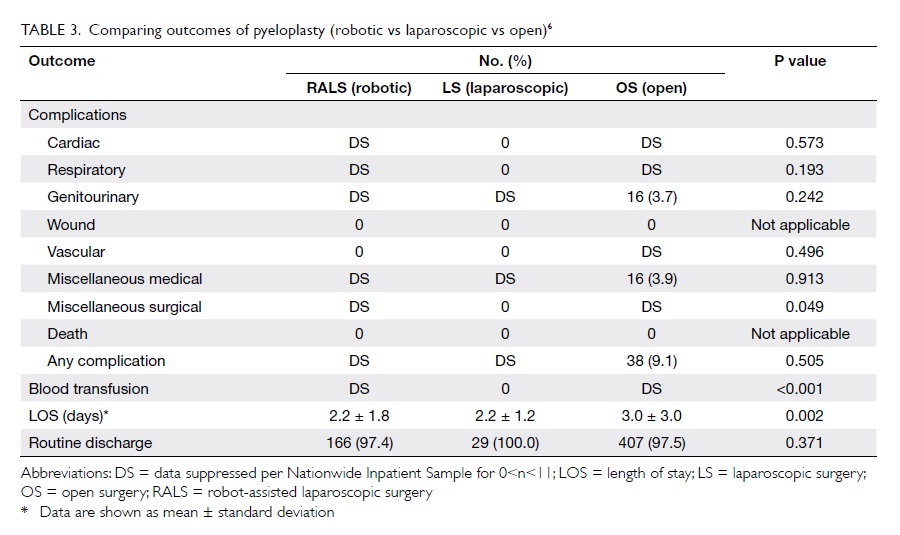
Use of viscoelastic haemostatic assay in emergency and elective surgery
Hong Kong Med J 2015 Feb;21(1):45–51 | Epub 1 Aug 2014
DOI: 10.12809/hkmj134147
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Use of viscoelastic haemostatic assay in
emergency and elective surgery
Maximus CF Yeung, MB, BS; Steven YT Tong, MB, BS; Paul YW Tong, MB, BS; Billy HH Cheung, MB, BS; Joanne YW Ng, MB, BS; Gilberto KK Leung, MB, BS, FHKAM (Surgery)
Department of Surgery, Li Ka Shing Faculty of Medicine, The University of
Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Gilberto KK Leung (gilberto@hku.hk)
Abstract
Objectives: To review the current evidence for the
use of viscoelastic haemostatic assays in different
surgical settings including trauma, cardiac surgery,
liver transplantation, as well as the monitoring
of antiplatelet agents and anticoagulants prior to
surgery.
Data sources: PubMed database.
Study selection: Key words for the literature search
were “thromboelastography” or “ROTEM” in
combination with “trauma”, “antiplatelet”, “cardiac
surgery”, “liver transplantation” or “anticoagulants”.
Data extraction: Original and major review articles
related to the use of viscoelastic haemostatic assays.
Data synthesis: Haemostatic function is a
critical factor determining patient outcomes in
emergency or elective surgery. The increasing use of
antiplatelet agents and anticoagulants has potentially
increased the risks of haemorrhages and the need
for transfusion. Conventional coagulation tests have
limitations in detecting haemostatic dysfunctions
in subgroups of patients and are largely ineffective
in diagnosing hyperfibrinolysis. The viscoelastic
haemostatic assays are potentially useful point-of-care
tools that provide information on clot formation,
clot strength, and fibrinolysis, as well as to guide goal-directed
transfusion and antifibrinolytic therapy.
They may also be used to monitor antiplatelet and
anticoagulant therapy. However, standardisation of
techniques and reference ranges is required before
these tests can be widely used in different clinical
settings.
Conclusions: Viscoelastic haemostatic assays,
as compared with conventional coagulation tests,
are better for detecting coagulopathy and are
the only tests that can provide rapid diagnosis of
hyperfibrinolysis. Goal-directed administration of
blood products based on the results of viscoelastic
haemostatic assays was associated with reduction
in allogeneic blood product transfusions in trauma,
cardiac surgery, and liver transplantation cases.
However, there is currently no evidence to support
the routine use of viscoelastic haemostatic assays for
monitoring platelet function prior to surgery.
Introduction
Conventional coagulation tests (CCTs) including
prothrombin time (PT), activated partial
thromboplastin time (aPTT), fibrinogen level, and
D-dimer have long been the standard laboratory
indicators of patients’ coagulation status. With the
introduction of the cell-based model of haemostasis,1
the role of platelets for intact thrombin generation,
and the limitations of CCTs have become increasingly
recognised. First of all, CCTs only measure individual
clotting components but do not take into account
the important interactions between platelets,
clotting factors, and other cellular components
in the generation of thrombin, nor the balance
between coagulation and fibrinolysis. Consequently,
results from CCTs may not correlate with clinically
significant coagulopathies or guide transfusion. Secondly, the quantity of individual elements does
not necessarily indicate how well haemostasis is
functioning, and qualitative dysfunction of platelets
and clotting factors is not taken into account. In
addition, PT/aPTT do not assess the overall strength
and stability of clots as they are read at the initiation
of fibrin polymerisation, which occurs when only
<5% of total thrombin has been generated.
