Beating ‘Guangdong cancer’: a review and update on nasopharyngeal cancer
Hong Kong Med J 2017 Oct;23(5):497–502 | Epub 1 Sep 2017
DOI: 10.12809/hkmj176834
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Beating ‘Guangdong cancer’: a review and update on nasopharyngeal cancer
CS Ho, BPharm
Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong
Kong
Corresponding author: Dr CS Ho (jasonho@link.cuhk.edu.hk)
Abstract
Once endemic in southern China, nasopharyngeal
cancer is becoming less prevalent in Hong Kong.
This is probably due to a better understanding of the
risk factors associated with the disease, its genomic
landscape, advances in radiotherapy technology,
and development of effective systemic agents. More
specifically, the close relationship between Epstein-Barr virus and nasopharyngeal cancer opens up
the possibility of using Epstein-Barr virus DNA
as a biomarker for early detection and monitoring
of the disease. On the other hand, the looming
genomic data for nasopharyngeal cancer aid in the
development of powerful biomarkers and promising
targeted therapy. Clinical use of a combination
of radiotherapy and chemotherapy continues to
increase, while the development of immunotherapy,
such as checkpoint inhibitors, offers hope in
improving treatment outcome.
Introduction
Nasopharyngeal cancer (NPC) was once considered
endemic in the southern part of China. This type of
cancer was so prevalent in Guangdong Province in
southern China in the early 20th century that it was
dubbed ‘Guangdong cancer’.1 Although the name is
now less popular and the incidence of NPC has been
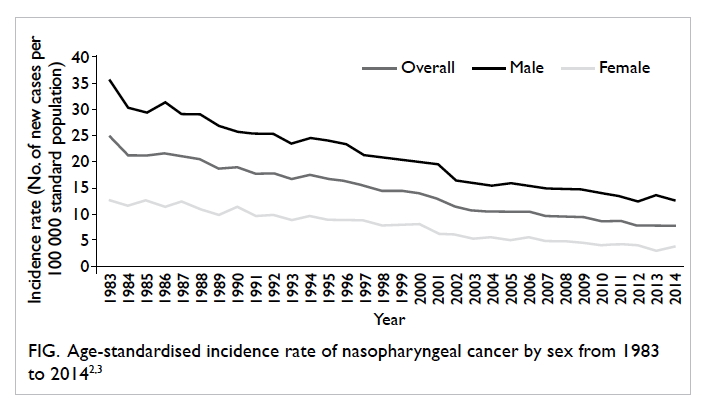
decreasing since then (Fig),2 3 its occurrence in Hong Kong and Southeast Asia is still considerably higher
than in other parts of the world: the age-standardised
incidence rate was 7.9 per 100 000 population in
2014 in Hong Kong,3 compared with less than 1.0 per
100 000 population in North America and Europe.4
The global data from GLOBOCAN in 2012 showed
that 38% of all new cases of NPC were registered in
China.5
While Hong Kong is one of the regions that
experience the most NPC,6 it has also become a centre
for NPC research. Indeed, many important and
landmark studies in NPC were performed in Hong
Kong, where local experts have been responsible for
developing practice guidelines with regard to the
diagnosis, management, and follow-up of NPC.7 8 It is thus interesting to review the updated knowledge
about the aetiology, risk factors, diagnosis, and
treatment strategies of this ‘Guangdong cancer’.
