Hong Kong Med J 2016 Dec;22(6):600–7 | Epub 31 Oct 2016
DOI: 10.12809/hkmj164969
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
A review of the clinical approach to persistent
pain following total hip replacement
YF Lam, MB, ChB, MRCS (HKIBSC)1;
PK Chan, FHKCOS, FHKAM (Orthopaedic Surgery)2;
Henry Fu, FHKCOS, FHKAM (Orthopaedic Surgery)2;
CH Yan, FHKCOS, FHKAM (Orthopaedic Surgery)2;
KY Chiu, FHKCOS, FHKAM (Orthopaedic Surgery)2
1 Department of Orthopaedics and Traumatology, Princess Margaret
Hospital, Laichikok, Hong Kong
2 Department of Orthopaedics and Traumatology, Queen Mary Hospital,
Pokfulam, Hong Kong
Corresponding authors: Dr YF Lam (pmjraphael@gmail.com), Dr PK Chan (cpk464@yahoo.com)
Abstract
Total hip arthroplasty is effective in reducing pain
and improving functional outcome for a variety of hip
pathologies. Approximately 27% patients, however,
complain of pain at 6 months’ follow-up following
surgery. The pain may worsen over time and can
become severe and chronic in around 4% of patients
who ultimately require revision surgery. Therefore, it
is important for clinicians to comprehensively assess
patients undergoing total hip arthroplasty in order
to identify the underlying pathology of a painful hip
and then offer prompt treatment. Causes of hip pain
after total hip arthroplasty are analysed in this article,
as well as the systematic approach to evaluation and
appropriate diagnostic investigations.
Introduction
Total hip arthroplasty (THA) is an effective means of
relieving pain and improving functional outcome1 2 3
in a variety of hip pathologies. In Hong Kong, the
most common reasons for THA are osteonecrosis,
osteoarthritis, and post-traumatic arthritis of the
hip.4 Although surgical techniques and implant
quality of THA have evolved over the last two
decades, approximately 27% patients complain of
pain at the first 6-month follow-up after THA.5 6
The pain may worsen with time: up to 4% of patients
develop severe chronic pain and require revision
surgery.5 Therefore, it is important for clinicians to
comprehensively assess patients undergoing THA to
determine the pathology of a painful hip and offer
prompt treatment.
In this article, we analyse the causes of hip pain
following THA, the systematic approach to evaluation
and the appropriate diagnostic investigations.
Several patients with similar complaints of painful
hip but different pathologies will be presented.
Causes
Traditionally, the causes of hip pain following THA
are classified as intrinsic or extrinsic. Intrinsic causes
include pathologies arising from the hip region, and
can be further classified as intra-capsular or extra-capsular.
Intra-capsular causes relate to components
of the implant and include infection, loosening,
instability, and implant failure. Extra-capsular
causes include pathologies from the surrounding
soft tissue such as iliopsoas tendon and trochanteric
bursa, as well as heterotrophic ossification. Extrinsic
causes include pathologies arising outside the hip
region. A very common example is lumbar spine
pathology such as lumbar stenosis, disc herniation,
or spondylosis. Common intrinsic and extrinsic
causes are summarised in the Table.
History
A comprehensive history can undoubtedly provide
most of the important clues to diagnosis. Pain
should be explored from different aspects including
temporal onset, its nature, location, exacerbating
factors, and severity. Whether the patient has a pain-free
period following THA is important. If there has
been an initial pain-free period followed by later onset
of hip pain, events that occurred before the onset of
pain should be carefully explored. For example, if
the patient has recently fallen or sustained a trauma
to the hip then pain may be due to a periprosthetic
fracture. A recent dental procedure or infection
elsewhere could lead to haematogenous spread of
bacteria and subsequent prosthetic joint infection.
Other causes of pain that appears after a pain-free
period include aseptic loosening, instability,
osteolysis, and soft tissue irritation. The nature of
pain when it is persistent should then be clarified.
If it is similar to that which was present before
surgery, then the initial indications for THA should
be reviewed. If they were misdiagnosed, then there
may be untreated hip pathology. Persistent infection
of the joint could also be a cause.
Different types of pain can indicate different
pathologies. Mechanical pain may reflect aseptic
loosening, stress fracture, or instability of implants.
Constant, nocturnal, and rest pain may be a sign of
infection or, rarely, malignancy. Burning pain at the
right hip, associated with numbness or radiation
from the back could be referred pain of lumbar
spine pathology. Sharp pain occurs following
periprosthetic fracture or soft tissue irritation. Deep
and dull pain may indicate intrinsic causes such as
infection or osteolysis.
Exacerbating factors should be sought
when assessing hip pain. Pain that increases with
initiation of movement or during weight bearing,
and is relieved by rest could indicate loosening
of components. Pain that begins after a certain
level of exacerbation or activity suggests vascular
or neurogenic claudication. Pain aggravated by
climbing stairs or rising from a seated position may
be due to iliopsoas tendinitis.
The location of pain may provide a clue as to the
location of the pathology or defective components.
Groin pain may indicate a failing acetabulum
component. Other intrinsic causes of groin pain
include iliopsoas impingement or tendinitis.