Last but not least, CCTs are not point-of-care assays
and the long processing time may lead to treatment
delay with associated morbidity and mortality.2
In recent years, there has been an increasing
use of viscoelastic haemostatic assays (VHAs),
mainly thrombelastography (TEG; Hemoscope
Corporation, Niles [IL], US) and the rotational
thromboelastometry (ROTEM; Tem International
GmbH, Munich, Germany), to provide global and functional assessment of coagulation. Both tests
can be performed at the point-of-care during both
emergency and elective surgery, and may be used
to target transfusion at specific abnormalities
with a positive impact on minimising unnecessary
transfusion of allogeneic blood products.3 4 5
In the following sections, we shall describe the
basic principles of VHAs, followed by a literature
review of current evidence for its use. We searched
the PubMed database for all original and major review
articles published in the English language, using the
keywords “thromboelastography” or “ROTEM” in
combination with “trauma”, “antiplatelet”, “cardiac
surgery”, “liver transplantation” or “anticoagulants”.
Abstracts were screened for eligibility, and the
reference lists of eligible articles were searched for
further related studies. The date of the last search was
31 May 2013. All original studies and major review
articles concerning the use of TEG and/or ROTEM
in trauma, cardiac surgery, liver transplantation, and
monitoring of antiplatelet agents and anticoagulants
were included.
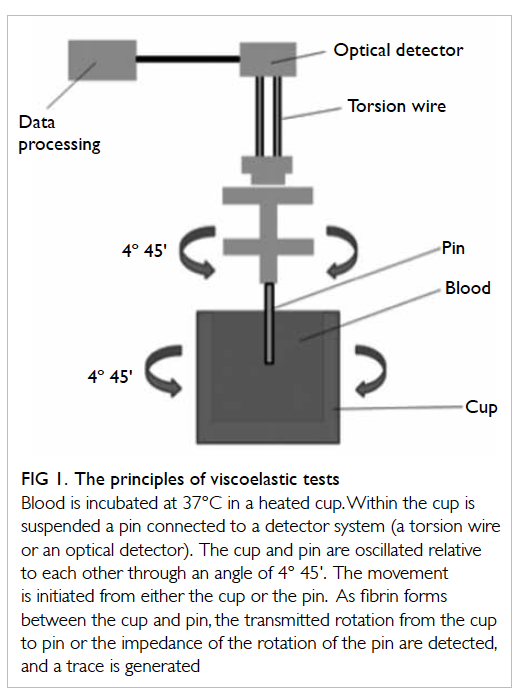
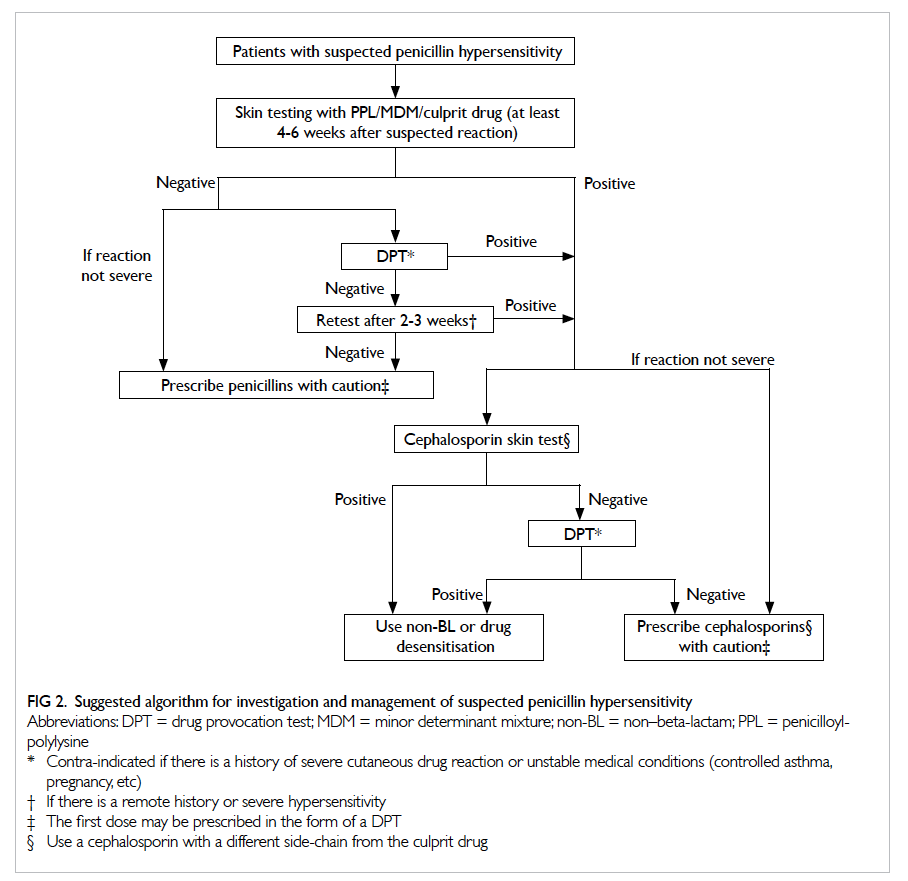
Principles of viscoelastic haemostatic assays
Thrombelastography was first described in 1948 by Hartert6 as a method to assess the viscoelastic
properties of coagulation in whole blood under
low shear conditions. The VHAs give a graphic presentation of clot formation and subsequent lysis.3
They measure the viscoelastic properties of a clot as
it forms in a cup after the addition of activators. As
the cup or pin oscillates, the torque of the rotational
cup, which is directly related to the strength of the
formed clot, is transmitted via the pin to a mechanoelectrical
transducer (Fig 1). The signals are then
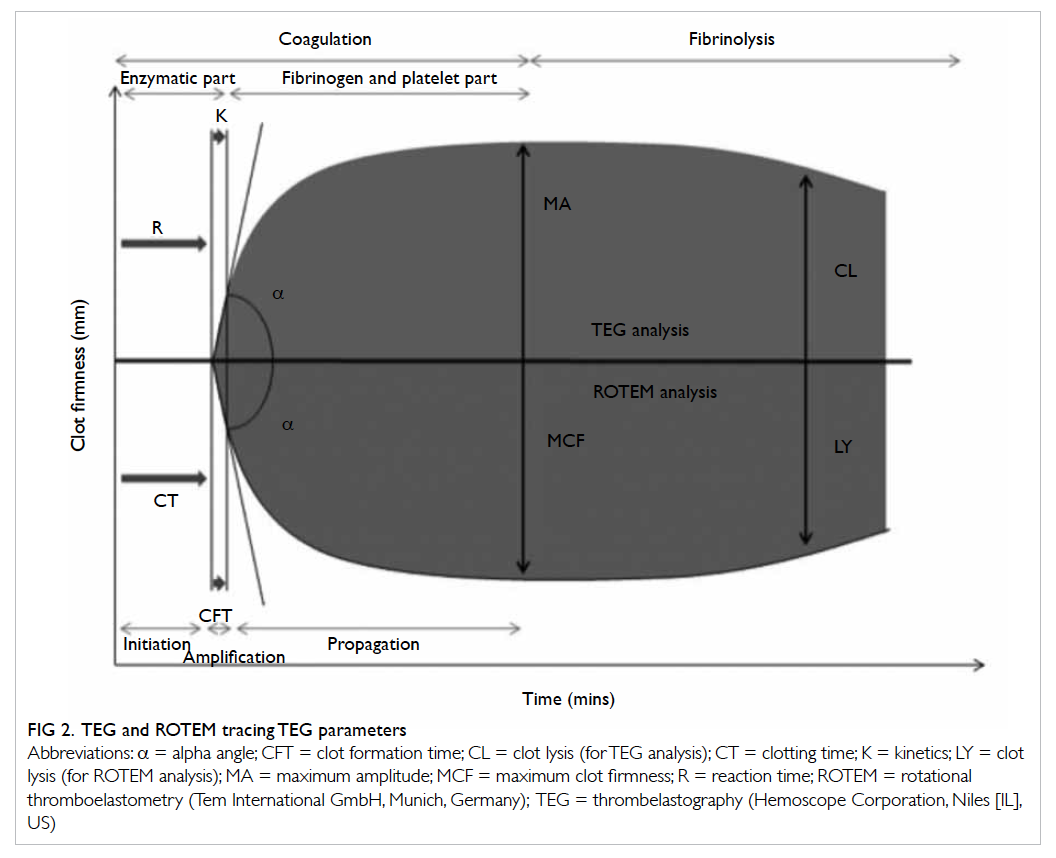
analysed by a computer that produces a trace graph.
