For
T2DM, the efficacy and application of the procedure
remain controversial but the outcomes are possibly
comparable to that in T1DM. Simultaneous
pancreas and kidney transplantation is a technically
demanding procedure that is associated with
significant complications, and should be undertaken
only in carefully selected patients. It should be
regarded as a ‘last resort’ treatment for patients in
whom diabetic complications have become life-threatening
or severely burdensome despite best
efforts in maintaining good diabetic control through
lifestyle modification and medications. Continued
efforts in patient education and the promotion of an
altruistic culture of organ donation among the public
are critical for the implementation of this treatment
paradigm in this locality.
References
1. Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas
and kidney transplantation: current trends and future
directions. Curr Opin Organ Transplant 2015;20:94-102. Crossref
2. Gruessner AC. 2011 Update on pancreas transplantation:
comprehensive trend analysis of 25,000 cases followed up
over the course of twenty-four years at the International
Pancreas Transplant Registry (IPTR). Rev Diabet Stud
2011;8:6-16. Crossref
3. Lindahl JP, Jenssen T, Hartmann A. Long-term outcomes
after organ transplantation in diabetic end-stage renal
disease. Diabetes Res Clin Pract 2014;105:14-21. Crossref
4. Young BY, Gill J, Huang E, et al. Living donor kidney
versus simultaneous pancreas-kidney transplant in type I
diabetics: an analysis of the OPTN/UNOS database. Clin J
Am Soc Nephrol 2009;4:845-52. Crossref
5. Morath C, Zeier M, Döhler B, et al. Transplantation of
the type 1 diabetic patient: the long-term benefit of a
functioning pancreas allograft. Clin J Am Soc Nephrol
2010;5:549-52. Crossref
6. Lindahl JP, Hartmann A, Horneland R, et al. Improved
patient survival with simultaneous pancreas and kidney
transplantation in recipients with diabetic end-stage renal
disease. Diabetologia 2013;56:1364-71. Crossref
7. Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM,
Pirsch JD. One thousand simultaneous pancreas-kidney
transplants at a single center with 22-year follow-up. Ann
Surg 2009;250:618-30. Crossref
8. Rayhill SC, D’Alessandro AM, Odorico JS, et al.
Simultaneous pancreas-kidney transplantation and
living related donor renal transplantation in patients
with diabetes: is there a difference in survival? Ann Surg
2000;231:417-23. Crossref
9. Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact
of simultaneous pancreas-kidney transplantation on long-term
patient survival. Transplantation 2001;71:82-90. Crossref
10. Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons
learned from more than 1,000 pancreas transplants at a
single institution. Ann Surg 2001;233:463-501. Crossref
11. Bunnapradist S, Cho YW, Cecka JM, Wilkinson A,
Danovitch GM. Kidney allograft and patient survival in
type I diabetic recipients of cadaveric kidney alone versus
simultaneous pancreas kidney transplants: a multivariate
analysis of the UNOS database. J Am Soc Nephrol
2003;14:208-13. Crossref
12. Reddy KS, Stablein D, Taranto S, et al. Long-term survival
following simultaneous kidney-pancreas transplantation
versus kidney transplantation alone in patients with type
1 diabetes mellitus and renal failure. Am J Kidney Dis
2003;41:464-70. Crossref
13. Waki K, Terasaki PI. Kidney graft and patient survival with
and without a simultaneous pancreas utilizing contralateral
kidneys from the same donor. Diabetes Care 2006;29:1670-2. Crossref
14. Weiss AS, Smits G, Wiseman AC. Twelve-month pancreas
graft function significantly influences survival following
simultaneous pancreas-kidney transplantation. Clin J Am
Soc Nephrol 2009;4:988-95. Crossref
15. Norman SP, Kommareddi M, Ojo AO, Luan FL. Early
pancreas graft failure is associated with inferior late
clinical outcomes after simultaneous kidney-pancreas
transplantation. Transplantation 2011;92:796-801. Crossref
16. Becker BN, Brazy PC, Becker YT, et al. Simultaneous
pancreas-kidney transplantation reduces excess mortality
in type 1 diabetic patients with end-stage renal disease.
Kidney Int 2000;57:2129-35. Crossref
17. Mohan P, Safi K, Little DM, et al. Improved patient survival
in recipients of simultaneous pancreas-kidney transplant
compared with kidney transplant alone in patients with
type 1 diabetes mellitus and end-stage renal disease. Br J
Surg 2003;90:1137-41. Crossref
18. Morath C, Zeier M, Döhler B, Schmidt J, Nawroth PP,
Opelz G. Metabolic control improves long-term renal
allograft and patient survival in type 1 diabetes. J Am Soc
Nephrol 2008;19:1557-63. Crossref
19. Sung RS, Zhang M, Schaubel DE, Shu X, Magee JC. A
reassessment of the survival advantage of simultaneous
kidney-pancreas versus kidney-alone transplantation.
Transplantation 2015;99:1900-6. Crossref
20. Israni AK, Feldman HI, Propert KJ, Leonard M, Mange
KC. Impact of simultaneous kidney-pancreas transplant
and timing of transplant on kidney allograft survival. Am J
Transplant 2005;5:374-82. Crossref
21. Bohman SO, Tydén G, Wilczek H, et al. Prevention of kidney
graft diabetic nephropathy by pancreas transplantation in
man. Diabetes 1985;34:306-8. Crossref
22. Wilczek HE, Jaremko G, Tydén G, Groth CG. Evolution
of diabetic nephropathy in kidney grafts. Evidence that a
simultaneously transplanted pancreas exerts a protective
effect. Transplantation 1995;59:51-7. Crossref
23. Bilous RW, Mauer SM, Sutherland DE, Najarian JS, Goetz
FC, Steffes MW. The effects of pancreas transplantation on
the glomerular structure of renal allografts in patients with
insulin-dependent diabetes. N Engl J Med 1989;321:80-5. Crossref
24. Nyberg G, Holdaas H, Brekke IB, et al. Glomerular
ultrastructure in kidneys transplanted simultaneously
with a segmental pancreas to patients with type 1 diabetes.
Nephrol Dial Transplant 1996;11:1029-33. Crossref
25. Navarro X, Sutherland DE, Kennedy WR. Long-term effects
of pancreatic transplantation on diabetic neuropathy. Ann
Neurol 1997;42:727-36. Crossref
26. Jukema JW, Smets YF, van der Pijl JW, et al. Impact of
simultaneous pancreas and kidney transplantation on
progression of coronary atherosclerosis in patients with
end-stage renal failure due to type 1 diabetes. Diabetes
Care 2002;25:906-11. Crossref
27. Robertson RP, Sutherland DE, Kendall DM, Teuscher AU,
Gruessner RW, Gruessner A. Metabolic characterization
of long-term successful pancreas transplants in type I
diabetes. J Investig Med 1996;44:549-55.
28. Robertson RP, Holohan TV, Genuth S. Therapeutic
controversy: Pancreas transplantation for type I diabetes.
J Clin Endocrinol Metab 1998;83:1868-74. Crossref
29. Drognitz O, Benz S, Pfeffer F, et al. Long-term follow-up
of 78 simultaneous pancreas-kidney transplants at
a single-center institution in Europe. Transplantation
2004;78:1802-8. Crossref
30. Martins LS, Outerelo C, Malheiro J, et al. Health-related
quality of life may improve after transplantation in
pancreas-kidney recipients. Clin Transplant 2015;29:242-51. Crossref
31. Sureshkumar KK, Mubin T, Mikhael N, Kashif MA,
Nghiem DD, Marcus RJ. Assessment of quality of life
after simultaneous pancreas-kidney transplantation. Am J
Kidney Dis 2002;39:1300-6. Crossref
32. Larsen JL. Pancreas transplantation: indications and
consequences. Endo Rev 2004;25:919-46. Crossref
33. Knight RJ, Lawless A, Patel SJ, Gaber AO. Simultaneous kidney-pancreas transplantation for end-stage renal disease patients with insulin-dependent diabetes and detectable C-peptide. Transplant Proc 2010;42:4195-6. Crossref
34. Weems P, Cooper M. Pancreas transplantation in type II
diabetes mellitus. World J Transplant 2014;4:216-21. Crossref
35. Sampaio MS, Kuo HT, Bunnapradist S. Outcomes of
simultaneous pancreas-kidney transplantation in type 2
diabetic recipients. Clin J Am Soc Nephrol 2011;6:1198-206. Crossref
36. Stratta RJ, Rogers J, Farney AC, et al. Pancreas
transplantation in C-peptide positive patients: does “type”
of diabetes really matter? J Am Coll Surg 2015;220:716-27. Crossref
37. Chakkera HA, Bodner JK, Heilman RL, et al. Outcomes
after simultaneous pancreas and kidney transplantation and
the discriminative ability of the C-peptide measurement
pretransplant among type 1 and type 2 diabetes mellitus.
Transplant Proc 2010;42:2650-2. Crossref
38. Wiseman AC, Gralla J. Simultaneous pancreas kidney
transplant versus other kidney transplant options in
patients with type 2 diabetes. Clin J Am Soc Nephrol
2012;7:656-64. Crossref
39. Grochowiecki T, Gałązka Z, Madej K, et al. Surgical
complications related to transplanted pancreas after
simultaneous pancreas and kidney transplantation.
Transplant Proc 2014;46:2818-21. Crossref
40. Sansalone CV, Maione G, Aseni P, et al. Surgical
complications are the main cause of pancreatic allograft
loss in pancreas-kidney transplant recipients. Transplant
Proc 2005;37:2651-3. Crossref
41. Michalak G, Kwiatkowski A, Czerwinski J, et al. Surgical
complications of simultaneous pancreas-kidney
transplantation: a 16-year-experience at one center.
Transplant Proc 2005;37:3555-7. Crossref
42. Gruessner AC, Sutherland DE, Gruessner RW. Pancreas
transplantation in the United States: a review. Curr Opin
Organ Transplant 2010;15:93-101. Crossref
43. Malaise J, Steurer W, Koenigsrainer A, et al. Simultaneous
pancreas-kidney transplantation in a large multicenter
study: surgical complications. Transplant Proc
2005;37:2859-60. Crossref
44. Grochowiecki T, Gałązka Z, Madej K, et al. Early
complications related to the transplanted kidney after
simultaneous pancreas and kidney transplantation.
Transplant Proc 2014;46:2815-7. Crossref
45. Campos Hernández JP, Gómez Gómez E, Carrasco Valiente J, et al. Influence of surgical complications on kidney graft
survival in recipients of simultaneous pancreas kidney
transplantation. Transplant Proc 2015;47:112-6. Crossref
46. Ounissi M, Gargah T, Barbouch S, et al. Acute tubular
necrosis in kidney transplantation [in French]. Tunis Med
2012;90:463-7.
47. Samhan M, Al-Mousawi M, Hayati H, Abdulhalim M,
Nampoory MR. Urologic complications after renal
transplantation. Transplant Proc 2005;37:3075-6. Crossref
48. Renoult E, Amicabile C, Jonon B, Kessler M, L’Hermite J.
Early and recurrent venous graft thrombosis after kidney
transplantation: benefit of an early surgery. Clin Nephrol
1990;34:236-7.
49. Bretan PN, Burke EC. Renal transplantation. In: Tanagho
EA, Mc Annich JW, editors. Smith’s general urology.
London: Appleton and Lange; 1995: 612-24.
50. Kribs SW, Rankin RN. Doppler ultrasonography after
renal transplantation: value of reversed diastolic flow in
diagnosing renal vein obstruction. Can Assoc Radiol J
1993;44:434-8.
51. Hawkins BR. A point score system for allocating cadaveric
kidneys for transplantation based on patient age, waiting
time and HLA match. Hong Kong J Nephrol 2004;2:79-83. Crossref