Extrinsic causes include local neurological or
vascular pathology, inguinal hernia, spinal pathology
or radiculopathy or, rarely, malignancy. Thigh pain
may suggest involvement of the femoral component
and relate to stem loosening, subsidence and
instability, modulus mismatch, or impingement on
bone cortex. Nerve injury, for example to the lateral
femoral cutaneous nerve, may present as thigh pain.
Buttock and leg pain could be secondary to spinal
stenosis or radiculopathy.
Perioperative details such as the model and
size of implant used, urinary catheterisation, wound,
and other systemic infections during the recovery
period could be important.
In addition to details about the pain and
surgical history, a routine general medical history
should not be ignored. In patients with a history
of immunosuppression, inflammatory arthritis,
obesity or diabetes, there may be a higher rate
of prosthetic joint infection. Patients who are
depressed or overemotional may be more prone to
chronic pain or complex regional pain syndrome.
Those prescribed long-term immunosuppressants,
biologics, or steroids are at high risk of infection and
hence adjustment of these drugs before operation is
necessary.
Physical examination
A complete physical examination of the hip should
include the painful as well as the contralateral
side, the spine and knees as well as a neurological
examination of the lower limbs—all critical to making
the right diagnosis. The conventional approach
to hip examination is to ‘look, feel, and move’. For
inspection, we examine the surgical incision that
will indicate the approach of the previous THA and
quality of postoperative wound healing. Hyperplasia
of the surgical scar may indicate previous wound
infection. Signs of infection such as erythema, pain,
swelling, and increased warmth should be noted
if present. The presence of sinus tracts indeed
is pathognomonic for prosthetic joint infection.
Muscle wasting may be due to deconditioning or
nerve injury. Gait analysis is also important as it may
reflect abductor insufficiency if the patient walks
with a Trendelenburg gait. Short limb gait may
indicate leg length discrepancy. When assessing leg
length, patients should be asked if they have noted
any progressive change in leg length discrepancy,
as it may suggest subsidence of the femoral stem.
For palpation, sites of local tenderness should be
sought as these may pinpoint the exact location of
hip pathology. Any swelling at the groin must be
carefully examined. Characteristics such as nature,
margin, tenderness, fluctuance, compressibility,
emptiability, pulsatility, and positive Tinel’s signs
should be noted. A reducible mass in the groin with
or without cough impulse could be an inguinal,
femoral, or obturator hernia. A pulsatile mass
could be a true or pseudo-aneurysm. A vague,
deeply seated tender swelling could be an ‘aseptic
lymphocyte-dominated vasculitis-associated lesion’
if a metal-on-metal bearing or a modular metal-on-metal head-neck articulation has been used. During
assessment of patient movement, the range and
any tenderness triggered by a specific movement
or manoeuvre should be noted. Reproducible pain
upon extreme range of movement may indicate
instability or impingement by implants. Pain elicited
during active movement may indicate instability or
loosening while that which appears during passive
movement could be due to infection. Any pain
triggered by active or resisted hip flexion may be
due to acetabulum component loosening, iliopsoas
tendinitis, or impingement.
Laboratory tests
Serological, microbiological, and cytological
investigations are important in the assessment
of patients with painful THA. Common serum
inflammatory markers that could indicate prosthetic
joint infection are white blood cell count (WBC),
erythrocyte sedimentation rate (ESR), and C-reactive
protein (CRP). Spangehl et al7 reported
the sensitivity and specificity of WBC of >11.0 x
109 /L as 0.2 and 0.96, that of ESR as 0.82 and 0.85,
and that of CRP as 0.96 and 0.92, respectively. It
has been suggested that interpretation of ESR and
CRP together improves sensitivity and specificity.
In the presence of abnormal serum parameters and/or clinical suspicion of infected THA, aspiration
should be performed to obtain synovial fluid for
microbiological and cytological examination.8 Gram
smear and bacterial culture are routinely requested,
while fungal and tuberculosis culture are ordered
selectively if indicated. Nonetheless, the sensitivity of
Gram staining is low, ranging from 10% to 67%.9 10 Hip aspirate culture is reported to have a variable
sensitivity of 50% to 86%.11 12 13 To increase the yield
of bacterial culture, all aspirated specimens should
be processed immediately.14 Font-Vizcarra et al15 advocated transport of aspirated synovial fluid in a
blood culture bottle to achieve higher sensitivity and
specificity. Cell count with neutrophil differential
also provides an important clue for prosthetic joint
infection. Different cut-off values of cell count and
neutrophil percentage have been suggested. An
international consensus on periprosthetic joint
infection in 2013 proposed 3000 cells/µL and
neutrophil differential of >80% as being indicative
of active infection.16 Parvizi et al17 suggested use of leukocyte esterase reagent strips as a rapid,
inexpensive, highly sensitive and specific test to
detect periprosthetic joint infection.
Radiological investigations
Plain radiographs are always the first-line
investigation for a painful hip following THA.
The anteroposterior view of the pelvis, and the
anteroposterior and lateral views of the affected hip,
including the tip of the stem area, are standard. These
often provide clues about pre-existing hip disease,
fixation method of the prosthesis, and design features
of the prosthesis and articulations—all of which are
important in determining the cause of hip pain.