The latter is divided into several parts with each
reflecting different stages of the haemostatic process
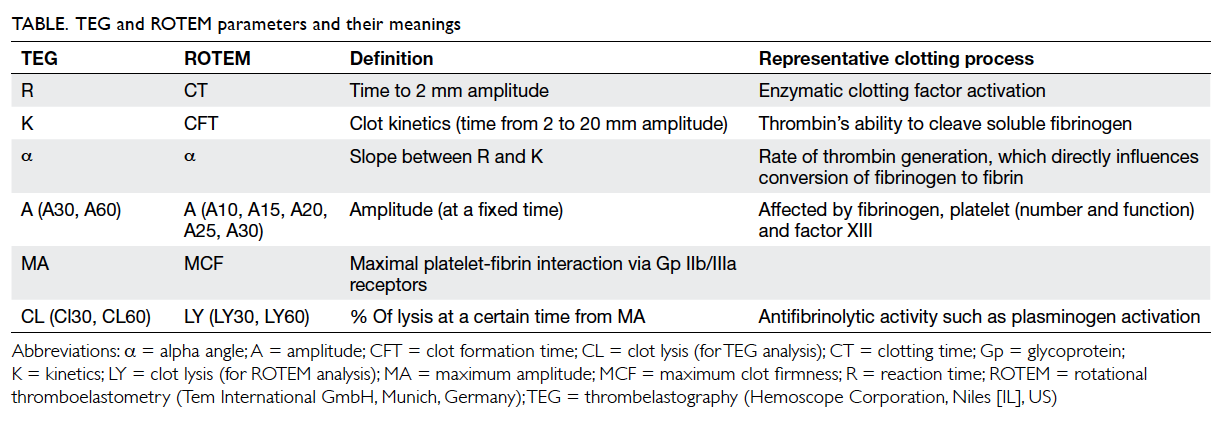
(Fig 2). Although the nomenclatures differ between
TEG and ROTEM, both provide information on
the speed of coagulation initiation (coagulation
factor activation), kinetics of clot growth (thrombin
generation and cleavage of fibrinogen), clot strength,
and fibrinolysis (Table). Different tracing morphology
and parameters will indicate which component of
the coagulation process may be dysfunctional.
Theoretical advantages of
viscoelastic haemostatic assays
over conventional coagulation
tests
Viscoelastic haemostatic assays assess the combined
influence of circulating plasmatic and cellular
(platelets, erythrocytes, leukocytes, microparticles)
elements. Results are available within 10 minutes, which can be utilised at the point-of-care in
different clinical settings. The end-points are clinically
relevant, allowing goal-directed coagulation factor
transfusions,7 8 9 with a reduction in allogeneic blood
product usage in trauma, cardiac, and liver surgery
cases.10 11 12 For example, fibrinogen concentrates could
be given in situations with decreased maximum
amplitude (MA)/maximum clot firmness (MCF).
Last but not least, as fibrinolysis plays an important role in both trauma-associated coagulopathy as well
as bleeding in patients undergoing elective cardiac
and liver surgery, VHAs serve as the only tests
available for rapid identification and quantification
of hyperfibrinolysis in those situations.
Moreover, VHAs can be used in diagnosing
surgical bleeding when the results of CCTs are normal,
and also play an important role in the assessment
of hypercoagulable states, prediction of bleeding risks in patients with liver and renal failures, and as
a research tool to improve the understanding of the
impact of various interventions on coagulation.
Trauma surgery
Uncontrolled haemorrhage accounts for more
than 50% of all trauma-related deaths within the
first 48 hours after hospitalisation.13 14 15 16 Current
evidence suggests that haemodilution, hypothermia,
acidaemia, and the consumption of clotting factors all play roles in the pathogenesis of coagulopathy in
trauma.17 Hyperfibrinolysis is associated with more
severe injury, greater coagulation abnormalities,
and a higher mortality rate. Kashuk et al18 suggested
that primary fibrinolysis occurred early in severely
injured patients, leading to rapid clot dissolution.
Massive transfusion of blood products in
trauma is not without risks, and may be associated
with increased risks of acute respiratory distress
syndrome, multi-organ failure, volume overload, and
transfusion-related infections. In particular, the risk
of transfusion-related acute lung injury following
fresh frozen plasma transfusion is recognised as
the most important cause of transfusion-associated
mortality and morbidity.19 Currently, the decision
of what, when, and how much blood products to
transfuse in massive trauma-related bleeding is
mainly empirical, though often supplemented by
results from CCTs. The laboratory diagnosis of
fibrinolysis, unfortunately, is often a difficult one,
requiring multiple tests such as euglobulin lysis
time (ELT), platelet count, fibrinogen, protein S,
protein C, and antithrombin levels, all of which
may not be readily available in the acute phase of
trauma management. Moreover, D-dimer (a marker
of fibrinolysis) is elevated in virtually all trauma
patients and is, therefore, not a useful indicator.