Classification, aetiology, and risk
factors
Classification and staging
Nasopharyngeal cancer can be categorised according
to its histopathology: keratinising, non-keratinising
(which can be further subdivided into differentiated
and undifferentiated forms), and basaloid squamous
cell carcinoma; all of which are to replace the old
numerical classification system.9 In endemic regions
such as Hong Kong, non-keratinising carcinoma
predominates, whereas the keratinising type is more
common in other parts of the world.10
Nasopharyngeal cancer is staged according to
the tumour, node, metastasis system. To assist with
the prognosis and guide treatment decisions, NPC
can be further stratified into five different stages
(stages I, II, III, IVA, and IVB), as suggested by the
latest American Joint Committee on Cancer (8th
edition) cancer staging manual.11
Viral factors
While it is widely believed that NPC is caused by
the interaction of several factors, Epstein-Barr virus
(EBV) infection is undoubtedly the most studied
aetiological factor for NPC. This virus—as a primary
aetiological agent of NPC, specifically the endemic
non-keratinising type—has been supported by a
large body of evidence12; a review in 2012 suggested
that EBV accounted for more than 85% of NPC cases
globally.13 Based on in-situ hybridisation techniques10
and the fact that EBV infects more than 90% of
the population,14 EBV reactivation is considered
necessary in the pathogenesis of NPC; inhibition of
EBV reactivation is currently being investigated as
a possible approach to preventing NPC relapse.15
What triggers the reactivation, however, is less well-defined,
although cigarette smoking is among the
possible reactivating factors.16 17
On the other hand, human papillomavirus
(HPV), a common aetiological agent causing
cervical cancer, is associated with the non-endemic,
keratinising type of NPC, although evidence is
limited due to its low prevalence.18 While EBV and
HPV infections are nearly always mutually exclusive
in the pathogenesis of NPC,9 studies have suggested
that HPV-positive NPC is associated with poorer
outcome when compared with EBV-positive NPC.19
Genetic factors
Genetic susceptibility has attracted intense
interest since the development of various genomic
techniques. A whole-exome sequencing study in
2014 revealed the genetic alterations that affect a
number of cellular pathways, including chromatin
modification, ErbB-phosphatidylinositol-3 kinase
signalling, and autophagy machinery in NPC.20
Epigenetic alterations of various chromosomal
regions, especially those regions with tumour-suppressor
genes, were also found in NPC patients.21
Li et al22 recently identified genomic aberrations
of multiple negative regulators of the nuclear
factor-κB (NF-κB) pathway in 111 EBV-positive
NPC samples in another whole-exome sequencing
study, suggesting the pivotal role of activating the
NF-κB signalling pathway in NPC and the potential
therapeutic applications of NF-κB inhibitors.22 The
researchers also revealed major histocompatibility
complex class I gene aberrations in some of the
samples, and the efficacy of immune checkpoint
inhibitors (discussed below) may be affected in this
subgroup of NPC patients.22 Although much in this
field remains to be elucidated, it is expected that
the genetic research will aid in the development of
powerful biomarkers for the diagnosis, prognosis,
and evaluation of the treatment for NPC.21
Environmental factors
An increased risk of NPC has been associated with a
number of lifestyle factors, among which a history of
salted fish consumption has the strongest association.
Various studies have confirmed its association with
NPC,23 24 and its relationship with the high prevalence of NPC in Hong Kong and neighbouring regions
in the 20th century.6 N-nitrosamine found in the
preserved salted fish is believed to be the carcinogen
concerned.25 Other factors such as the use of Chinese
medicinal herbs and high consumption of fermented
food were also suggested, but the associations were
often inconsistent among studies.24
Diagnosis
Nasendoscopy for a biopsy sample is essential for a
definitive diagnosis of NPC. Detecting and diagnosing
NPC at an early stage is of paramount importance:
the disease stage is significantly correlated with the
outcome in NPC, and early diagnosis may improve
outcomes.12 Cell-free EBV DNA analysis was shown
to have high sensitivity and specificity in detecting
NPC, and has been further validated by various
studies.26 A local study further showed that the
analysis was useful in detecting early-stage NPC in
asymptomatic individuals.27 An expanded phase II
study involving over 20 000 participants to evaluate
its feasibility as a screening tool (NCT02063399) has
just been completed, showing excellent sensitivity
and specificity (97.1% and 98.6%, respectively).28
Participants who were identified with NPC by this
screening tool were detected significantly earlier and
with better outcome when compared with those in a
historical control.28
Other roles of Epstein-Barr virus DNA
With the substantial involvement of EBV in the
pathogenesis of NPC, it is sensible to exploit EBV
DNA as a biomarker in managing patients with NPC.
One such application is the prediction of disease
recurrence after treatment. Post-treatment EBV
DNA level has been shown to be the most powerful
predictor for disease recurrence and long-term
survival in NPC patients of different ethnic origins,
clinical stages, and treatment modalities.29 30 31 32 33 34 35 36 Recently
Lee et al37 demonstrated that serial post–intensity
modulated radiation therapy (IMRT) undetectable
plasma EBV DNA was prognostic of all predefined
survival end-points at 3 years in the modern IMRT era.