For example, cementless femoral stems, especially
extensively porous-coated long stems, could cause
mid-thigh pain due to modulus mismatch and stress
shielding. Osteolysis is not uncommonly present in
metal-on-polyethylene articulation. Details of the
procedure should also be evaluated. The abduction
angle, horizontal and vertical positions, and version
for the socket, as well as the coronal alignment,
grades of cement mantle for cemented stem and canal
filling for cementless stem are important and should
be reviewed. Malalignment of the socket and/or
stem can result in instability and increase the risk of
early loosening, polyethylene wear, and dislocation.
Quality of the cement mantle can be assessed by the
Barrack classification that grades according to the
percentage of radiolucency present in the medullary
canal.18 A poor grade of cementation may lead to
loosening and early failure of the implant. To assess
loosening of a cemented femoral stem, Harris criteria
described three categories: definite, probable and
possible, depending on the size of radiolucent zone at
the cement-bone interface, subsidence, and presence
of fractured cement mantle or stem.19 DeLee and
Charnley20 divided the cement-bone area around
the cemented socket into three types—radiolucent
lines at the lateral one third as type I, involvement
at the middle one third as type II, and complete
involvement of the cement-bone interface as type
III. If one zone is involved, the rate of loosening
of the cup is 7%. The risk significantly increases
to 71% and 94% in type II and III, respectively.21
For cementless stems, as described by Engh’s
classification,22 presence of spot welding and parallel
demarcation lines indicates stable bone ingrowth
and fibrous fixation, respectively. Subsidence, calcar
hypertrophy, and pedestal at the tip of the stem are
signs of unstable stem fixation. For cementless cups,
signs of loosening include change in abduction angle
of >8°, migration of ≥3 mm, implant failure, halo
around screws, and shredding of porous coating.23
Endosteal scalloping and periosteal reaction are
classic signs of infection. Presence of osteolysis on
plain radiographs may indicate particle disease.
Computed tomography (CT) can be useful
in evaluating the complications of THA, provided
proper parameter modifications are adopted to
reduce artefact from the prosthesis.24 25 Accurate measurement of the acetabulum cup version can be
achieved with CT because of the ability to measure
in multiple orthogonal planes.26 27 28 29 Other potential
uses of CT include preoperative assessment of
bone loss for acetabulum and femur, evaluation of
bone density for stress shielding, and detection
of osteolysis, liner wear, and metallosis. Magnetic
resonance imaging (MRI) is excellent for evaluation
of the periprosthetic soft tissue and hence detection
of THA complications. Nonetheless, its use, as with
CT, is limited by the occurrence of artefact from
the prosthesis. To improve the diagnostic value of
MRI in the evaluation of THA complications, metal
artefact reduction sequence (MARS)–MRI has
been developed and achieves better visualisation
of the periprosthetic soft tissue structure that
is obscured by signal void in conventional MRI
sequences.30 31 The imaging, MARS-MRI, has a high sensitivity to detect particle diseases that can result
in proliferative synovitis, pseudotumours, loosening,
and osteolysis.32 33 34 Involvement of superficial and
deep soft tissue surrounding the prosthesis can also
be assessed by MRI.
A nuclear medicine scan such as technetium-99
is often advocated when there is no obvious diagnosis
despite extensive investigations. It has a high
sensitivity to detect a wide variety of complications
including infection, loosening, instability, and stress
fractures. Nonetheless, the specificity is rather
low and increased uptake can occur for 2 years in
uncomplicated THA.35 If a technetium-99 scan is
positive, indium-111 white cell scan may be used to
differentiate between an infective or non-infective
pathology.36
Local anaesthetic test
To differentiate between the intrinsic or extrinsic
source of pain, a local anaesthetic agent such as
marcaine 0.5% can be injected with an 18-Gauge
spinal needle under fluoroscopic guidance to the
tender spots. Immediate pain relief following
injection will confirm the exact site of pathology.
Crawford et al37 reported sensitivity of up to 96% for
this technique that offered a rapid, reliable diagnostic
test with low morbidity.
Illustrative cases
Case 1
A 66-year-old woman prescribed a long-term
steroid for systemic lupus erythematous underwent
Austin-Moore arthroplasty in 1978 for avascular
necrosis of bilateral femoral heads. She underwent
multiple revision surgeries on both hips due to
infective loosening. The latest operation in 2011
was revision of the loosened right acetabulum cup
due to infection. The femoral stem was retained at
that time as it was well fixed. She enjoyed a pain-free
period and could walk with a stick. Serial
radiographs showed no loosening of components.