The VHAs, on the other hand, may provide
distinct advantages by promptly diagnosing
trauma coagulopathy (especially hyperfibrinolysis),
guiding blood transfusions, and providing potential
prognostic indicators. In a systematic review of
12 reported studies, Sankarankutty et al20 found
that TEG/ROTEM could be used to diagnose
hyperfibrinolysis accurately. There were otherwise
inconsistent correlations between VHAs and
CCTs; the most consistent correlation identified
was between TEG MA and ROTEM MCF with
platelet count and aPTT. Davenport et al21 showed
that ELT, which was used as a gold standard test
for the detection of fibrinolysis, was correlated
with clot amplitude at 10 minutes and 15 minutes, MCF, and clot lysis index at 60 minutes. This has
important implications in the use of antifibrinolytic
agents and fibrinogen for targeted management.22
As demonstrated in the CRASH-2 study, trauma
patients who received an antifibrinolytic agent
within 3 hours of trauma experienced a significant
reduction in mortality.23
Other studies also provided evidence to
support VHA-guided transfusion practice. Schöchl
et al24 25 reported that ROTEM-guided haemostatic
therapy with coagulation factor concentrates (eg
fibrinogen concentrate, prothrombin complex
concentrate) could facilitate early and effective
correction of coagulopathy, as well as reduce
transfusion of allogeneic blood products. Kashuk
et al26 27 showed that goal-directed resuscitation
based on rapid-TEG (rTEG) could reduce the use of fresh frozen
plasma. In terms of prognostication, TEG MA and
ROTEM MCF have been found to be associated with
mortality, while excessive fibrinolysis diagnosed by
either TEG clot lysis (CL) or ROTEM maximum
lysis (ML) was an independent predictor of mortality.
Cardiac surgery
Heparin is used during cardiopulmonary bypass
in open-heart surgery, and excessive postoperative
bleeding has been attributed to the insufficient
reversal of heparinisation with protamine sulfate.
This is partly due to the fact that conventional
monitoring by means of activated clotting time may
fail to differentiate between the contributions from
heparinisation, dilution, and platelet dysfunction.28 29
Johansson et al30 reviewed over 3250 cardiac patients
reported in 16 studies, and demonstrated superiority
of VHAs over CCTs in predicting bleeding and
the need for re-operation. The total number of
blood transfusions was reduced with VHA-guided
transfusion compared with CCT-based practices.
The degree of heparinisation could be evaluated
with assays that neutralise the heparin (heparinase
in TEG and HepTEM [heparinase-modified thromboelastometry] in ROTEM), so that non-heparin–related haemostatic problems could be
detected. These findings were corroborated in a
recent Cochrane analysis.11
Liver transplantation
A temporary hypercoagulable state is common after
liver transplantation due to imbalance between the
procoagulant and anticoagulant systems, as well as
fibrinolytic shutdown.31 This may play a role in the
early development of hepatic artery thrombosis.32
The use of CCT monitoring alone in the first
week post-transplantation may lead to significant
bleeding complications in certain patients.33 34 In
contrast, VHAs can accurately assess postoperative
hypercoagulability and thrombosis. Cerutti et al35
showed that TEG monitoring could demonstrate
hypercoagulability in the majority of the subjects
after living donor liver transplantation and may,
therefore, be used to guide antithrombotic treatment
in the perioperative period.
Monitoring antiplatelet therapy
With the rising prevalence of atherosclerotic diseases such as ischaemic heart disease and stroke,
it is not uncommon for surgical patients to be on
antiplatelet drugs. The latter may worsen bleeding
in trauma, increase transfusion requirement, and
increase the need for re-operation after elective
surgery.36 37 Unfortunately, CCTs do not test for
platelet inhibition due to antiplatelet therapy,
and tests for platelet function such as platelet
aggregometry do not provide results rapidly enough
for intra-operative monitoring and assessment of
coagulopathy in emergency surgery. Furthermore,
although antiplatelet drugs are thought to work
primarily by decreasing platelet aggregation,
they may also have anticoagulant effects.36 In
this respect, the MA/MCF from TEG/ROTEM
may serve to reveal the overall platelet function
and fibrinogen levels. In differentiating whether
reduced clot strength is due to low fibrinogen or low
platelet concentration/functionality, both TEG and
ROTEM have specific assays (functional fibrinogen
and FIBTEM [fibrin-based clotting], respectively) that block platelet
contributions with the addition of platelet inhibitors.
At present, however, there is no clinical evidence to
support the routine use of VHAs for monitoring
platelet function prior to surgery. Further studies
are required to demonstrate the clinical benefits of
these tests in reducing transfusion requirement and
improving surgical outcomes.