Leung et al38 further showed that detectable plasma
EBV DNA level at midcourse of radiotherapy (RT)
or chemoradiotherapy (CRT) is adversely associated
with worse overall survival (OS) and progression-free
survival (PFS). This suggests the possibility of
shifting prognostication from a post-therapy time-point
to midcourse of therapy, and selecting high-risk
patients for therapy intensification by measuring
midcourse plasma EBV DNA level.38
Another notable application is the prediction of
treatment outcome by measuring the clearance rate
of plasma EBV DNA. Following the observation that
EBV DNA was rapidly cleared from the circulation
after surgical resection of NPC,39 subsequent
studies demonstrated that patients with more rapid
clearance of plasma EBV DNA responded better to
chemotherapy or CRT compared with patients with
a slower clearance.40 41 A prospective trial evaluating
the response to chemotherapy by measuring plasma
EBV DNA half-life together with tumour metabolic
response (via fluorodeoxyglucose positron emission
tomographic scan) is currently underway.
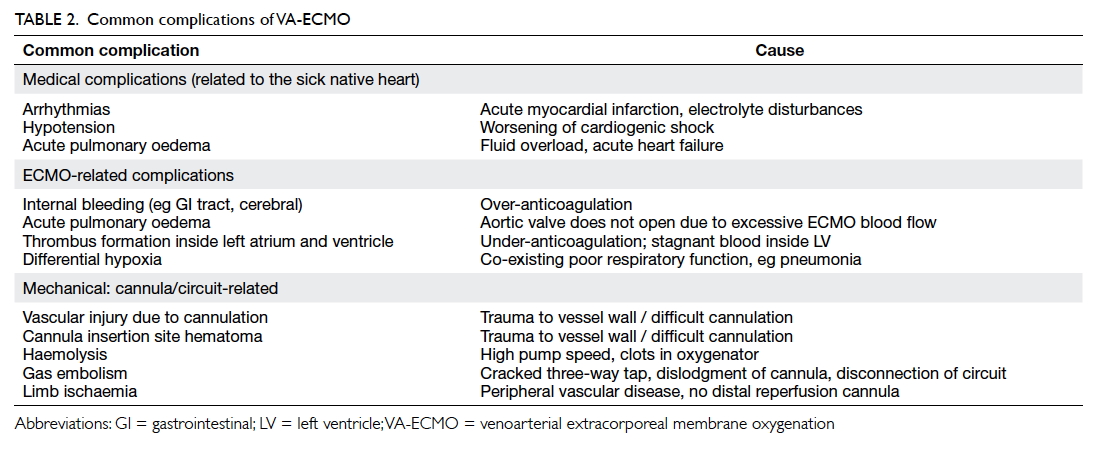
Treatment strategies
Radiotherapy
Radiotherapy has long been regarded as the mainstay
of NPC treatment, due to the radiosensitive nature of
the tumour, and the anatomical position of NPC that
limits a surgical approach.10 Of note, IMRT is currently
the preferred approach, with its improved OS and
decreased toxicity,42 advantages in preserving parotid
function and reducing severe xerostomia,43 and
improved quality of life compared with conventional
two-dimensional (2D) RT.44 It is currently used as a
monotherapy for the early stage of NPC.
Since the pre-IMRT era, re-irradiation has
been shown to be effective in non-metastatic,
recurrent NPC (rNPC) patients after primary RT.45 46 47
With its introduction, IMRT has quickly emerged
as the radiation modality of choice for rNPC as
well, with or without the use of chemotherapy. Its
efficacy has been established in various studies,
with documented long-term OS rates ranging from
45% to 65%.48 49 50 51 52 53 54 55 56 Yet, most of the patients in those
studies were treated with conventional 2D-RT in
the pre-IMRT era. In a recent study conducted by
Kong et al,56 77 patients received salvage IMRT for
rNPC after a definitive course of primary IMRT.
While the median OS and PFS were 37.0 and 20.5
months, respectively, of particular note is the re-irradiation
toxicity. Of 34 patients, 18 died from
treatment-induced severe adverse effects without
evidence of disease progression during the study,
including mucosal necrosis, temporal lobe necrosis,
and cranial neuropathy,56 reflecting the limitations
of salvage IMRT in the modern IMRT era. Other
radiation modalities have been proposed, including
particle therapy using proton and carbon ions,57 but
long-term data are not yet available.
Chemotherapy
Chemotherapy is another important modality in
managing NPC, and it is often combined with RT
in the intermediate and advanced stages of NPC.
The benefit of CRT was well-illustrated in a meta-analysis
of seven trials, which showed significantly
improved OS and 10-year PFS in the CRT group
compared with the RT-alone group.58 A platinum-based
regimen is often used as the chemotherapy of
choice, in which cisplatin is most commonly used.10
While it is clear that chemotherapy is essential
in the treatment of advanced NPC, its value as an
add-on induction therapy (preceding CRT) and
adjuvant therapy (following CRT) is less clear.