She complained of right hip pain during follow-up
in 2014, however, and radiographs of the right hip
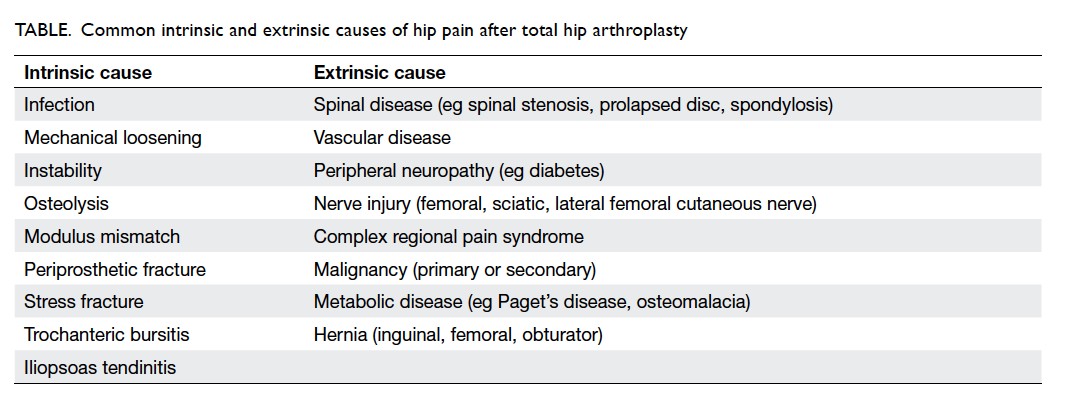
showed endosteal scalloping over the THA (Fig 1a, 1b). Blood tests revealed an elevated ESR and CRP.
Hip aspiration was performed and 2 mL of turbid
synovial fluid was aspirated. Bacterial culture was
negative but cell count was 33 400 cells/µL. The
provisional diagnosis was an infected right THA and
a two-stage revision was proposed. While waiting for
revision, she was admitted for worsening right hip
pain for 2 weeks. Radiographs showed a radiolucent
line across all Gruen zones and lucent lines were
present at zones I and II around the acetabulum cup.
Periosteal reaction and endosteal scalloping were
also noted. Serum inflammatory markers were all
elevated. Extended trochanteric osteotomy, removal
of implant, and placement of antibiotic-loaded
cement spacer was performed (Fig 1c). Multiple specimens were taken for culture. Erysipelothrix
rhusiopathiae was cultured from the anterior capsule
granulation tissue. Postoperatively she was given
intravenous ampicillin for 4 weeks and switched to
oral ampicillin for a further 8 weeks. Levels of ESR
and CRP returned to normal. Repeated right hip
aspiration, after antibiotics had been stopped for 2
weeks, were negative on bacterial culture. Cell count
was 325 cells/µL with neutrophils of 27%. Second-stage
revision with cementless acetabulum cup and
extensive porous-coated long stem prosthesis was
performed and was uneventful (Fig 1d). After 3 months, she had no hip pain and could walk with a
stick for more than 30 minutes. Radiographs showed
no interval change in alignment nor loosening.
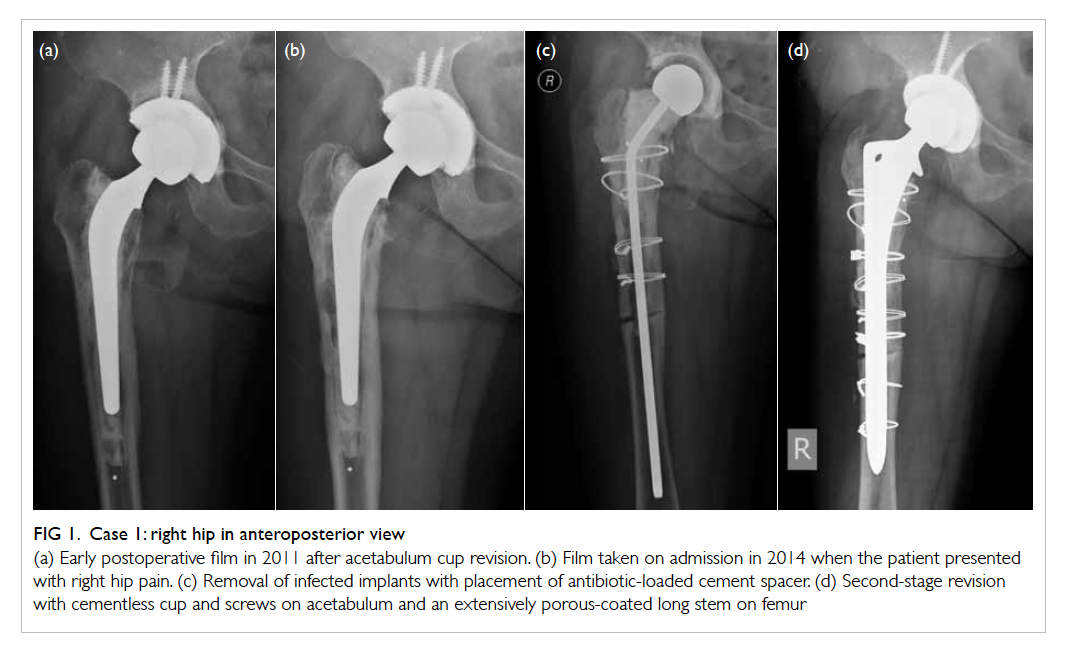
Figure 1. Case 1: right hip in anteroposterior view
(a) Early postoperative film in 2011 after acetabulum cup revision. (b) Film taken on admission in 2014 when the patient presented with right hip pain. (c) Removal of infected implants with placement of antibiotic-loaded cement spacer. (d) Second-stage revision with cementless cup and screws on acetabulum and an extensively porous-coated long stem on femur
Case 2
A 65-year-old woman had a medical history of
tuberculosis of the right hip with auto-fusion,
followed by conversion to THA in 1995. She
underwent acetabulum cup revision in 2004 due to
aseptic loosening. The procedure was uneventful and
she was asymptomatic afterwards. Twelve years later
she complained of right hip pain for 2 weeks with no
history of trauma. She had been febrile for several
days with chills and rigor. She denied any respiratory,
abdominal, or urinary symptoms. She walked with a
limping gait after onset of pain. Examination upon
admission revealed a high fever with stable vital
signs. Palpation of her right groin revealed a vague,
tender swelling that was neither compressible,
reducible, nor pulsatile. Active and passive range of
movement of the right hip was significantly limited by
pain. Pain was aggravated by internal rotation of the
affected hip. Neurovascular status appeared intact.