Monitoring anticoagulation
therapy
Anticoagulation therapy has similar negative
impact on emergency and elective surgery as
antiplatelet therapy. Point-of-care VHAs have
been shown to be useful in monitoring treatments
with low-molecular-weight heparin (LMWH),
heparinoids (eg danaparoid), and unfractionated
heparin.38 However, to increase the sensitivity of
VHAs for the effects of LMWH and heparinoids,
both standard and heparinase-modified tests
have to be carried out. Recently, direct thrombin
inhibitors such as dabigatran are being used
increasingly for the prevention and treatment of
venous thromboembosis, acute coronary syndrome,
and heparin-induced thrombocytopenia.39 The
monitoring of this group of patients is facilitated by
VHAs with the use of ecarin clotting time, in which
ecarin is used to activate thrombin directly.40 41
Limitations of viscoelastic haemostatic assays
There is at present a lack of universally agreed
algorithms for the use of VHAs. In a recent
systematic review of nine randomised clinical
trials (eight in cardiac surgery and one in liver
transplantation) comparing VHA-based algorithms
with standard treatments, the only supporting evidence identified for the use of VHAs was for
detection of hyperfibrinolysis.2
On an operational level, VHAs have been
criticised for not having undergone the same
evaluation process as CCTs. There are wide
technical variations in how VHAs are performed,
and the machine requires calibrations 2 to 3 times a
day which causes significant inconvenience in daily
point-of-care usage.42 While originally designed for
fresh whole blood with no additional activators,
subsequent modifications have included sample
anticoagulation and the use of different activators
to standardise the initiation of coagulation. Patients’
gender, age, and alcohol drinking may also affect
the result. Moreover, the normal reference ranges
for VHAs were derived from hospitalised surgical
patients in one study, and from a small sample of 12
healthy volunteers in another.43 Hence, each centre
is recommended to generate its own reference
range by specially trained personnel according
to the guidelines from the Clinical Laboratory
Improvement Amendments.44 The latter, which
regulates all laboratory tests in the United States,
demands a minimum of 30 to 40 subjects for special
coagulation tests.45 All these necessitate an active and
tightly controlled quality assurance programme.42
Despite its proposed role as a point-of-care
test, a complete viscoelastic test can take up to 60
minutes although useful information can be obtained
in approximately 10 minutes, when MCF is reached. This may be further increased by
a 30- to 40-minute wait for anticoagulated samples.42
To compensate for this, a modified rTEG has been developed. It uses tissue factors
as activators in addition to kaolin, and can provide
results on MCF more rapidly. However, information
on coagulation and clot formation time is limited.
Although there is a strong correlation between rTEG
and conventional TEG in terms of
overall clot strength and platelet function, there is
only moderate correlation in the degree of fibrin
cross-linking and poor correlation in evaluating
thrombolysis.46
There are conditions in which VHAs may fail
to detect haemostatic dysfunction. The test setting
is at 37°C. Therefore, the effect of hypothermia,
which has a well-recognised negative impact on
coagulation, may not be recognised.47 48 Viscoelastic
haemostatic assays do not assess the endothelial
contribution to haemostasis since an activator is
directly added to initiate coagulation during the test.
This means that the diagnosis of certain conditions
such as von Willebrand disease is not possible with
VHAs.48
Lastly, the interchangeability of results between
TEG and ROTEM has been questioned. Although
they share the same fundamental principles, and
similar parameters, hardware and techniques, the results generated may not be directly comparable,
possibly due to the use of different activators.
Consistent correlations are limited to that between
TEG MA and ROTEM MCF measurements, and that
between TEG CL and ROTEM ML in diagnosing
hyperfibrinolysis and predicting mortality.
Conclusions
Haemostatic function is a critical factor determining patient outcomes in emergency or elective
surgery. The increasing use of antiplatelet agents
and anticoagulants has potentially increased the
risks of haemorrhages and need for transfusion.
Conventional coagulation tests have limitations in
detecting haemostatic dysfunction in subgroups
of patients and are largely ineffective in diagnosing
hyperfibrinolysis. The VHAs are potentially useful
point-of-care tools to provide information on
clot formation, clot strength, and fibrinolysis, as well as to
guide goal-directed transfusion and antifibrinolytic
therapy. They may also be used to monitor
antiplatelet and anticoagulant therapy. However,
standardisation of techniques and reference ranges
is required before these tests can be widely used
in different clinical settings. Further studies are
needed to validate different algorithms, and address
coagulopathies in situations like hypothermia or
endothelial dysfunction.
Declaration
No conflicts of interest were declared by the authors.
References
1. Roberts HR, Hoffman M, Monroe DM. A cell-based model
of thrombin generation. Semin Thromb Hemost 2006;32
Suppl 1:32-8. CrossRef
2. Wikkelsoe AJ, Afshari A, Wetterslev J, Brok J, Moeller AM.
Monitoring patients at risk of massive transfusion with
thrombelastography or thromboelastometry: a systematic
review. Acta Anaesthesiol Scand 2011;55:1174-89. CrossRef
3. Ganter MT, Hofer CK. Coagulation monitoring: current
techniques and clinical use of viscoelastic point-of-care
coagulation devices. Anesth Analg 2008;106:1366-75. CrossRef
4. Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL.
