Medical manslaughter in Hong Kong—how, why, and why not
Hong
Kong Med J 2018 Aug;24(4):384–90 | Epub 27 Jul 2018
DOI: 10.12809/hkmj187346
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Medical manslaughter in Hong Kong—how, why, and why not
Gilberto KK Leung, LLM, FHKAM (Surgery)
Department of Surgery, The University of Hong Kong,
Pokfulam, Hong Kong
Corresponding author: Prof Gilberto KK Leung (gilberto@hku.hk)
Abstract
The increasing number of medical
manslaughter cases in recent years raises concerns about the concept of
criminal liability in medical negligence. Contemporary cases in Hong
Kong have also generated debate on whether criminal law intervention is
justified and effective at dealing with substandard medical practices.
This paper examines the legal principles underlying the applicable legal
offence of gross negligence manslaughter and the implications that
recent events may have on patient care and the medical profession. The
author argues that the criminalisation of medical mistakes can have a
detrimental effect on clinical practice and patient welfare. At stake is
the potential for a loss of mutual trust between the medical profession
and the rest of society. Gross negligence manslaughter is an unstable
legal concept, and criminal sanctions should at most be applied to
conscious violations of established rules and standards but not
unintentional errors. As we await the outcomes of ongoing cases in Hong
Kong, there is an urgent need to uphold standards of practice and to
nurture a robust culture of ethical awareness, compassionate care, and
professionalism.
Introduction
Medical manslaughter is involuntary manslaughter by
gross negligence where patient death has resulted from a grossly negligent
(but otherwise lawful) act or omission.1
Although related prosecutions remain uncommon, the emergence of recent
cases in Hong Kong presents an opportunity to reflect upon whether
criminal sanctions are justifiable and effective at dealing with
substandard medical practices. This paper begins with an examination of
the underlying legal principles, followed by a discussion on how the
current direction of travel might impact patient care and the medical
profession.
Gross negligence manslaughter
The applicable legal offence in medical
manslaughter is that of gross negligence manslaughter (GNM).1 As for civil claims in medical negligence, the legal
test to satisfy is that the affected party is owed a duty of care and that
a breach of that duty has occurred and caused the injury at issue.2 In addition, it must be further established in GNM that
the degree of negligence has gone:
“…beyond a mere matter of compensation between
subjects and has showed such disregard for the life and safety of others
as to amount to a crime against the state and conduct deserving
punishment”.3
The legal test was affirmed in the landmark case of
Adomako, in which an anaesthesiologist failed to respond to a
disconnection of the oxygen supply during general anaesthesia, whereby the
jury had to decide whether “the degree of negligence is so great that
a criminal penalty is warranted”.4
It is ultimately about the transformation of a private wrong into a public
one.
Contemporary cases
Medical manslaughter cases have historically been
rare, but a rising trend has been observed in recent years. In the UK, 85
doctors were charged between 1795 and 2005,5
while 11 cases have already materialised between 2006 and 2013.6 A similar situation has occurred in the United States,
where over 50 prosecutions have been brought since 1990.7 The majority of UK cases involved obstetrics and errors
in the prescription or administration of drugs.5
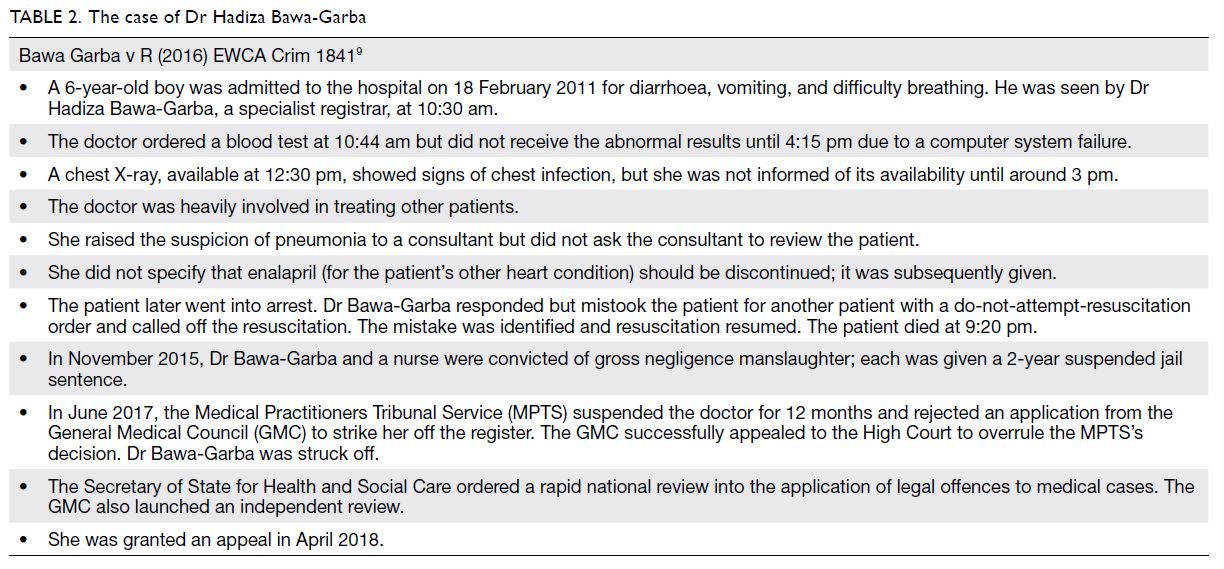
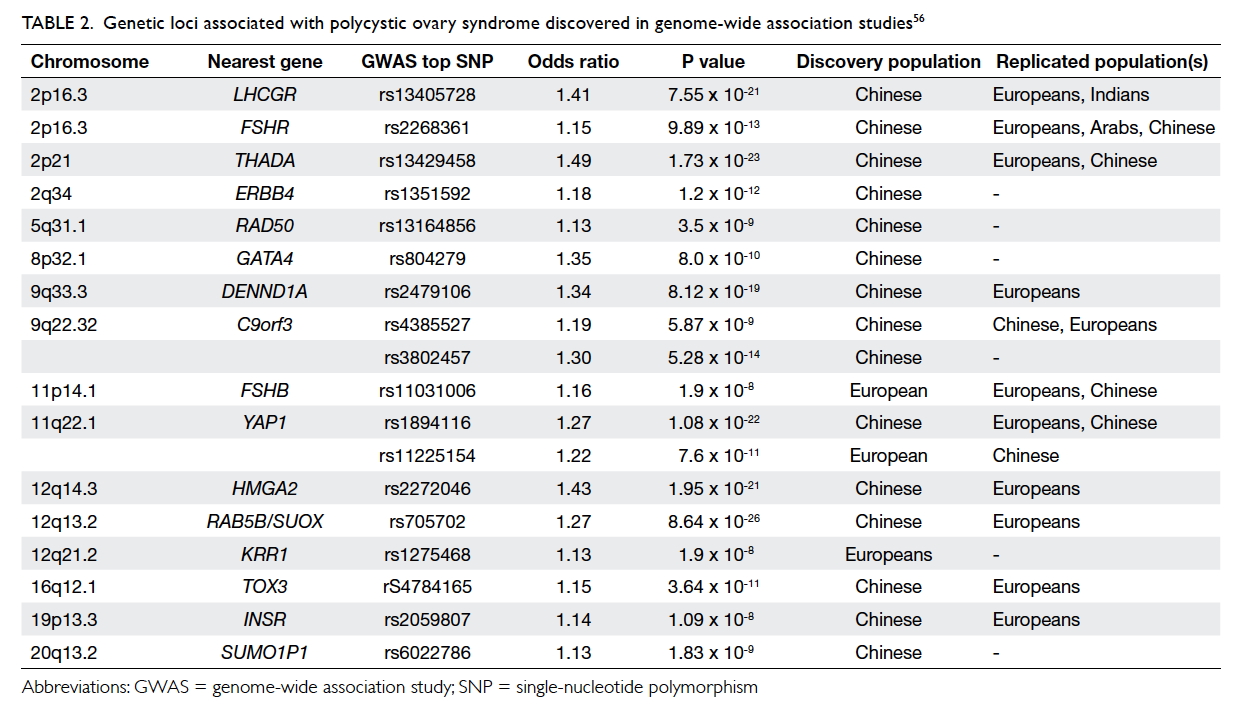
Tables 1 and 2 outline the two recent and controversial cases of
Mr David Sellu8 and Dr Hadiza
Bawa-Garba,9 respectively.
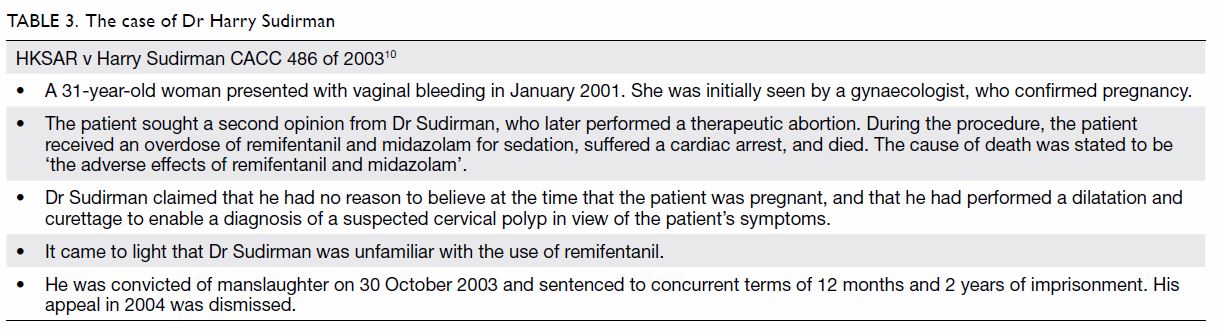
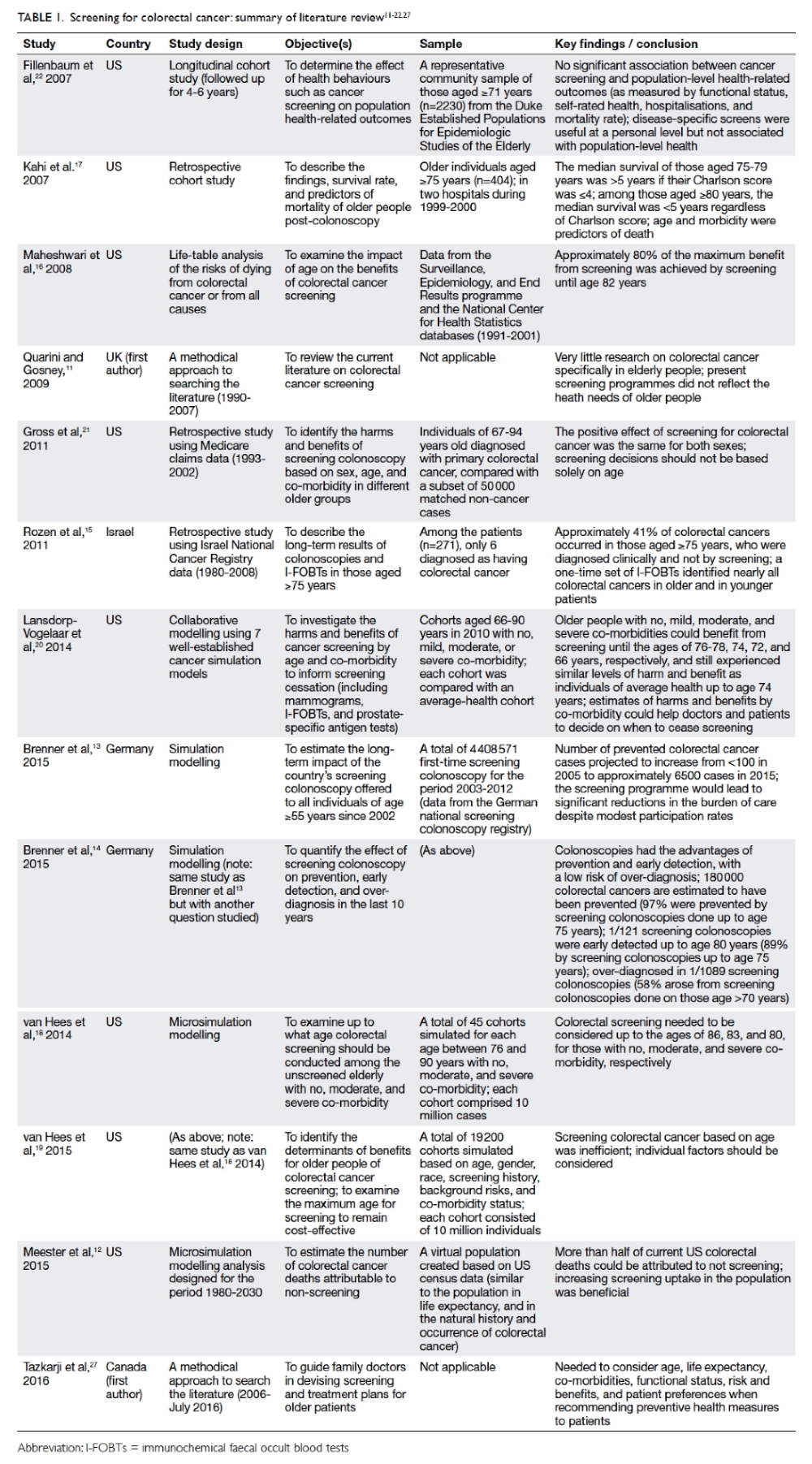
The first case in Hong Kong involved the misuse of
sedative drugs during an illegal abortion for which the doctor was jailed
for 2 years in 2003 (Table 3).10
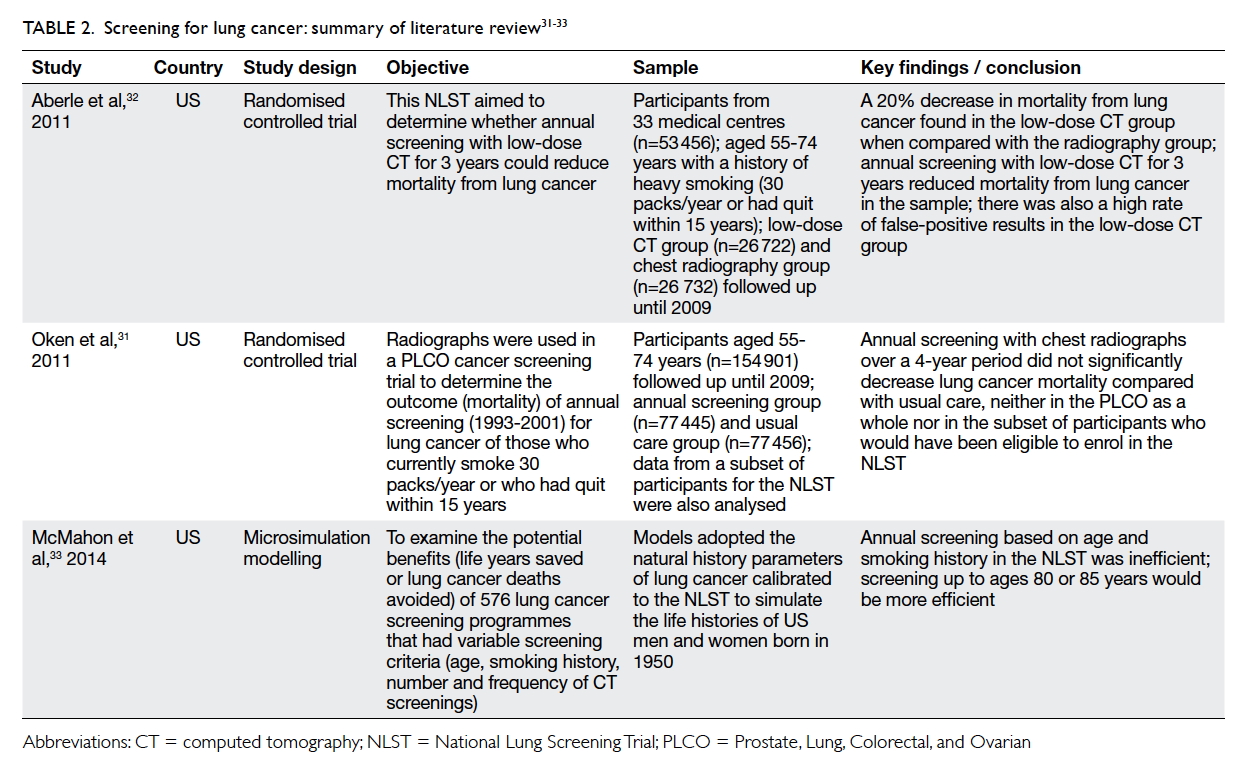
A hiatus followed. The highly publicised “DR Group” case saw the
conviction of a beauty clinic’s owner and technician in 2017; the third
defendant, a doctor who gave the treatment, is awaiting a re-trial (Table
4).11 In March 2018, a
general practitioner was charged after her patient died following a
liposuction 4 years prior.12
Presently, the family of a 73-year-old man is alleging criminal
responsibility on the part of a group of nurses13
and a doctor14 who were already
found guilty of professional misconduct in connection with his death.
Could this be the beginning of a trend in Hong
Kong? If so, what could we be wrestling with here?
Medical negligence as crime
The central and hotly debated question is whether
criminal law intervention is justified in cases of fatal medical
incidents.15 On the one hand, a
doctor’s license to practice has a legal foundation. Criminal sanctions
serve an important punitive function and a symbolic role in restoring
public trust when that foundation is not being respected. By holding
individuals publicly accountable, criminal sanctions may ensure compliance
with professional standards and deter poor and dangerous practices.16 In France, for example, a range of criminal offences
is available to punish medical mistakes, even when they are non-fatal.
Jail terms are uncommon; the ultimate force of deterrence lies in the
stigma of the charge or conviction itself.17
On the other hand, it can be argued that the
criminal law is supposed to punish those who cause damages with a morally
blameworthy state of mind, whereas misjudgement, inadvertence, or sheer
incompetence may be the reasons for a doctor failing to meet standards of
care.18 Criminal sanctions
presume, if not forcibly embed, the role of choice in situations where no
choice or decision has been consciously made. This overlooks the notion of
blameworthiness as the foundation of the criminal law, and prosecutions
were arguably unjustified in at least some cases.19
At issue is the appropriateness of the legal test and the threshold for
prosecution.
The legal test
The term “gross negligence” has not been defined,
and the legal test established in Adomako has been criticised for
its circularity, in that an act or omission constitutes a crime if the
jury finds it a crime.7 The
determination of “grossness” is one for the jury, as directed by the
judge; it is a question of law and not for medical experts to address.
However, medical experts have occasionally employed their varied
understanding of the legal term and provided opinions that could
potentially usurp the jury’s role.20
In the UK, the conviction of Mr David Sellu was reversed because the trial
judge had failed to direct the jury properly with regard to the
determination of “grossness” and the weight given to the expert opinions
on that issue (Table 1).8
Clearer guidance has since become available; how this will affect judicial
practice and outcomes within the UK and other common law jurisdictions,
such as Hong Kong, remains to be discovered.
Another contentious aspect of the existing law
concerns the mens rea (or “guilty mind”) requirement for GNM. A
detailed discussion on this highly complex topic is beyond the scope of
this paper, and readers are referred to previous works by legal scholars.18 21
22 23
It suffices to say that the culpable mental state in GNM is one of
indifference. This can be understood as a state of “wilful blindness” or a
high degree of negligence in considering and avoiding obvious risks.18 It is (arguably) distinguishable from recklessness,
which is acting in the face of subjectively known risks, but less readily
so from what is commonly regarded as “inadvertence” or “absent-mindedness”
by lay people.23
Take for example a hypothetical scenario in which a
patient died from septicaemia following a transfusion procedure. While
those who consciously disregarded standard safety procedures in preparing
the blood product should probably be held criminally liable, it is
debatable whether the doctor who gave the transfusion should be so
treated. Did the doctor know or suspect the risk of contamination? If not,
should he or she have been aware of or considered the possibility of that
risk? Was he or she “indifferent”? What is the level of due diligence
reasonably expected from a doctor administering a treatment passed to him
or her by someone else? How much checking is needed, and how should
“grossness” be determined in a situation like this? And from an ethical
point of view, should the severity of the consequence alone (ie, patient
death) transform a common human failing such as “inattention” into a
crime? If so, what could this mean in daily clinical practice?
There is no ready answer, and the concept of
liability in criminal negligence remains controversial, especially in the
medical context.23 In the UK,
charges against two pharmacists, whose dispensing of a defective medicine
caused a child’s death, were dropped because of the absence of malicious
intent to cause harm,24 whereas
two junior doctors who inadvertently injected vincristine into a patient’s
spine were convicted on the grounds that criminal liability may be found
not at the time of the injection but when they had “chosen” not to be more
careful before acting.25 The
distinction between these individuals’ corresponding mental states is not
overly clear, and the legal bar for gross negligence remains disputable.14
The situation is further complicated by the fact
that a defendant’s mental state may be judged either objectively (ie, what
a reasonably competent doctor should have been thinking at the time) or
subjectively (ie, what the doctor in question was actually thinking at the
time).18 In the sensationalised
case of Dr Conrad Murray, convicted for causing the death of the singer
Michael Jackson, it was sufficient for the prosecution to prove that the
doctor “should have been aware” of the risks associated with using
Propofol outside of a hospital setting, not whether he had actual
knowledge of those risks (ie, an objective test).26
In contrast, the High Court of Hong Kong had consciously departed from the
established English authorities and applied the subjective test in a
recent non-medical case.27 The
debate continues.28
Threshold for prosecution
A charge of GNM even without conviction can be
devastating for the doctor involved. In principle, prosecution is brought
when it is in the public interest to do so and when there is a realistic
prospect of success, but the loosely defined concept of gross negligence
affords prosecutors considerable discretion.29
Importantly, the criteria for distinguishing between honest mistakes and
conscious violations of professional standards are “tests unknown to
the criminal law”.30 As a
result, doctors (or even lawyers) can have little confidence in knowing
what kinds of behaviour will attract the attention of the criminal law.
Indeed, prosecutors in the UK have been criticised
for prosecuting many doctors who should not have been charged in the first
place.19 Such prosecutions have
caused significant disruptions to the personal and professional lives of
innocent individuals and negative feelings within the medical community.31 The small number of cases in
Hong Kong does not permit a valid assessment, but a reasonable,
consistent, and transparent threshold for prosecution would certainly be
welcomed. The principle reaffirmed in Mr David Sellu’s successful appeal
is that a prosecution should not be brought unless the conduct of the
doctor involved was “truly exceptionally bad”. The mere commission
of an error, even if fatal, does not begin to satisfy that test.
Criminal sanctions as a quality assurance measure
From the public’s point of view, an important
question is whether criminalisation improves patient safety. There is no
empirical evidence to show that it does, and the current understanding of
the nature of human error challenges the premise that punishment can
prevent mistakes.32 Again, a
distinction can be made between errors and violations.
Human errors are by nature unintentional. Even the
most able and conscientious clinician can commit errors in a complex
hospital environment; inexperience, exhaustion, lack of supervision, or
systemic failures may be responsible.33
Because errors are committed without awareness of the associated risks,
they are unlikely to respond positively to the threat of criminal
prosecution.34 This is
particularly the case where health care delivery involves multiple
disciplines and professionals whose roles and responsibilities, and hence
their duties of care and liabilities, cannot be easily delineated.
Moreover, human error is often the last part of a chain of events leading
up to an adverse outcome; the proper response should be the adoption of a
culture of open disclosure, learning, risk management, and system
improvement measures.32 A case in
point is that of Dr Bawa-Garba, in which system factors such as
understaffing, lack of supervision, and hardware malfunction are thought
to be at least partially responsible for a tragic patient outcome (Table
2).
In contrast, violations are associated with
deliberate disregard for patient safety and unjustified risk taking.
Adverse outcomes occur primarily because of individual doctors’ autonomous
decisions rather than the cumulative effects of system and latent factors.
The predominance of human agency renders system improvement measures
ineffective if not irrelevant; deterrence targeting individuals’ attitudes
and mental states is needed.22
Criminal sanctions in these situations can potentially discourage some
doctors’ “couldn’t care less” attitude and promote a greater sense of
responsibility and carefulness. The conduct of Dr Sudirman and the two
convicted individuals in the “DR Group” case in Hong Kong fall squarely
into this category (Table 3 and 4).
Admittedly, the distinction between error and
violation is not always straightforward, especially when there are
questions about clinical competency. Clinical competency involves a range
of human qualities, from skills and knowledge to conscientiousness and
ethical standards, only some of which are influenced by threat of criminal
penalties. It is notable that criminal sanctions in the early
vincristine-related cases did not prevent a recurrence: numerous cases
have occurred since.33
Diligence or vengeance?
It has been suggested that the wider use of the
criminal law in the present context represents an attempt by the public to
exact retribution rather than a desire to improve patient safety.35 In the UK, findings from several public inquiries,
such as the Bristol Royal Infirmary Report36
and the Francis Report,37 revealed
widespread unethical and substandard practices within the National Health
Service. Public dissatisfaction and a deepening blame culture have
allegedly created a greater tendency to hold individuals criminally
responsible, turning what was once a private matter of civil litigation
into a public act of criminal prosecution by the state.38
There has at the same time been a gradual but
fundamental shift in the way that the medical profession is perceived by
society.39 Better access to
medical information and a stronger emphasis on patients’ rights means that
doctors are no longer held as high priests of the mysterious art and
science of healing but partners in patient journey or providers of
services to which taxpayers are entitled. Mistakes are not deemed
acceptable simply because medical peers say so; society expects to have
the final word. When society thinks that certain behaviours are
unacceptable, criminal sanctions can be seen as a ready and legitimate
solution.40
The medical profession has not taken this well. In
the UK, the initial conviction of Mr David Sellu was met with fervent
protests.31 This surgeon had an
otherwise unblemished track record, was held in high regard by his peers
and patients, and the penalty imposed on him was seen by some as
unjustifiable and disproportionate (Table 1). Similarly, as mentioned previously, the
court in Dr Bawa-Garba’s case has been strongly criticised for its failure
to give due consideration to system factors.41
The insistence of the General Medical Council on removing Dr Bawa-Garba
from its register caused such an outcry that the UK government decided to
launch a national review into the application of the existing law to
medical cases (Table 2).42
The loss of mutual trust between the medical profession, its regulatory
body and the criminal justice system encapsulated in these cases is
probably the most damaging effect of the prevailing climate of blame and
fear. Patient care may also suffer as doctors become reluctant to disclose
their mistakes. Instead of promoting high-quality care, criminalisation
could in fact encourage the practice of defensive medicine, stifle
compassionate care, alienate the medical profession, and hamper the
promotion of a safety culture.43
The road ahead
Reactions from medical peers in Hong Kong towards
the two convictions in the “DR Group” case have been restrained. Few
appear to condone the negligent practices in that case or disapprove of
the penalties imposed (Table 4). There is arguably a sense of detachment,
as the circumstances in this case (ie, the preparation of an experimental
blood product) are far removed from those commonly experienced by most
doctors. As such, we have not seen the kind of emotional responses from
within our medical sector that have been found in the UK, where doctors
perform routine duties with the knowledge that jail sentences could arise
from a single missed diagnosis or a few hours’ delay in performing
life-saving surgery.
The outcome of the re-trial of the third defendant
in the “DR Group” case could generate more lively discussions for several
reasons. First, general opinions vary more widely regarding the
wrongfulness of the doctor’s conduct. Second, practising clinicians can
relate more readily to the circumstances in this part of the case and see
the relevance and implications of the re-trial. Third, the legal arguments
involved are more complex and subject to debate with respect to the
culpability of the doctor’s mental state.28
Irrespective of the outcome, the ruling will send a strong message on how
similar cases will be handled in the future and raise concerns within
different sectors of society one way or the other. Ahead of us could be a
challenging time.
In the greater scheme of things, there are perhaps
good reasons to believe that the general attitude towards the medical
profession in Hong Kong has not (yet) become hostile, and that the
catalogue of recent cases here is a rare exception. A previous survey
showed that the majority of our patients were very satisfied with the
quality of care received.44 The
number of complaints submitted to the Medical Council of Hong Kong has
remained steady.45 We have not had
any public scandal at a comparable scale to those in the UK, and our
health care professionals still enjoy a reasonable level of respect.44 There is also a handsome degree of transparency and
accountability within our system, while institutional measures are in
place to ensure that our medical students are properly trained, foreign
graduates suitably qualified, and requirements for continuous education
diligently followed.46 47 All of these factors must be acknowledged, treasured,
and enhanced.
But society’s trust in us is not a given; it has to
be earned and maintained. Our doctors and nurses work under challenging
conditions and need to be supported.48
The recent controversy about the suspension of a doctor by the Medical
Council mentioned above represents a serious trust crisis that must be
addressed urgently.14 Meanwhile,
we need to train our medical students and trainees well and impart a
strong sense of ethical awareness and responsibility so that our patients
will remain safe, and know that they are safe, in our hands.49 Public education should culture a better
understanding of the nature of human error and the acknowledgment of the
fact that Medicine is not a perfect science. Lastly, underpinning our
right to practice and power to self-regulate is a social contract with
society that is built on trust.50
The expert opinions we give, how we discuss and handle medical incidents,
and the ways in which we respond to legislative efforts to improve patient
safety will eventually affect how society perceives and reacts to our
mistakes and failings.
Conclusion
The rising number of medical manslaughter charges
and convictions in Hong Kong and overseas poses a concern. Criminal
liability for medical negligence is an arguably unstable legal concept,
and the prosecutorial threshold and legal test for GNM are imbued with
uncertainty. The criminalisation of medical mistakes can potentially
create a climate of blame and fear that is damaging to the medical
profession and detrimental to patient welfare. From the perspectives of
providing deterrence and punishment in the medical context, criminal
sanctions should probably be limited to conscious violations of
established standards; unintentional errors are better dealt with through
professional disciplinary actions or litigation based on the ordinary
civil test of negligence. However, this necessitates a fundamental
jurisprudential shift that is unlikely to materialise in the near future.
As we await the outcomes of ongoing cases in Hong Kong, there is much that
we can do to maintain society’s trust in the medical profession by
upholding standards of care and nurturing a robust culture of
professionalism.
Author contributions
The author has made substantial contributions to
the concept or design; acquisition of data; analysis or interpretation of
data; drafting of the article; and critical revision for important
intellectual content.
Funding/support
This article received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
The author has disclosed no conflicts of interest.
The author had full access to the data, contributed to the paper, approved
the final version for publication, and takes responsibility for its
accuracy and integrity.
References
1. Crown Prosecution Service, UK
Government. Homicide: murder and manslaughter. Available from:
http://www.cps.gov.uk/legal/h_to_k/homicide_murder_and_manslaughter/#gross.
Accessed 16 Mar 2018.
2. Mason JK, Laurie GT. Mason & McCall
Smith’s Law and Medical Ethics. 9th ed. Oxford: Oxford University Press;
2013.
3. R v Bateman [1925] 19 Cr App R 8.
4. R v Adomako [1995] 1 AC 171.
5. Ferner RE, McDowell SE. Doctors charged
with manslaughter in the course of medical practice, 1795-2005: a
literature review. J R Soc Med 2006;99:309-14. Crossref
6. White P. More doctors charged with
manslaughter are being convicted, shows analysis. BMJ 2015;351:h4402. Crossref
7. Quick Q. Medicine, mistakes and
manslaughter: a criminal combination? Cam Law J 2010;69:186-203. Crossref
8. David Sellu v R [2016] EWCA Crim 1716.
9. Bawa Garba v R [2016] EWCA Crim 1841.
10. HKSAR v Harry Sudirman CACC 486/2003.
11. HKSAR v Chow Heung-Wing, Stephen and 2
others HCCC 437/2015.
12. Mok D. Hong Kong doctor arrested on
manslaughter charge over death of dancer after beauty treatment. South
China Morning Post. 2018 Mar 13. Available from:
http://www.scmp.com/news/hong-kong/law-crime/article/2137050/hong-kong-doctor-arrested-manslaughter-charge-over-death.
Accessed
19 Mar 2018.
13. Tsang E. Hong Kong nurses found guilty
of misconduct in fatal blunder or one month. South China Morning Post.
2016 June 13. Available from:
http://www.scmp.com/news/hong-kong/health-environment/article/1974286/hong-kong-nurses-found-guilty-misconduct-fatal.
Accessed 16 Mar 2018.
14. Cheung E. Hong Kong doctor Wong
Cheuk-yi banned for six months over death of elderly cancer patient Wang
Keng-kao at Kowloon Hospital. South China Morning Post. 2018 May 9.
Available from:
http://www.scmp.com/news/hong-kong/health-environment/article/2145352/hong-kong-doctor-wong-cheuk-yi-found-guilty.
Accessed 10 Mar 2018.
15. Edwards S. Medical manslaughter: a
recent history. Ann R Coll Surg Engl 2014;96:118-9. Crossref
16. Dekker SA. Criminalization of medical
error: who draws the line? ANZ J Surg 2007;77:831-7. Crossref
17. Spencer JR, Brajeux MA. Criminal
liability for negligence—a lesson from across the Channel? Int Comp Law
Quart 2010;59:1-24. Crossref
18. McCall Smith A. Criminal negligence
and the incompetent doctor. Med Law Rev 1993;1:336-49. Crossref
19. Hubbeling D. Criminal prosecution for
medical manslaughter. J R Soc Med 2010;103:216-8. Crossref
20. Quick O. Expert evidence and medical
manslaughter: vagueness in action. J Law Soc 2011;38:496-518. Crossref
21. Virgo G. Reconstructing manslaughter
on defective foundations. Cam Law J 1995;54:14-6. Crossref
22. Horder J. Gross negligence and
criminal culpability. U Toronto Law J 1997;47:495-521. Crossref
23. Smith AM. Criminal or merely human?:
the prosecution of negligent doctors. J Cont Health Law Policy
1996;12:131-46.
24. March PJ. Boots pharmacist and trainee
cleared of baby’s manslaughter, but fined for dispensing a defective
medicine. Pharmaceutical J 2000;264:390-2.
25. R v Prentice, R v Adomako [1995] 1 AC
171.
26. Kim CJ. The trial of Conrad Murray:
prosecuting physicians for criminally negligent over-prescription. Am Crim
Law Rev 2014;51:517-40.
27. HKSAR v Lai Shui Yin HCCC 29/2011.
28. Leung JA, Liu HT. Gross negligence
manslaughter after Lai Shui Yin. Hong Kong Law J 2014;44:709-17.
29. Quick O. Prosecuting ‘gross’ medical
negligence: manslaughter, discretion, and the crown prosecution service. J
Law Soc 2006;33:421-50. Crossref
30. O’Doherty S. Doctors and
manslaughter—response from the Crown Prosecution Service. J R Soc Med
2006;99:544. Crossref
31. McDonald P. Doctors and manslaughter.
Bull R Coll Surgeons 2014;96:112-3. Crossref
32. Reason J. Human error: models and
management. BMJ 2000;320:768-70. Crossref
33. Leape LL. Error in medicine. JAMA
1994;272:1851-7. Crossref
34. Merry AF. How does the law recognize
and deal with medical errors? J R Soc Med 2009;102:265-71. Crossref
35. Holbrook J. The criminalisation of
fatal medical mistakes. BMJ 2003;327:1118-9. Crossref
36. Department of Health, UK Government.
Learning from Bristol: The Department of Health’s response to the report
of the Public Inquiry into Children’s Heart Surgery at the Bristol Royal
Infirmary 1984-1995. 2002. Available from:
https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/273320/5363.pdf.
Accessed 18 Feb 2018.
37. UK Government Report of the Mid
Staffordshire NHS foundation trust public inquiry. London: The Stationery
Office; 2013.
38. Radhakrishna S. Culture of blame in
the National Health Service; consequences and solutions. Br J Anaesth
2015;115:653-5. Crossref
39. Edwards N, Komacki MJ, Silversin J.
Unhappy doctors: what are the causes and what can be done? BMJ
2002;324:835-8. Crossref
40. Leung GK. If we do not take charge of
ourselves, some else would (have to). Surg Pract 2016;20:141. CrossRef
41. Cohen D. Back to blame: the Bawa-Garba
case and the patient safety agenda. BMJ 2017;359:j5534. Crossref
42. Donnelly L. Hunt orders review of
medical malpractice amid doctors’ outcry over manslaughter case. The
Telegraph. 6 Feb 2018. Available from:
https://www.telegraph.co.uk/news/2018/02/06/hunt-orders-review-medical-malpractice-amid-doctors-outcry-manslaughter/.
Accessed 26 Mar 2018.
43. Kessler D, McClellan M. Do doctors
practice defensive medicine? Q J Econ 1996;111:353-90. Crossref
44. Wong EL, Coulter A, Cheung AW, Yam CH,
Yeoh EK, Griffiths SM. Patient experiences with public hospital care:
first benchmark survey in Hong Kong. Hong Kong Med J 2012;18:371-80.
45. The Medical Council of Hong Kong.
Annual reports 2016. Available from:
https://www.mchk.org.hk/files/annual/files/2016/MCAR_2016_e.pdf. Accessed
26 Mar 2018.
46. The Medical Council of Hong Kong. Hong
Kong doctors. October 2017. Available from:
https://www.mchk.org.hk/english/publications/files/HKDoctors.pdf. Accessed
29 Mar 2018.
47. The Hong Kong Academy of Medicine.
Positional paper on postgraduate medical education. 2010. Available from:
https://www.hkam.org.hk/publications/HKAM_position_paper.pdf. Accessed 27
Mar 2018.
48. Chow CB. Satisfied patients, burnout
doctors! Hong Kong Med J 2012;18:360-1.
49. Wong DS, Lai PB. Malpractice claims:
prevention is often a better strategy. Hong Kong Med J 2011;17:425-6.
50. Cruess SR, Cruess RL. Professionalism:
a contract between medicine and society. CMAJ 2000;162:668-9.