Radiographs of the right hip showed no loosening
or migration of THA components. No periosteal
reaction or endosteal scalloping was noted. Serum
WBC, ESR, and CRP were all elevated (WBC, 14 x 109 /L; ESR, 104 mm/h; CRP, 9.26 mg/L). In view
of her febrile state and tender groin swelling, CT
right hip with contrast was arranged. No abnormal
increase in periprosthetic hypodensities was noted
and loosening was unlikely but a rim-enhancing
lesion of 3.3 x 6.8 x 11 cm in size at the right iliopsoas
was noted and psoas abscess was diagnosed. Then
CT-guided drainage was performed by radiologists
and 30 mL of blood-stained purulent fluid was
aspirated. The aspirate was sent immediately for
bacterial culture and revealed Parabacteroides
merdae sensitive to rifampicin. She was treated
with antibiotics according to the sensitivity
tests. Colonoscopy was arranged as the cultured
bacteria is usually of gastrointestinal origin. After
aspiration of the psoas abscess and administration
of antibiotics, she improved clinically. Hip pain
resolved, fever subsided, and she was able to walk
unaided without pain. Blood tests showed reducing
ESR and CRP. Serial CT abdomen and pelvis showed
regression of psoas abscess (Fig 2). Despite her clinical improvement and reassuring radiological
and serological tests, it remained uncertain whether
the right THA was infected. Hip aspiration posed
a risk of introducing the bacteria into the hip joint
because of the close proximity of the psoas abscess,
causing ‘iatrogenic’ prosthetic joint infection. She is
being closely monitored and surgical drainage can be
offered if she deteriorates in future.
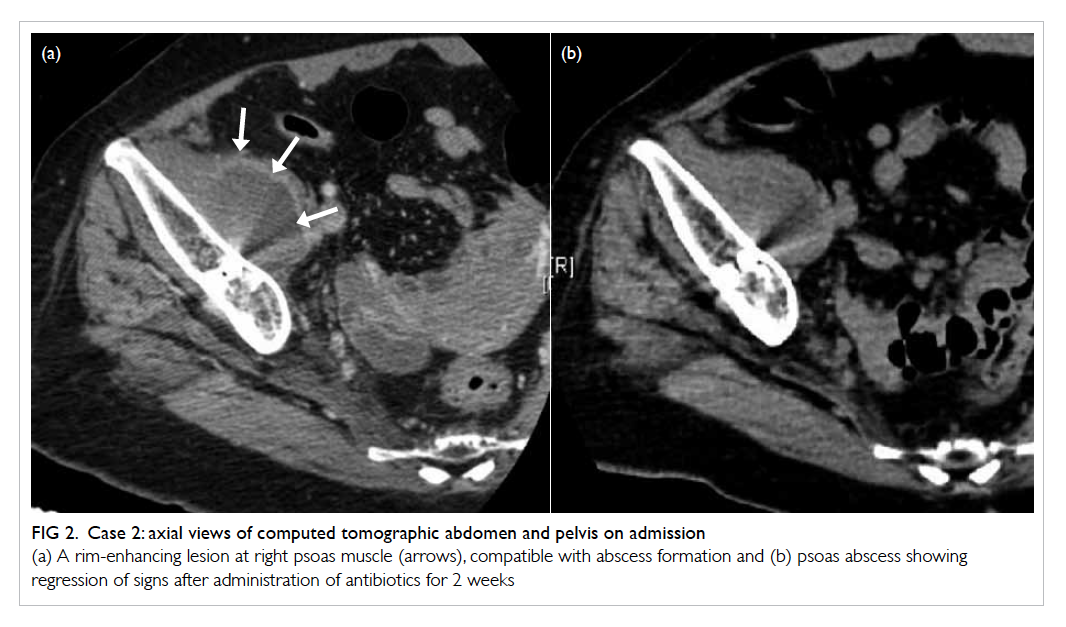
Figure 2. Case 2: axial views of computed tomographic abdomen and pelvis on admission
(a) A rim-enhancing lesion at right psoas muscle (arrows), compatible with abscess formation and (b) psoas abscess showing regression of signs after administration of antibiotics for 2 weeks
Case 3
A 75-year-old woman underwent dynamic hip
screw for fixation in 1991 due to intertrochanteric
fracture of the right proximal femur. In 1992 she
underwent cementless THA for the cut-through
dynamic hip screw. Unfortunately during follow-up
she was noted to have subsidence of the femoral stem
with impingement at the lateral cortex. Infection
was excluded and revision THA was offered but
refused by the patient who could walk with a stick
for 30 minutes and had no hip pain. In 2010, she was
diagnosed with carcinoma of the transverse colon
and right hemicolectomy was performed. Intra-operative
specimens showed clear margins and there
was no evidence of local or distant metastasis. She
defaulted from surgical follow-up, however. In 2014,
22 years after the THA, she complained of insidious
onset of right groin and thigh pain for several months.