Regarding induction therapy, a phase III trial recently
showed that the addition of docetaxel, cisplatin, and
fluorouracil prior to CRT was superior to CRT alone
in terms of OS and PFS at 3 years,59 although another
trial using cisplatin and fluorouracil as induction
therapy failed to show significant differences in
OS.60 The role of induction therapy requires further
confirmation from other ongoing phase III trials.
Meanwhile, the use of adjuvant chemotherapy
following CRT is debatable. A phase III trial with
a median follow-up of 68.4 months failed to show
significantly improved OS and PFS after adding
cisplatin and fluorouracil as adjuvant therapy post-CRT in locally advanced NPC,61 but another study
suggested adjuvant chemotherapy might be reserved
for high-risk patients defined by post-treatment
residual EBV DNA.62 63 It should be noted, however, that the benefit of more intensive therapy may be
limited by the late toxicities of high cumulative doses
of chemotherapy, most notably cisplatin, which are
not reported in some of the studies.60 64
Platinum-containing doublet regimens remain
the first-line systemic treatment for recurrent or
metastatic NPC. Cisplatin and fluorouracil have
been the conventional choices.10 A recent study by
Zhang et al65 demonstrated that the combination
of cisplatin plus gemcitabine was superior to the
combination of cisplatin and fluorouracil, in terms
of median PFS (7.0 vs 5.6 months; hazard ratio=0.55;
95% confidence interval, 0.44-0.68), although the
cisplatin-gemcitabine group experienced more
haematological toxicity, such as grade-3 or higher
leukopenia, neutropenia, and thrombocytopenia.65
This randomised controlled trial has thus established
the role of cisplatin and gemcitabine combination
as the chemotherapy of choice in recurrent or
metastatic NPC.
Surgery and targeted therapy
As mentioned above, surgery is usually not
considered in the routine management of NPC; yet
salvage therapy can be considered an option for
selected patients with local recurrence in the neck.66
Molecular targeted therapy is considered hopeful
for many other types of carcinoma, but its efficacy
in treating NPC has been disappointing; studies
of inhibitors of epidermal growth factor receptor
(eg cetuximab) and vascular endothelial growth
factor (eg sunitinib) failed to show superiority over
standard treatments, and were largely limited to
phase II trials.8 Lee et al8 attributed its failure to
the scarcity of authentic NPC models that can be
utilised in the preclinical studies of new drugs, and
increased incidence of drug-related toxicities such
as bleeding. The development of immunotherapy
is therefore exciting as it presents a new hope for
managing NPC.
Immunotherapy
The presence of EBV and the expression of viral
antigens in almost all NPC cases make this
disease an attractive target for the development of
immunotherapy. For example, EBV nuclear antigen
I (EBNA1) and latent membrane protein 2 (LMP2)
are frequently expressed in EBV-associated NPC,
and a recombinant virus–based vaccine that encodes
an inactive fusion protein containing fragments
of EBNA1 and LMP2 was shown to be effective in
inducing T-cell response in a local phase I trial.67 The
vaccine is currently being tested in a phase II clinical
trial (NCT01094405).
As EBV that persists as a latent infection is
controlled by cytotoxic T lymphocytes (CTL),68 it
follows that the use of EBV-specific CTL for NPC
appears logical as a treatment strategy. Adoptive
immunotherapy that includes infusion of autologous
CTL has been tested in a number of clinical trials,
and the results have been promising. For example,
a study in Singapore showed that chemotherapy
followed by EBV-specific CTL achieved a response
rate (full or partial) of 71.4% in 38 patients,69 and
a phase III trial is currently underway to assess its
efficacy (NCT02578641).
Among all the immunotherapies available,
checkpoint inhibitors seem to be the most rapidly
developing. Programmed death ligand–1 (PD-L1)
was found to be expressed on antigen-presenting
cells, and its interaction with the programmed
death–1 (PD-1) receptor on T cells inhibits
downstream signalling of T cell receptors.70 Tumour-associated
PD-L1 was also found to mediate immune
suppression by various other mechanisms, such as
facilitating T cell apoptosis and inducing regulatory
T cells.71 With PD-L1 expressed in many different
carcinomas,72 blockade of PD-L1 and/or the PD-1
receptor has become the focus of new cancer drug
development in the past 5 years.