Usefulness of thrombelastography in assessment of trauma
patient coagulation. J Trauma 1997;42:716-20. CrossRef
5. Rugeri L, Levrat A, David JS, et al. Diagnosis of early
coagulation abnormalities in trauma patients by rotation
thrombelastography. J Thromb Haemost 2007;5:289-95. CrossRef
6. Hartert H. Blutgerinnungsstudien mit
der thrombelastographie, einem neuen
untersuchungsverfahren [in German]. Klin Wochenschr 1948;26:577-83. CrossRef
7. Bontempo FA, Lewis JH, Van Thiel DH, et al. The relation of
preoperative coagulation findings to diagnosis, blood usage
and survival in adult liver transplantation. Transplantation
1985;39:532-6. CrossRef
8. Steib A, Gengenwin N, Freys G, Boudjema K, Levy S,
Otteni JC. Predictive factors of hyperfibrinolytic activity
during liver transplantation in cirrhotic patients. Br J
Anaesth 1994;73:645-8. CrossRef
9. Dzik WH, Arkin CF, Jenkins RL, Stump DC. Fibrinolysis
during liver transplantation in humans: role of tissue-type
plasminogen activator. Blood 1988;71:1090-5.
10. Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev
J. Thrombelastography (TEG) or thromboelastometry
(ROTEM) to monitor haemotherapy versus usual care in
patients with massive transfusion. Cochrane Database Syst
Rev 2011;(3):CD007871.
11. Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided
transfusion decreases intraoperative blood
transfusion during orthotopic liver transplantation:
randomized clinical trial. Transplant Proc 2010;42:2590-3. CrossRef
12. Aoki K, Sugimoto A, Nagasawa A, Saito M, Ohzeki H.
Optimization of thromboelastography-guided platelet
transfusion in cardiovascular surgery. Gen Thorac
Cardiovasc Surg 2012;60:411-6. CrossRef
13. Sauaia A, Moore FA, Moore EE, et al. Epidemiology of
trauma deaths: a reassessment. J Trauma 1995;38:185-93. CrossRef
14. Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of
trauma: mechanism, identification and effect. Curr Opin
Crit Care 2007;13:680-5. CrossRef
15. Maegele M, Lefering R, Yucel N, et al. Early coagulopathy
in multiply injury: an analysis from the German Trauma
Registry on 8724 patients. Injury 2007;38:298-304. CrossRef
16. MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha
M. Early coagulopathy predicts mortality in trauma. J
Trauma 2003;55:39-44. CrossRef
17. Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of
trauma: a review of mechanisms. J Trauma 2008;65:748-54. CrossRef
18. Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis
is integral in the pathogenesis of the acute coagulopathy of
trauma. Ann Surg 2010;252:434-4.
19. Stainsby D, Jones H, Asher D, et al. Serious hazards of
transfusion: a decade of hemovigilance in the UK. Transfus
Med Rev 2006;20:273-82. CrossRef
20. Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli
S. TEG® and ROTEM® in trauma: similar test but different
results. World J Emerg Surg 2012;7 Suppl 1:S3. CrossRef
21. Davenport R, Manson J, De’Ath H, et al. Functional
definition and characterization of acute traumatic
coagulopathy. Crit Care Med 2011;39:2652-8.
22. Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use
for minimising perioperative allogeneic blood transfusion.
Cochrane Database Syst Rev 2011;(1):CD001886.
23. Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a
randomised controlled trial and economic evaluation of
the effects of tranexamic acid on death, vascular occlusive
events and transfusion requirement in bleeding trauma
patients. Health Technol Assess 2013;17:1-79.
24. Schöchl H, Nienaber U, Maegele M, et al. Transfusion in
trauma: thromboelastometry-guided coagulation factor
concentrate-based therapy versus standard fresh frozen
plasma-based therapy. Crit Care 2011;15:R83. CrossRef
25. Schöchl H, Nienaber U, Hofer G, et al. Goal-directed
coagulation management of major trauma patients using
thromboelastometry (ROTEM®)-guided administration
of fibrinogen concentrate and prothrombin complex
concentrate. Crit Care 2010;14:R55. CrossRef
26. Kashuk JL, Moore EE, Le T, et al. Noncitrated whole
blood is optimal for evaluation of postinjury coagulopathy
with point-of-care rapid thrombelastography. J Surg Res
2009;156:133-8. CrossRef
27. Kashuk JL, Moore EE, Wohlauer M, et al. Initial experiences
with point-of-care rapid thrombelastography for
management of life-threatening postinjury coagulopathy.