She experienced nocturnal pain at the right hip and
an intermittent low-grade fever. Unexplained weight
loss over 1 month was noted. She could only walk
with a stick for 5 minutes since the onset of thigh
pain. Examination showed shortening of the right
lower limb by 2 cm and tenderness at the right femur
shaft. Serum WBC was slightly elevated (12.5 x 109 /L),
and both ESR and CRP were markedly increased
(ESR, 111 mm/h; CRP, 13.3 mg/L). Serum tumour
marker levels were normal and carcinoembryonic
antigen level was static. Radiographs showed
extensive osteolytic lesions at the anterior and
posterior aspects of the acetabulum cup. Migration
of the cup position was noted (Fig 3). In view of the history of malignancy of the transverse colon and
abnormal radiographs of the right hip, CT pelvis and
right hip with contrast was performed and revealed
a large soft tissue mass in the right pelvis with
extensive bony erosion of the acetabulum, ilium,
ischium, and superior pubic ramus with loosening
of the implant (Fig 4). The diagnosis was bone metastasis to the pelvis with erosion. Exploration
was performed and a large friable soft tissue mass
with extensive destruction of the acetabulum was
noted. Intra-operative specimens were revealed on
frozen section to be metastatic adenocarcinoma. In
view of the massive bone loss over the acetabular
side, her advanced age and underlying medical
condition, excision arthroplasty was performed in
the same operation. Further histopathological tests
of intra-operative specimens confirmed metastatic
adenocarcinoma that was likely of colorectal origin.
All other specimens for microbiological culture,
including tuberculosis culture, were negative.
She was referred to oncologists and underwent
radiotherapy for local control of disease. Her right
groin and thigh pain was much relieved after
operation. She tolerated sitting well and could
ambulate in a wheelchair. The patient was referred
to a hospice and finally succumbed 4 months later
due to a chest infection.
Figure 3. Case 3: (a) radiograph of right hip before onset of right groin and thigh pain. (b) Film repeated after admission for right hip pain showing extensive osteolytic lesion over the anterior and posterior column of acetabulum with migration of cup (arrows)
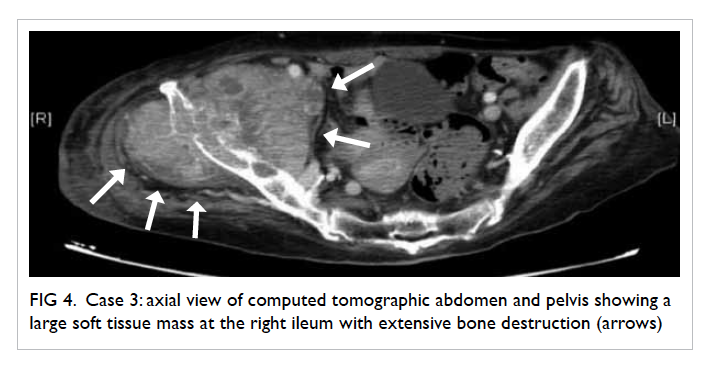
Figure 4. Case 3: axial view of computed tomographic abdomen and pelvis showing a large soft tissue mass at the right ileum with extensive bone destruction (arrows)
Case 4
A 50-year-old woman with osteoarthritis of the
left hip secondary to untreated developmental
hip dysplasia underwent total hip replacement
in the private sector in January 2012. She gained
satisfactory pain relief at her left hip until March
2012 when she presented with increased left hip
pain that was aggravated by active flexion, stair
walking, getting out of bed, and getting on public
transport. Examination showed her left lower limb
to be lengthened by 1 cm. No local tender spot at
the left hip was noted. Active range of flexion was
0° to 110°. Severe pain was noted during active
flexion of hip. Blood tests were all unremarkable.
Ultrasound-guided aspiration of the left hip showed
no growth. Computed tomography of the left
hip showed protrusion of the anterior rim of the
acetabular cup (Fig 5). After excluding infection, a working diagnosis of iliopsoas tendon impingement
due to severe pain triggered by active hip flexion
was proposed. Ultrasound-guided injection of local
anaesthetic to the left iliopsoas tendon insertion to
the lesser trochanter was performed to relieve the
pain although it returned 1 week later. Arthroscopic
release of the left iliopsoas tendon was performed
and was uneventful. Upon follow-up at 6 weeks after
operation, her left hip pain was much improved and
no pain was noted on walking upstairs or active
flexion of left hip.
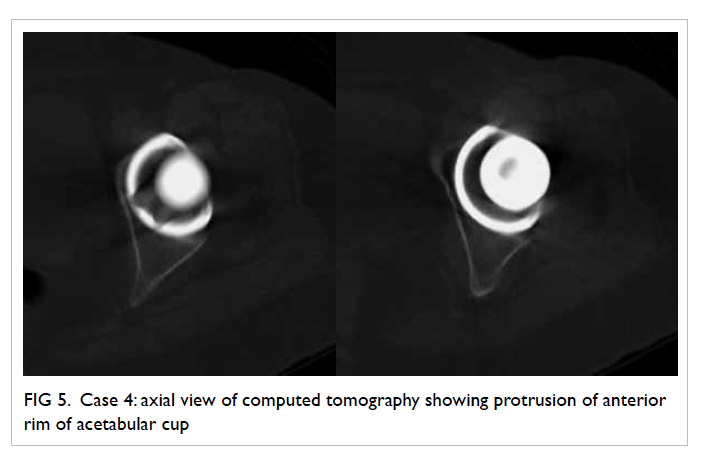
Figure 5. Case 4: axial view of computed tomography showing protrusion of anterior rim of acetabular cup
Conclusion
Any pain that appears after THA should not be
overlooked. Making an accurate diagnosis of the
pain requires a detailed history, thorough clinical
examination, and appropriate investigations. Large-scale
reviews in the literature report instability,
mechanical loosening, and infection as the three
main causes of implant failure necessitating revision
surgery38 39 and all should be considered during
evaluation. Patients who have undergone THA but
have postoperative hip pain should be reviewed by
the operating surgeon for further management after
an initial assessment. The ultimate goal is to unearth
the underlying cause and offer timely treatment,
hence preventing unnecessary revision surgery and
facilitating the patient’s return to normal activity.
Acknowledgement
We would like to thank Dr HC Cheng, Chief of
Service of the Department of Orthopaedics and
Traumatology, United Christian Hospital, Hong
Kong for providing an illustrative case of iliopsoas
tendon impingement after total hip replacement in
this article.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Visuri T, Koskenvuo M, Honkanen R. The influence of total
hip replacement on hip pain and the use of analgesics. Pain
1985;23:19-26. Crossref
2. Laupacis A, Bourne R, Rorabeck C, et al. The effect of
elective total hip replacement on health related quality of
life. J Bone Joint Surg Am 1993;75:1619-26.
3. Alberta Hip Improvement Project, MacKenzie JR,
O’Connor GJ, et al. Functional outcomes for 2 years
comparing hip resurfacing and total hip arthroplasty. J
Arthroplasty 2012;27:750-7.e2. Crossref
4. Chan VW, Chan PK, Chiu KY, Yan CH, Ng FY. Why do
Hong Kong patients need total hip arthroplasty? An
analysis of 512 hips from 1998 to 2010. Hong Kong Med J
2016;22:11-5. Crossref
5. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent
pain after joint replacement: prevalence, sensory qualities,
and postoperative determinants. Pain 2011;152:566-72. Crossref
6. Britton AR, Murray DW, Bulstrode CJ, McPherson K,
Denham RA. Pain levels after total hip replacement: their
use as endpoints for survival analysis. J Bone Joint Surg Br
1997;79:93-8. Crossref
7. Spangehl MJ, Masri BA, O’Connel JX, Duncan CP.
Prospective analysis of preoperative and intraoperative
investigations for the diagnosis of infection at the sites of
two hundred and two revision total hip arthroplasties. J
Bone Joint Surg Am 1999;81:672-83.
8. O’Neill DA, Harris WH. Failed total hip replacement:
assessment by plain radiographs, arthrograms, and
aspiration of the hip joint. J Bone Joint Surg Am
1984;66:540-6.
9. Barrack RL, Harris WH. The value of aspiration of the hip
joint before revision total hip arthroplasty. J Bone Joint
Surg Am 1993;75:66-76.
10. Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E,
Meurman O, Nelimarkka O. The reliability of diagnosis
of infection during revision arthroplasties. Scand J Surg
2002;91:178-81.
11. Fehring TK, Cohen B. Aspiration as a guide to sepsis in
revision total hip arthroplasty. J Arthroplasty 1996;11:543-7. Crossref
12. Ali F, Wilkinson JM, Cooper JR, et al. Accuracy of joint
aspiration for the preoperative diagnosis of infection in
total hip arthroplasty. J Arthroplasty 2006;21:221-6. Crossref
13. Lachiewicz PF, Rogers GD, Thomason HC. Aspiration of
the hip joint before revision total hip arthroplasty. Clinical
and laboratory factors influencing attainment of a positive
culture. J Bone Joint Surg Am 1996;78:749-54.
14. Wilson ML, Winn W. Laboratory diagnosis of bone, joint,
soft-tissue, and skin infections. Clin Infect Dis 2008;46:453-7. Crossref
15. Font-Vizcarra L, Garcia S, Martinez-Pastor JC, Sierra
JM, Soriano A. Blood culture flasks for culturing synovial
fluid in prosthetic joint infections. Clin Orthop Relat Res
2010;468:2238-43. Crossref
16. Parvizi J, Gehrke T, Chen AF. Proceedings of the
International Consensus on Periprosthetic Joint Infection.
Bone Joint J 2013;95-B:1450-2. Crossref
17. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of
periprosthetic joint infection: the utility of a simple yet
unappreciated enzyme. J Bone Joint Surg Am 2011;93:2242-8. Crossref
18. Barrack RL, Mulroy RD Jr, Harris WH. Improved cementing
techniques and femoral component loosening in young
patients with hip arthroplasty. A 12-year radiographic
review. J Bone Joint Surg Br 1992;74:385-9.
19. Harris WH, McCarthy JC Jr, O’Neill DA. Femoral
component loosening using contemporary techniques
of femoral cement fixation. J Bone Joint Surg Am
1982;64:1063-7.
20. DeLee JG, Charnley J. Radiological demarcation of
cemented sockets in total hip replacement. Clin Orthop
Relat Res 1976;(121):20-32. Crossref
21. Hodgkinson JP, Shelley P, Wroblewski BM. The correlation
between the roentgenographic appearance and operative
findings at the bone-cement junction of the socket in
Charnley low friction arthroplasties. Clin Orthop Relat Res
1988;(228):105-9. Crossref
22. Engh CA, Glassman AH, Suthers KE. The case for porous
coated hip implants. The femoral side. Clin Orthop Relat
Res 1990;(261):63-81.
23. Massin P, Schmidt L, Engh CA. Evaluation of cementless
acetabular component migration. An experimental study. J
Arthroplasty 1989;4:245-51. Crossref
24. Buckwalter KA, Parr JA, Choplin RH, Capello WN.
Multichannel CT imaging of orthopedic hardware and
implants. Semin Musculoskelet Radiol 2006;10:86-97. Crossref
25. Mahnken AH, Raupach R, Wildberger JE, et al. A new
algorithm for metal artifact reduction in computed
tomography: in vitro and in vivo evaluation after total hip
replacement. Invest Radiol 2003;38:769-75. Crossref
26. Marx A, von Knoch M, Pförtner J, Wiese M, Saxler
G. Misinterpretation of cup anteversion in total hip
arthroplasty using planar radiography. Arch Orthop
Trauma Surg 2006;126:487-92. Crossref
27. Kalteis T, Handel M, Herold T, Perlick L, Paetzel C, Grifka
J. Position of the acetabular cup—accuracy of radiographic
calculation compared to CT-based measurement. Eur J
Radiol 2006;58:294-300. Crossref
28. Blendea S, Eckman K, Jaramaz B, Levison TJ, Digioia
AM 3rd. Measurements of acetabular cup position and
pelvic spatial orientation after total hip arthroplasty using
computed tomography/radiography matching. Comput
Aided Surg 2005;10:37-43. Crossref
29. Wines AP, McNicol D. Computed tomography
measurement of the accuracy of component version in
total hip arthroplasty. J Arthroplasty 2006;21:696-701. Crossref
30. Toms AP, Smith-Bateman C, Malcolm PN, Cahir J, Graves
M. Optimization of metal artefact reduction (MAR)
sequences for MRI of total hip prostheses. Clin Radiol
2010;65:447-52. Crossref
31. Potter HG, Foo LF, Nestor BJ. What is the role of
magnetic resonance imaging in the evaluation of total hip
arthroplasty? HSS J 2005;1:89-93. Crossref
32. Chen Z, Pandit H, Taylor A, Gill H, Murray D, Ostlere
S. Metal-on-metal hip resurfacings—a radiological
perspective. Eur Radiol 2011;21:485-91. Crossref
33. Cooper HJ, Ranawat AS, Potter HG, Foo LF, Koob TW,
Ranawat CS. Early reactive synovitis and osteolysis after
total hip arthroplasty. Clin Orthop Relat Res 2010;468:3278-85. Crossref
34. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and
soft tissue mass around total hip arthroplasty: description
of four cases and review of the literature with respect to
clinico-radiographic and pathologic differential diagnosis.
Eur J Radiol 2011;77:43-50. Crossref
35. Utz JA, Lull RJ, Galvin EG. Asymptomatic total hip
prosthesis: natural history determined using Tc-99m MDP
bone scans. Radiology 1986;161:509-12. Crossref
36. Merkel KD, Brown ML, Dewanjee MK, Fitzgerald RH Jr.
Comparison of indium-labeled-leukocyte imaging with
sequential technetium-gallium scanning in the diagnosis
of low-grade musculoskeletal sepsis. A prospective study.
J Bone Joint Surg Am 1985;67:465-76.
37. Crawford RW, Gie GA, Ling RS, Murray DW. Diagnostic
value of intra-articular anaesthetic in primary osteoarthritis
of the hip. J Bone Joint Surg Br 1998;80:279-81. Crossref
38. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The
epidemiology of revision total hip arthroplasty in the
United States. J Bone Joint Surg Am 2009;91:128-33. Crossref
39. Ulrich SD, Seyler TM, Bennett D, et al. Total hip
arthroplasties: what are the reasons for revision? Int
Orthop 2008;32:597-604. Crossref