While PD-L1 inhibitor has recently gained
much attention in the treatment of non–small-cell
lung cancer,73 its progress in the treatment
of advanced NPC is exciting and much awaited.
Pembrolizumab was shown to be well-tolerated
with significant anti-tumour activity in NPC in a
phase Ib trial,74 and is currently in a phase II trial
to confirm the response rate and efficacy in terms
of improvement in OS (NCT02611960). Nivolumab
has just completed phase II trials; the preliminary
results showed that it is active in heavily pre-treated
recurrent or metastatic patients,75 76 and that PD-L1 expression may predict benefits from nivolumab.75
Conclusion
Once a nightmare in the eyes of many Hong Kong
inhabitants, NPC has become less prevalent
in southern China, but it still poses a threat to
Hong Kong citizens as it was ranked as the 10th
most common cancer in the city.3 With clearer
understanding of its pathophysiology and advances
in technology, it is expected that more refined
treatment strategies and novel therapeutic agents
will be available in the near future.
Acknowledgement
I would like to thank Prof Brigette Ma from
Department of Clinical Oncology, The Chinese
University of Hong Kong for her comments and
advice.
References
1. Gibb AG, van Hasselt CA, editors. Nasopharyngeal
carcinoma. 2nd ed. Hong Kong: The Chinese University
Press; 1999.
2. Nasopharyngeal cancer. Available from: http://www.chp.gov.hk/en/content/9/25/54.html. Accessed 12 Apr 2017.
3. Hong Kong Cancer Registry, Hospital Authority. Available
from: www3.ha.org.hk/cancereg/statistics.html. Accessed
12 Apr 2017.
4. 2014 Review of cancer medicines on the WHO list of
essential medicines: Nasopharyngeal carcinoma. Geneva:
World Health Organization; 2014.
5. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN
2012: Estimated cancer incidence, mortality and prevalence
worldwide in 2012 v1.0. Lyon, France: International Agency
for Research on Cancer; 2013.
6. Tang LL, Chen WQ, Xue WQ, et al. Global trends in
incidence and mortality of nasopharyngeal carcinoma.
Cancer Lett 2016;374:22-30. Crossref
7. Chan AT, Grégoire V, Lefebvre JL, et al. Nasopharyngeal
cancer: EHNS-ESMO-ESTRO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol 2012;23
Suppl 7:vii83-5. Crossref
8. Lee AW, Ma BB, Ng WT, Chan AT. Management of
nasopharyngeal carcinoma: current practice and future
perspective. J Clin Oncol 2015;33:3356-64. Crossref
9. Pathology and genetics of head and neck tumours. In:
Barnes L, Eveson JW, Reichart P, Sidransky D, editors.
World Health Organization classification of tumours.
Lyon: IARC Press; 2005.
10. Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal
carcinoma. Lancet 2016;387:1012-24. Crossref
11. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck
cancers—major changes in the American Joint Committee
on Cancer eighth edition cancer staging manual. CA
Cancer J Clin 2017;67:122-37. Crossref
12. Raghupathy R, Hui EP, Chan AT. Epstein-Barr virus as a
paradigm in nasopharyngeal cancer: from lab to clinic. Am
Soc Clin Oncol Educ Book 2014: 149-53. Crossref
13. de Martel C, Ferlay J, Franceschi S, et al. Global burden
of cancers attributable to infections in 2008: a review and
synthetic analysis. Lancet Oncol 2012;13:607-15. Crossref
14. Raab-Traub N. Epstein-Barr virus in the pathogenesis of
NPC. Semin Cancer Biol 2002;12:431-41. Crossref
15. Wu CC, Fang CY, Hsu HY, et al. EBV reactivation as a target
of luteolin to repress NPC tumorigenesis. Oncotarget
2016;7:18999-9017. Crossref
16. Xu FH, Xiong D, Xu YF, et al. An epidemiological and
molecular study of the relationship between smoking,
risk of nasopharyngeal carcinoma, and Epstein-Barr virus
activation. J Natl Cancer Inst 2012;104:1396-410. Crossref
17. Hsu WL, Chen JY, Chien YC, et al. Independent effect of
EBV and cigarette smoking on nasopharyngeal carcinoma:
a 20-year follow-up study on 9,622 males without family
history in Taiwan. Cancer Epidemiol Biomarkers Prev
2009;18:1218-26. Crossref
18. Lo EJ, Bell D, Woo JS, et al. Human papillomavirus and
WHO type I nasopharyngeal carcinoma. Laryngoscope
2010;120:1990-7. Crossref
19. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic
HPV-positive nasopharyngeal carcinoma: association with
poor prognosis. Int J Radiat Oncol Biol Phys 2014;88:580-8. Crossref
20. Lin DC, Meng X, Hazawa M, et al. The genomic landscape
of nasopharyngeal carcinoma. Nat Genet 2014;46:866-71. Crossref
21. Dai W, Zheng H, Cheung AK, Lung ML. Genetic and
epigenetic landscape of nasopharyngeal carcinoma. Chin
Clin Oncol 2016;5:16. Crossref
22. Li YY, Chung GT, Lui VW, et al. Exome and genome
sequencing of nasopharynx cancer identifies NF-κB
pathway activating mutations. Nat Commun 2017;8:14121. Crossref
23. Ho JH. Nasopharyngeal carcinoma (NPC). Adv Cancer Res
1972;15:57-92. Crossref
24. Yu MC, Ho JH, Lai SH, Henderson BE. Cantonese-style
salted fish as a cause of nasopharyngeal carcinoma:
report of a case-control study in Hong Kong. Cancer Res
1986;46:956-61.
25. Guo X, Johnson RC, Deng H, et al. Evaluation of nonviral
risk factors for nasopharyngeal carcinoma in a high-risk
population of Southern China. Int J Cancer 2009;124:2942-7. Crossref
26. Fung SY, Lam JW, Chan KC. Clinical utility of circulating
Epstein-Barr virus DNA analysis for the management of
nasopharyngeal carcinoma. Chin Clin Oncol 2016;5:18. Crossref
27. Chan KC, Hung EC, Woo JK, et al. Early detection of
nasopharyngeal carcinoma by plasma Epstein-Barr
virus DNA analysis in a surveillance program. Cancer
2013;119:1838-44. Crossref
28. Chan KC, Woo JK, King A, et al. Analysis of Plasma
Epstein–Barr Virus DNA to Screen for Nasopharyngeal
Cancer. N Engl J Med 2017;377:513-22. Crossref
29. Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr
virus DNA and residual disease after radiotherapy for
undifferentiated nasopharyngeal carcinoma. J Natl Cancer
Inst 2002;94:1614-9. Crossref
30. Lin JC, Wang WY, Chen KY, et al. Quantification of
plasma Epstein-Barr virus DNA in patients with advanced
nasopharyngeal carcinoma. N Engl J Med 2004;350:2461-70. Crossref
31. Le QT, Jones CD, Yau TK, et al. A comparison study
of different PCR assays in measuring circulating
plasma Epstein-Barr virus DNA levels in patients with
nasopharyngeal carcinoma. Clin Cancer Res 2005;11:5700-7. Crossref
32. Lin JC, Wang WY, Liang WM, et al. Long-term prognostic
effects of plasma Epstein-Barr virus DNA by minor
groove binder-probe real-time quantitative PCR on
nasopharyngeal carcinoma patients receiving concurrent
chemoradiotherapy. Int J Radiat Oncol Biol Phys
2007;68:1342-8. Crossref
33. An X, Wang FH, Ding PR, et al. Plasma Epstein-Barr
virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with
palliative chemotherapy. Cancer 2011;117:3750-7. Crossref
34. Hou X, Zhao C, Guo Y, et al. Different clinical significance
of pre- and post-treatment plasma Epstein-Barr virus
DNA load in nasopharyngeal carcinoma treated with
radiotherapy. Clin Oncol (R Coll Radiol) 2011;23:128-33. Crossref
35. Ferrari D, Codecà C, Bertuzzi C, et al. Role of plasma EBV
DNA levels in predicting recurrence of nasopharyngeal
carcinoma in a western population. BMC Cancer
2012;12:208. Crossref
36. Zhao FP, Liu X, Chen XM, et al. Levels of plasma Epstein-Barr virus DNA prior and subsequent to treatment predicts the prognosis of nasopharyngeal carcinoma. Oncol Lett
2015;10:2888-94. Crossref
37. Lee VH, Kwong DL, Leung TW, et al. Prognostication
of serial post-intensity-modulated radiation therapy
undetectable plasma EBV DNA for nasopharyngeal
carcinoma. Oncotarget 2017;8:5292-308.
38. Leung SF, Chan KC, Ma BB, et al. Plasma Epstein-Barr
viral DNA load at midpoint of radiotherapy course predicts
outcome in advanced-stage nasopharyngeal carcinoma.
Ann Oncol 2014;25:1204-8. Crossref
39. To EW, Chan KC, Leung SF, et al. Rapid clearance of
plasma Epstein-Barr virus DNA after surgical treatment of
nasopharyngeal carcinoma. Clin Cancer Res 2003;9:3254-9.
40. Hsu CL, Chang KP, Lin CY, et al. Plasma Epstein-Barr virus
DNA concentration and clearance rate as novel prognostic
factors for metastatic nasopharyngeal carcinoma. Head
Neck 2012;34:1064-70. Crossref
41. Wang WY, Twu CW, Chen HH, et al. Plasma EBV DNA
clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res
2010;16:1016-24. Crossref
42. Peng G, Wang T, Yang KY, et al. A prospective, randomized
study comparing outcomes and toxicities of intensity-modulated
radiotherapy vs. conventional two-dimensional
radiotherapy for the treatment of nasopharyngeal
carcinoma. Radiother Oncol 2012;104:286-93. Crossref
43. Kam MK, Leung SF, Zee B, et al. Prospective randomized
study of intensity-modulated radiotherapy on salivary
gland function in early-stage nasopharyngeal carcinoma
patients. J Clin Oncol 2007;25:4873-9. Crossref
44. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and
quality of life after intensity-modulated radiotherapy vs.
conventional radiotherapy for early-stage nasopharyngeal
carcinoma: initial report on a randomized controlled
clinical trial. Int J Radiat Oncol Biol Phys 2006;66:981-91. Crossref
45. Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent
nasopharyngeal carcinoma: factors affecting the
therapeutic ratio and ways for improvement. Int J Radiat
Oncol Biol Phys 1997;38:43-52. Crossref
46. Teo PM, Kwan WH, Chan AT, Lee WY, King WW, Mok
CO. How successful is high-dose (>or = 60 Gy) reirradiation
using mainly external beams in salvaging local failures of
nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys
1998;40:897-913. Crossref
47. Chen C, Fee W, Chen J, et al. Salvage treatment for locally
recurrent nasopharyngeal carcinoma (NPC). Am J Clin
Oncol 2014;37:327-31. Crossref
48. Lu TX, Mai WY, Teh BS, et al. Initial experience
using intensity-modulated radiotherapy for recurrent
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys
2004;58:682-7. Crossref
49. Chua DT, Sham JS, Leung LH, Au GK. Re-irradiation of
nasopharyngeal carcinoma with intensity-modulated
radiotherapy. Radiother Oncol 2005;77:290-4. Crossref
50. Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity-modulated
radiation therapy in the salvage of locally
recurrent nasopharyngeal carcinoma. Int J Radiat Oncol
Biol Phys 2012;83:676-83. Crossref
51. Hua YJ, Han F, Lu LX, et al. Long-term treatment outcome
of recurrent nasopharyngeal carcinoma treated with
salvage intensity modulated radiotherapy. Eur J Cancer
2012;48:3422-8. Crossref
52. Han F, Zhao C, Huang SM, et al. Long-term outcomes and
prognostic factors of re-irradiation for locally recurrent
nasopharyngeal carcinoma using intensity-modulated
radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:569-76. Crossref
53. Qiu S, Lu J, Zheng W, et al. Advantages of intensity
modulated radiotherapy in recurrent T1-2 nasopharyngeal
carcinoma: a retrospective study. BMC Cancer 2014;14:797. Crossref
54. Xiao W, Liu S, Tian Y, et al. Prognostic significance of tumor
volume in locally recurrent nasopharyngeal carcinoma
treated with salvage intensity-modulated radiotherapy.
PLoS One 2015;10:e0125351. Crossref
55. Tian YM, Guan Y, Xiao WW, et al. Long-term survival and
late complications in intensity-modulated radiotherapy
of locally recurrent T1 to T2 nasopharyngeal carcinoma.
Head Neck 2016;38:225-31. Crossref
56. Kong L, Wang L, Shen C, Hu C, Wang L, Lu JJ. Salvage
intensity-modulated radiation therapy (IMRT) for locally
recurrent nasopharyngeal cancer after definitive IMRT: a
novel scenario of the modern era. Sci Rep 2016;6:32883. Crossref
57. Kong L, Hu J, Guan X, Gao J, Lu R, Lu JJ. Phase I/II trial
evaluating carbon ion radiotherapy for salvaging treatment
of locally recurrent nasopharyngeal carcinoma. J Cancer
2016;7:774-83. Crossref
58. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and
radiotherapy in nasopharyngeal carcinoma: an update of
the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. Crossref
59. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy
plus concurrent chemoradiotherapy versus concurrent
chemoradiotherapy alone in locoregionally advanced
nasopharyngeal carcinoma: a phase 3, multicentre,
randomised controlled trial. Lancet Oncol 2016;17:1509-20. Crossref
60. Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy
followed by concurrent chemoradiotherapy versus
concurrent chemoradiotherapy alone in locoregionally
advanced nasopharyngeal carcinoma: a phase III
multicentre randomised controlled trial. Eur J Cancer
2017;75:14-23. Crossref
61. Chen L, Hu CS, Chen XZ, et al. Adjuvant chemotherapy
in patients with locoregionally advanced nasopharyngeal
carcinoma: Long-term results of a phase 3 multicentre
randomised controlled trial. Eur J Cancer 2017;75:150-8. Crossref
62. Chan AT, Ngan RK, Hui EP, et al. A multicenter
randomized controlled trial of adjuvant chemotherapy in
nasopharyngeal carcinoma with residual plasma EBV DNA
following primary radiotherapy or chemoradiotherapy. J
Clin Oncol 2012;30(Suppl):abstract 5511.
63. Hui EP, Ma BB, Chan KC, et al. Clinical utility of plasma
Epstein-Barr virus DNA and ERCC1 single nucleotide
polymorphism in nasopharyngeal carcinoma. Cancer
2015;121:2720-9. Crossref
64. Lee AW, Tung SY, Chua DT, et al. Randomized trial of
radiotherapy plus concurrent-adjuvant chemotherapy vs
radiotherapy alone for regionally advanced nasopharyngeal
carcinoma. J Natl Cancer Inst 2010;102:1188-98. Crossref
65. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: a multicentre, randomised,
open-label, phase 3 trial. Lancet 2016;388:1883-92. Crossref
66. Chan JY. Surgical salvage of recurrent nasopharyngeal
carcinoma. Curr Oncol Rep 2015;17:433. Crossref
67. Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant
modified vaccinia ankara encoding Epstein-Barr viral
tumor antigens in nasopharyngeal carcinoma patients.
Cancer Res 2013;73:1676-88. Crossref
68. Khanna R, Burrows SR. Role of cytotoxic T lymphocytes
in Epstein-Barr virus-associated diseases. Annu Rev
Microbiol 2000;54:19-48. Crossref
69. Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer
and chemotherapy in the first-line treatment of metastatic
and/or locally recurrent nasopharyngeal carcinoma. Mol
Ther 2014;22:132-9. Crossref
70. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The
future of cancer treatment: immunomodulation, CARs
and combination immunotherapy. Nat Rev Clin Oncol
2016;13:273-90. Crossref
71. Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer:
past, present, and future. J Clin Invest 2015;125:3384-91. Crossref
72. Chen DS, Mellman I. Oncology meets immunology: the
cancer-immunity cycle. Immunity 2013;39:1-10. Crossref
73. Reck M, Rodríguez-Abreu D, Robinson A, et al.
Pembrolizumab versus chemotherapy for PD-L1–positive
non–small-cell lung cancer. N Engl J Med 2016;375:1823-33. Crossref
74. Hsu C, Lee SH, Ejadi S, et al. Antitumor activity and
safety of pembrolizumab in patients with PD-L1-positive
nasopharyngeal carcinoma: Interim results from a phase
1b study. Eur J Cancer 2015;51 Suppl 3:S558. Crossref
75. Ma BB, Goh BC, Lim WT, et al. Multicenter phase II
study of nivolumab in previously treated patients with
recurrent and metastatic non-keratinizing nasopharyngeal
carcinoma [abstract]. Proceedings of the 107th Annual
Meeting of the American Association for Cancer
Research; 2017 Apr 1-5; Washington, DC: Philadelphia;
2017. Available from: http://www.abstractsonline.com/pp8/#!/4292/presentation/12342. Accessed Aug 2017.
76. Delord JP, Hollebecque A, De Boer JP, et al. An
open-label, multicohort, phase I/II study to evaluate
nivolumab in patients with virus-associated tumors
(CheckMate 358): Efficacy and safety in recurrent or
metastatic nasopharyngeal carcinoma. J Clin Oncol
2017;35(Suppl):abstract 6025.