Transfusion 2012;52:23-33. CrossRef
28. Koster A, Fischer T, Praus M, et al. Hemostatic activation
and inflammatory response during cardiopulmonary
bypass: impact of heparin management. Anesthesiology
2002;97:837-41. CrossRef
29. Koster A, Despotis G, Gruendel M, et al. The plasma
supplemented modified activated clotting time for
monitoring of heparinization during cardiopulmonary
bypass: a pilot investigation. Anesth Analg 2002;95:26-30. CrossRef
30. Johansson PI, Sølbeck S, Genet G, Stensballe J, Ostrowski
SR. Coagulopathy and hemostatic monitoring in cardiac
surgery: an update. Scand Cardiovasc J 2012;46:194-202. CrossRef
31. Stahl RL, Duncan A, Hooks MA, Henderson JM, Millikan
WJ, Warren WD. A hypercoagulable state follows
orthotopic liver transplantation. Hepatology 1990;12:553-8. CrossRef
32. Lisman T, Porte RJ. Hepatic artery thrombosis after liver
transplantation: more than just a surgical complication?
Transpl Int 2009;22:162-3. CrossRef
33. Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein
thrombosis in candidates for liver transplantation:
usefulness of screening and anticoagulation. Gut
2005;54:691-7. CrossRef
34. Widen A, Rolando N, Manousou P, et al. Anticoagulation
after liver transplantation: a retrospective audit and case-control
study. Blood Coagul Fibrinolysis 2009;20:615-8. CrossRef
35. Cerutti E, Stratta C, Romagnoli R, et al. Thromboelastogram
monitoring in the perioperative period of hepatectomy for
adult living liver donation. Liver Transpl 2004;10:289-94. CrossRef
36. Jacob M, Smedira N, Blackstone E, Williams S, Cho L. Effect
of timing of chronic preoperative aspirin discontinuation
on morbidity and mortality in patients having combined
coronary artery bypass grafting and valve surgery. Am J
Cardiol 2012;109:824-30. CrossRef
37. Jacob M, Smedira N, Blackstone E, Williams S, Cho L. Effect
of timing of chronic preoperative aspirin discontinuation
on morbidity and mortality in coronary artery bypass
surgery. Circulation 2011;123:577-83. CrossRef
38. Coppell JA, Thalheimer U, Zambruni A, et al. The effects
of unfractionated heparin, low molecular weight heparin
and danaparoid on the thromboelastogram (TEG): an in-vitro
comparison of standard and heparinase-modified
TEGs with conventional coagulation assays. Blood Coagul
Fibrinolysis 2006;17:97-104. CrossRef
39. Di Nisio M, Middeldorp S. Büller HR. Direct thrombin
inhibitors. N Engl J Med 2005;353:1028-40. CrossRef
40. Nielsen VG, Steenwyk BL, Gurley WQ, Pereira SJ, Lell
WA, Kirklin JK. Argatroban, bivalirudin, and lepirudin do
not decrease clot propagation and strength as effectively
as heparin-activated antithrombin in vitro. J Heart Lung
Transplant 2006;25:653-63. CrossRef
41. Carroll RC, Chavez JJ, Simmons JW, et al. Measurement of
patients’ bivalirudin plasma levels by a thrombelastograph
ecarin clotting time assay: a comparison to a standard
activated clotting time. Anesth Analg 2006;102:1316-9. CrossRef
42. da Luz LT, Nascimento B, Rizoli S. Thrombelastography
(TEG): practical considerations on its clinical use in
trauma resuscitation. Scand J Trauma Resusc Emerg Med
2013;21:29. CrossRef
43. Scarpelini S, Rhind SG, Nascimento B, et al. Normal
range values for thromboelastography in healthy adult
volunteers. Braz J Med Biol Res 2009;42:1210-7. CrossRef
44. Chan KL, Summerhayes RG, Ignjatovic V, Horton SB,
Monagle PT. Reference values for kaolin-activated
thromboelastography in healthy children. Anesth Analg
2007;105:1610-3. CrossRef
45. Centers for Disease Control and Prevention. Considering
the clinical laboratory improvement amendment. CDC
website: http://wwwn.cdc.gov/cliac/pdf/Addenda/cliac0210/Addendum%20Y.pdf. Accessed 9 Feb 2010.
46. Lee TH, McCully BH, Underwood SJ, Cotton BA,
Cohen MJ, Schreiber MA. Correlation of conventional
thrombelastography and rapid thrombelastography in
trauma. Am J Surg 2013;205:521-7. CrossRef
47. Rundgren M, Engström M. A thromboelastometric
evaluation of the effects of hypothermia on the coagulation
system. Anesth Analg 2008;107:1465-8. CrossRef
48. Johansson PI, Stissing T, Bochsen L, Ostrowski SR.
Thrombelastography and thromboelastometry in assessing
coagulopathy in trauma. Scand J Trauma Resusc Emerg
Med 2009;17:45. CrossRef
Find HKMJ in MEDLINE: