Congenital infections in Hong Kong: an overview of TORCH
Hong Kong Med J 2020 Apr;26(2):127–38 | Epub 2 Apr 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Congenital infections in Hong Kong: an overview
of TORCH
Karen KY Leung, MB, BS, MRCPCH1; KL Hon, MB, BS, MD1; Alice Yeung2; Alexander KC Leung, FRCP (UK), FRCPCH3; Elim Man, MB, BS, MRCPCH1
1 Department of Paediatrics and Adolescent Medicine, The Hong Kong Children’s Hospital, Kowloon Bay, Hong Kong
2 Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Pediatrics, University of Calgary and Alberta Children’s Hospital, Calgary, Canada
Corresponding author: Dr KL Hon (ehon@hotmail.com)
Abstract
Congenital infections refer to a group of
perinatal infections that may have similar clinical presentations, including rash and ocular findings. TORCH is the acronym that covers these infections (toxoplasmosis, other [syphilis], rubella, cytomegalovirus, herpes simplex virus). There are, however, other important causes of intrauterine/perinatal infections, including enteroviruses, varicella zoster virus, Zika virus, and parvovirus B19. Intrauterine and perinatal infections are significant causes of fetal and neonatal mortality and important contributors to childhood morbidity. A high index of suspicion for congenital infections and awareness of the prominent features of the most common congenital infections can help to facilitate early diagnosis, tailor appropriate diagnostic evaluation, and if appropriate, initiate early treatments. In the absence of maternal laboratory results diagnostic of intrauterine infections, congenital infections should be suspected in newborns with certain clinical features or combinations of clinical features, including hydrops fetalis, microcephaly, seizures, cataract, hearing loss, congenital heart disease, hepatosplenomegaly, jaundice, or rash. Primary prevention of maternal infections during pregnancy is the cornerstone of prevention of congenital infection. Available resources should focus on the promotion of public health.
Introduction
Congenital infections are those that can cross the
placenta and damage the fetus in utero or transmit
to the infant during the peripartum period of
birth, resulting in neonatal infection.1 Apart from
miscarriage, stillbirths, and neonatal deaths,
congenital infections account for 2% to 3% of all
congenital anomalies and are a significant cause
of childhood morbidity.2 3 4 Immunologist Andres
Nahmias first used the acronym ToRCH in 1971
to describe perinatal infections associated with
toxoplasma (To), rubella (R), cytomegalovirus (C),
and herpes simplex virus (H); these infections are
difficult to differentiate from one another clinically.5
In 1975, Harold Fuerst proposed adding syphilis,
another important congenital infection, to the list and
revising the acronym into STORCH.6 Also in 1975,
Roger Brumback recommended replacing STORCH
with TORCHES, as the latter term was more readily
accepted and recognised by paediatricians familiar
with the older acronym.7 Subsequently, the ‘O’ in
TORCH has been broadened and now stands for
‘Others’ to include the following pathogens: syphilis,
parvovirus, coxsackievirus, listeriosis, hepatitis
virus, varicella-zoster virus, Trypanosoma cruzi, enterovirus, human immunodeficiency virus (HIV),
and the latest addition, Zika virus.1 4
Congenital infections have remained a major
public health issue globally, especially in developing
countries. These infections can lead to significant
consequences, such as severe disabilities or even
fetal deaths, but most of them are preventable
by preventing primary maternal infection during
pregnancy. In view of this, public awareness is crucial.
The World Health Organization has proposed
strategies to eliminate mother-to-child transmission
of HIV by 2020 and syphilis and hepatitis B by 2030.8
This review discusses congenital infections
in terms of the clinical features, medical
management, implications to public health, and
neurodevelopmental outcomes pertinent to the
local situation. This review will focus on the classic
TORCH infections: toxoplasmosis, syphilis, rubella,
cytomegalovirus (CMV), and herpes simplex virus
(HSV). References were searched using key terms
‘congenital infection’ and ‘Hong Kong’ or ‘TORCH’
and ‘Hong Kong’ in PubMed, limited to ‘human’,
with no filters on article type or publication date.
Discussion is based on, but not limited to, the search
results.
T – Congenital toxoplasmosis
Toxoplasma gondii is the protozoan parasite
responsible for congenital toxoplasmosis.
Toxoplasma infection usually occurs through
contact with faeces of infected cats or consumption
of raw or undercooked meat, raw oysters, clams,
mussels, fruits, vegetables, goat’s milk, or water
contaminated with the parasite.9 Approximately 30%
of primary Toxoplasma infections during pregnancy
result in vertical transmission to the fetus through
the transplacental route.9 10 Congenital infection
occurs predominantly after primary infection
during the parasitemic phase in a pregnant woman.
However, cases of transmission from women
infected shortly before pregnancy and reactivation
from immunosuppressed women have also been
reported.11 The sequelae tend to be more frequent
and severe if the mother is infected during the first
or second trimester of pregnancy, which may result
in intrauterine death, spontaneous abortion, or
premature birth.11 The risk of developing the classic
triad of congenital toxoplasmosis (intracranial
calcifications, hydrocephalus, and chorioretinitis)
decreases from 61% at 13 weeks to 25% at 26
weeks and 9% at 36 weeks. However, if maternal
infection occurs later during gestation, the risk of
transmission to the fetus is higher, increasing sharply
from 6% at 13 weeks to 40% at 26 weeks and 72% at
36 weeks.10 12
The seroprevalence of toxoplasmosis also
varies greatly by geographical area, ranging from
less than 1% to more than 95%. The highest rates
are found in Latin American countries while the
lowest rates are reported in the Southeast Asia.9 In
1980, the overall seroprevalence of toxoplasmosis in Hong Kong was reported to be 9.8%; this low rate is
probably attributed to the local habit of eating well-cooked
meat as part of traditional Chinese meals
and the relatively low number of households that
keep cats as pets.13 The estimated global incidence
of congenital toxoplasmosis is approximately
190 100 cases annually, with an incidence rate of
approximately 1.5 cases per 1000 live births.14
Although congenital toxoplasmosis is
asymptomatic in approximately 75% of affected
newborns, common manifestations in symptomatic
neonates include fever, maculopapular rash,
chorioretinitis, intracranial calcification,
hydrocephalus, abnormal cerebrospinal fluid
(CSF), jaundice, thrombocytopenia, anaemia,
hepatosplenomegaly, lymphadenopathy, pneumonitis,
seizures, microphthalmia, and microcephaly.10 15 The
classic triad of chorioretinitis, hydrocephalus, and
intracranial calcifications occur in less than 10% of
cases of congenital toxoplasmosis.16 Infants who are
asymptomatic at birth may develop chorioretinitis,
blindness, cerebral palsy, cerebellar dysfunction,
microcephaly, seizures, mental retardation,
sensorineural deafness, or growth retardation later
in life, and untreated cases are at higher risk of
developing these serious sequelae.17 18
Acute maternal infection with T gondii is
usually asymptomatic. Symptoms include transient,
mild fever, headaches, myalgias, maculopapular rash,
sore throat, lymphadenopathy, and hepatomegaly.
When toxoplasmosis is suspected, three tests can
be performed for prenatal diagnosis. Serological
tests to detect the levels of Toxoplasma-specific
immunoglobulin G (IgG) and IgM antibodies in the
maternal serum are widely used to assess immunity
to the parasite and any recent infection in the
pregnant mother. After infection, IgG appears in 1
to 2 weeks and persists throughout life, therefore
leading to immunity in the mother. In contrast,
IgM becomes detectable earlier after infection than
IgG and persists for a variable period from months
to years, such that positive results for both IgG
and IgM antibodies can be difficult to interpret.9
Ultrasonography of affected fetuses can be normal
or non-specific, but intracranial calcifications,
ventricular dilatations, hepatic enlargement,
ascites, and increased placental thickness are
present in approximately 6% of infected fetuses.19 20
Because maternal infection does not necessarily
result in fetal infection, suspected or established
maternal infection should be confirmed prenatally
by polymerase chain reaction (PCR) amplification
of Toxoplasma DNA in amniotic fluid. Diagnostic
performance after 18 weeks of gestation and at least
4 weeks after maternal seroconversion is likely to be
more reliable.10 21
Postnatal diagnosis of congenital toxoplasmosis
can be confirmed by detection of T gondii in the infant’s umbilical cord blood, urine, peripheral
blood, or CSF; Toxoplasma DNA in the infant’s
amniotic fluid, peripheral blood, urine, or CSF;
IgG, IgM, and IgA antibodies in peripheral blood
or CSF; or Toxoplasma IgG antibody at 12 months
of life. For infants positive for IgG but negative for
IgM and IgA, follow-up serology testing for IgG
should be repeated every 4 to 6 weeks until complete
disappearance of IgG.9 21 22
When primary maternal infection is diagnosed
before 18 weeks of gestation, antiparasitic treatment
with spiramycin should be initiated as soon as
possible to prevent transplacental transmission.9 10
If PCR on amniotic fluid is positive for T gondii
DNA after 18 weeks of gestation, treatment
should be replaced by pyrimethamine-sulfadiazine
with leucovorin (folinic acid). If PCR is negative,
following the prophylaxis regimen in the US and
France, continuation of spiramycin is recommended
until delivery; or following the prophylaxis regimen
in Austria and Germany, spiramycin is used by a
4-week course of pyrimethamine-sulfadiazine at 17
weeks of gestation.10
For infants with symptomatic congenital
toxoplasmosis, a 12-month treatment with
pyrimethamine-sulfadiazine is indicated. Folinic
acid is also given to minimise pyrimethamine
toxicity.10 22 As sulfadiazine is not available in Hong
Kong, clindamycin can be used instead.23 The same
regimen is used for asymptomatic infants, but the
treatment duration is 3 months.22
Education on prevention of Toxoplasma
infection is important for pregnant mothers.
They should be advised to avoid eating raw or
undercooked meat/shellfish or drinking unfiltered
water; to clean fruits and vegetables thoroughly
before consumption24; and to employ proper hand
hygiene to reduce the risk of infection.25 If the family
keeps cats as pets, the cats should not be fed raw or
undercooked meat and contact with cat litter should
be avoided.26
Universal screening for maternal
toxoplasmosis is incorporated into the maternal-child
care programme in some countries, including
Austria, France, and Italy.27 However, such screening
is not practised in the US, Canada, or the United
Kingdom, mainly because of the lower prevalence of
toxoplasmosis, uncertainty about the effectiveness
of maternal treatment at preventing congenital
infection, high cost of frequent testing required
for early detection of infections, low screening
sensitivity, cost-ineffectiveness of treating women
with false positive results, and possible low
adherence to frequent rescreening.28 29 In Hong
Kong, as the prevalence of toxoplasmosis and the cost
effectiveness of antenatal screening for toxoplasmosis
are relatively low, patient education for prevention of Toxoplasma infection should be sufficient to reduce
the risk of congenital toxoplasmosis.
O – Congenital syphilis
Syphilis is a sexually transmitted disease caused by Treponema pallidum, a spirochete. Congenital
syphilis affects approximately 2 million pregnancies
annually, and approximately 25% of these pregnancies
result in spontaneous abortion or stillbirths.30
Syphilis is not a notifiable disease in Hong
Kong. The Social Hygiene Service reported only 100
new cases in 1991, increasing to 1095 cases in 2018,
accounting for 14.6 cases per 100 000 population.31
This is much higher than the 9.5 cases per 100 000
population reported in the US in 2017.32 In Hong
Kong, congenital syphilis decreased from over
100 new cases annually in the early 1970s to three
cases of congenital syphilis in 2017, 0 cases in 2018,
and one case in 2019 (through August). All recent
reported cases were late congenital syphilis.31
Congenital syphilis usually results from
transplacental transmission of T pallidum, mostly
in untreated or inadequately treated mothers,
especially with concomitant HIV infection. The
risk of fetal infection increases along with the
progression of gestation.9 In contrast, the risk of
vertical transmission in untreated mothers is highest
during the first stage and lowest in the late stage.33
Congenital syphilis can result in spontaneous
abortion (usually after the first trimester), stillbirth,
premature birth, impaired fetal growth, and neonatal
mortality.9,34 Approximately two thirds of infected
neonates born alive are asymptomatic at birth.9 34
However, symptoms usually develop by the third
month if these infants are left untreated.35
Congenital syphilis can be divided into two
stages: early congenital syphilis with symptoms onset
during the first 2 years of life and late congenital
syphilis with manifestations after age 2 years.35
Hepatomegaly with or without splenomegaly,
jaundice, syphilitic rhinitis (snuffles), maculopapular
and vesicular rash, generalised lymphadenopathy,
osteochondritis, and periostitis are common
manifestations of early congenital syphilis.9 36 Other
manifestations of early congenital syphilis include
non-immune hydrops fetalis, fever, pneumonia,
secondary sepsis, myocarditis, inability to move
an extremity because of pain (“pseudoparalysis of
Parrot”), chorioretinitis, cataract, glaucoma, loss
of eyebrows, uveitis, nephrotic syndrome, rectal
bleeding from ileitis, malabsorption, keratoderma of
the hands and feet, and onychauxis of the fingernails
and toenails.37 38 39 Laboratory abnormalities may
include anaemia, thrombocytopenia, leukopenia,
and leukocytosis. Radiological abnormalities may
include erosions (osseous destruction) and lucencies
(demineralisation) of the proximal medial tibial metaphysis (Wimberger sign), metaphyseal lucent
bands, metaphyseal serrated appearance at the
epiphyseal margin of long bones (Wegner sign),
irregular areas of increased density and rarefaction
(‘moth-eaten’ appearance), diaphyseal periostitis,
and multiple sites of osteochondritis.40
Late congenital syphilis occurs in
approximately 40% of untreated infants and is often
related to scarring and deformities resulting from
early infection.9 Manifestations include saddle nose;
perioral fissures (rhagades); frontal bossing; Clutton
joints (symmetrical, sterile, and painless synovial
effusions); thickening of sternoclavicular joint
(Higoumenakis sign); scaphoid scapula; anterior
bowing of shins (saber shins); perforation of the hard
palate; multicusped first molars (mulberry molars);
peg-shaped, notched, widely spaced permanent
upper central incisors (Hutchinson’s teeth);
interstitial keratitis; glaucoma; mental retardation;
sensorineural deafness; and hydrocephalus.9 36 37
Hutchinson’s triad, including Hutchinson’s teeth,
interstitial keratitis, and sensorineural deafness,
specific to late congenital syphilis, is rather rare.36
Diagnosis of gestational syphilis can be
established by serological tests, including both non-treponemal
(rapid plasma regain [RPR] and venereal
disease research laboratory [VDRL]) and treponemal
tests (T pallidum particle agglutination and
automated treponemal assay, eg enzyme immunoassay). Non-treponemal
assays are recommended for screening, followed by
a treponemal test if the screening result is positive. If
the reverse sequence screening algorithm is used and
the pregnant woman is reactive to the treponemal
test, confirmatory testing with a non-treponemal test
should be performed. However, if these two results
are discordant, a different treponemal test using a
different T pallidum should be performed.22 Nonspecific
ultrasonographic abnormalities include
hepatomegaly, placentomegaly, polyhydramnios,
and hydrops fetalis.36 Congenital syphilis should be
suspected in infants with a history of untreated or
inadequately treated maternal syphilis, especially
when they have a reactive treponemal test, and the
physical examination showing signs of infection,
abnormal long bone radiography, elevated cell count,
protein in the CSF, or a quantitative non-treponemal
test with titre at least four-fold higher than that of
the mother. Diagnosis can be established by the
presence of T pallidum in body fluids or samples
from lesions when viewed by dark-field microscopy
or fluorescent antibody staining.41
Parenterally administered penicillin G is the
standard treatment for syphilis.41 Pregnant patients
with immediate-type penicillin allergy should be
treated with penicillin after desensitisation because
there is no satisfactory alternative for treating
syphilis in pregnancy.41 The overall success rate
of maternal treatment at all gestational ages in preventing congenital syphilis is as high as 98% and
maternal secondary syphilis has the highest risk of
fetal treatment failure compared with other stages
of maternal infection.42 Mother-to-child infection is
more likely to occur in adequately treated mothers
in the conditions that the interval from treatment
to delivery is short (<30 days), stage of maternal
infection is early, delivery occurs before 36 weeks
of gestation, or non-treponemal titre is high at the
initiation of treatment and delivery.43
If congenital syphilis is diagnosed or suspected
in an infant, treatment can be made with reference
to the mother’s treatment history for syphilis, the
infant’s physical examination findings, and maternal
and infant RPR/VDRL titres. For proven or highly
probable congenital syphilis, penicillin G should
be given intravenously for 10 days.22 41 For possible
congenital syphilis cases with either incomplete or
abnormal evaluations, the same regimen should be
administered. In cases where congenital syphilis is
less likely positive (eg, infant RPR/VDRL are less than
four-fold of the maternal RPR/VDRL), the infant
should be followed-up every 3 months until nontreponemal
tests become non-reactive; alternatively,
a single dose of penicillin G can be given.22
Screening for syphilis during early pregnancy
remains an important preventive measure against
congenital syphilis since early diagnosis facilitates
timely treatment that can prevent perinatal loss and
potential severe disabilities.44 In Hong Kong and
many other countries, the VDRL test for syphilis is
performed during the first antenatal visit.
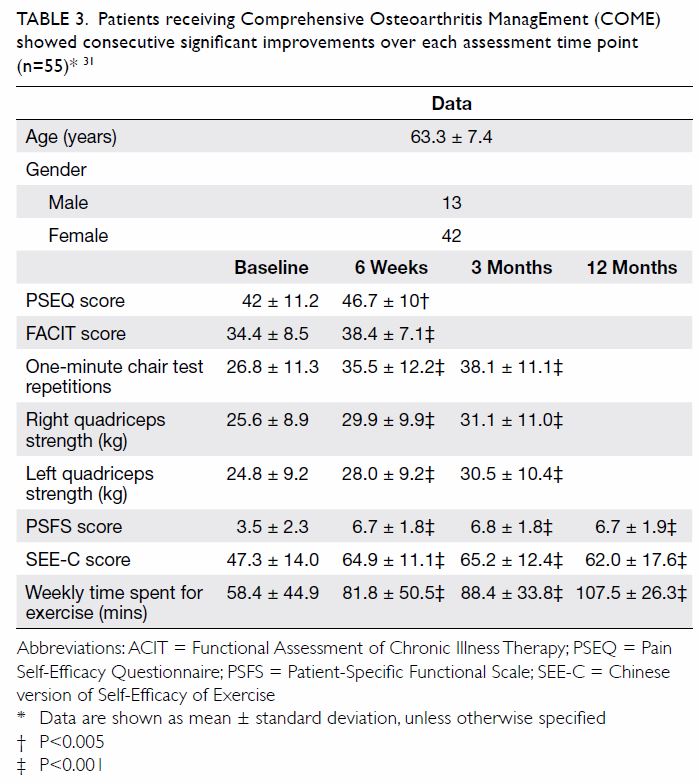
R – Congenital rubella
Rubella, also known as German measles, is a viral illness that is often mild or even asymptomatic when
acquired. However, catastrophic consequences may
result from congenital infections and is therefore of
particular concern in pregnant women.
Rubella has been a notifiable disease in Hong
Kong since 1994, and the number of cases has
fluctuated drastically, from eight cases in 1994 up
to 4958 cases in 1997.45 After a spike of 2338 cases
recorded in 2000, the number of rubella cases has
decreased and remained low in recent years. There
were 11 reported cases in 2018 and 46 cases in
2019 (through August).46 From 2001 to 2019, there
were only four reported cases of congenital rubella
syndrome (CRS): one in 2008 and three in 2012.46 The
three cases in 2012 were mothers born in mainland
China who had either uncertain proof or no history
of rubella vaccination.45
Although rubella virus can spread by a
respiratory route, vertical transmission from
an infected mother to her fetus can occur via
haematogenous spread during maternal viraemia.
The risk of transmission differs depending on the
timing of maternal infection.47 A study conducted in England from 1976 to 1978 found that when
maternal infection occurred within the first 12
weeks of gestation, the fetal infection rate reached
>80%; at the end of second trimester, it could drop to
25%; at 27 to 30 weeks, it could be 35%; and when it
occurred during the last month of gestation, the rate
could get close to 100%.48
Rubella infection has an incubation period
of 14 to 21 days and can be asymptomatic (ie,
subclinical) in 25% to 50% of individuals.49 When
symptoms occur, they are usually mild and self-limiting.
Prodromal symptoms include low-grade
fever, malaise, anorexia, nausea, non-exudative
conjunctivitis, coryza, cough, sore throat, headache,
petechiae on the soft palate (Forchheimer spots) and
postauricular area, and occipital and/or posterior
cervical lymphadenopathy.47 49 These symptoms,
though common in adolescents and adults, are
unusual in children.47 Prodromal symptoms are
usually followed by the characteristic pinpoint,
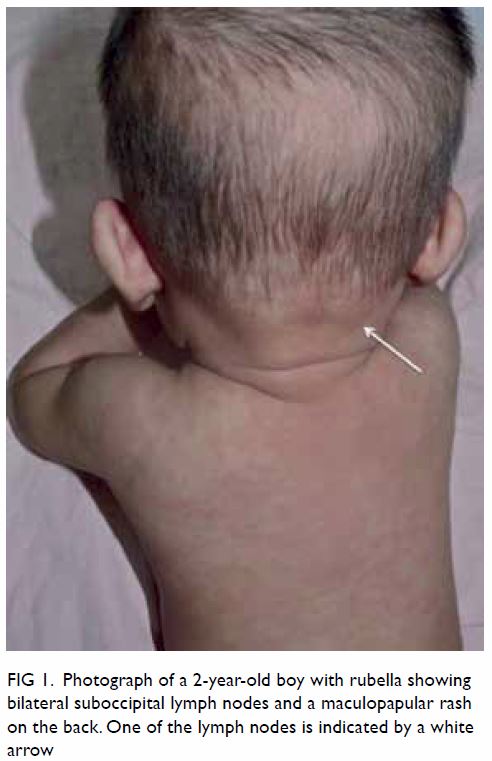
erythematous, maculopapular rash in 50% to 80%
of cases, starting on the face, later spreading to the
trunk and limbs, and becoming generalised within 24
hours.47 The rash usually subsides in 3 days and fades
in the same directional pattern as it appears. Some
individuals, mostly adolescents and adult women,
may also experience polyarthritis and polyarthralgia
approximately 1 week after the rash.49
Maternal rubella infection during the first 8
to 10 weeks of gestation can lead to catastrophic
consequences, including spontaneous abortion,
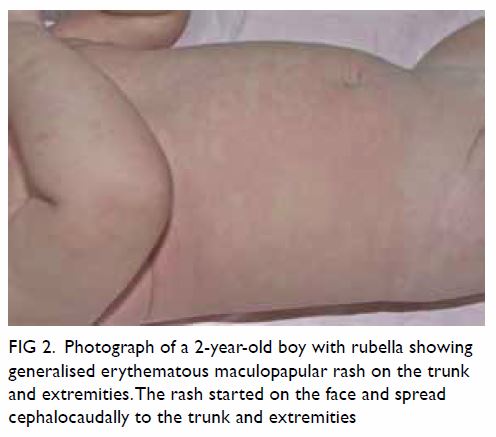
stillbirth, and prematurity.9 50 51 Cataracts,
congenital heart defects (eg, patent ductus
arteriosus, branch pulmonary artery hypoplasia/stenosis), and sensorineural hearing loss are the
classic triad of CRS.47 Other manifestations of
CRS include intrauterine growth retardation,
retinopathy, infantile glaucoma, mental retardation,
microcephaly, meningoencephalitis, hepatitis,
hepatomegaly, splenomegaly, haemolytic anaemia,
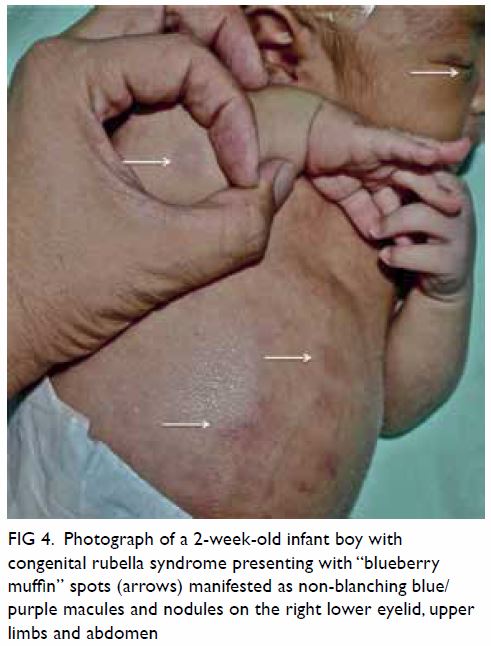
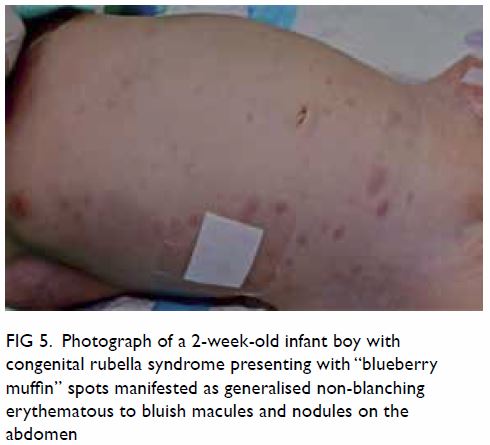
thrombocytopenia, and purpura (“blueberry muffin
spots”).9 50 51 52 Some of these clinical manifestations
are transient and non-specific; others may evolve
over time into adulthood, even when the infection
is subclinical at birth.9 50 51 52 The risk of congenital
defects after maternal infection is essentially limited
to the first 16 weeks of gestation. Beyond 20 weeks
of gestation, focal growth restriction seems to be the
only significant sequela.49
Diagnosis of maternal infection can be
confirmed by using serological tests when there
is a four-fold increase in rubella-specific IgG titre
between acute and convalescent serum samples,
a positive test for rubella-specific IgM antibodies,
or a positive culture for the rubella virus.49 Fetal
infection can be confirmed with a positive PCR
assay on a chorionic villus sample when other
clinical findings are consistent with the features of congenital rubella.49 If congenital rubella infection
is suspected, the diagnosis can be confirmed by
the presence of rubella-specific IgM antibodies in
the cord blood or neonatal serum within the first
6 months of life, detection of rubella virus RNA by
reverse transcription PCR on nasopharyngeal swab
or urine, or isolation of the rubella virus.47 53
No specific antiviral treatment for rubella is
now available. When maternal rubella is confirmed
before 20 weeks of gestation, treatment with Ig and
termination of pregnancy should be discussed as
options based on local legislation.47 Treatment of
CRS mainly involves long-term interdisciplinary
supportive care for managing clinical manifestations,
close monitoring of neurodevelopmental progress,
and long-term audiological and ophthalmic follow-up.
47
Vaccination is the only practical and effective
way of preventing congenital rubella infection; a single
dose of vaccine can offer long-lasting immunity in
>95% of cases, while two doses given at an appropriate
interval offers close to 100% protection.47 52 While
the rubella vaccine is available as an isolated vaccine,
it is often administered as a combined vaccine with
measles (MR), both measles and mumps (MMR),
or together with varicella (MMRV). Yet the vaccine
is contra-indicated in pregnant women because of
potential transplacental transfer of live rubella virus.
Since 1969 the immunisation against rubella with
live, attenuated virus was first introduced, some
countries have implemented national immunisation
programmes against rubella although no cases of
CRS have been reported due to rubella vaccination
during early pregnancy,54 55 the number of cases of
rubella infection has declined globally.
Under the Hong Kong Childhood
Immunisation Programme, the inclusion of eligible
children who are entitled to free immunisation
against 11 infectious diseases including rubella has
been incorporated into the programme progressively
since 1978.45 Starting from 1982, the two-dose
protocol has covered all eligible children in Hong
Kong. The first dose of MMR vaccine is given at
age 1 year, and the second dose at primary one. The
second dose of MMR vaccine has been replaced
with MMRV vaccine for children born on or after
1 January 2013. The estimated coverage of the first
dose of MMR vaccine in children from aged 2 to 5
years remains high (over 98%).56
Screening for rubella IgG antibodies is
performed in pregnant women during their first
antenatal visit. If the pregnant woman is found to
be non-immune to rubella during the screening,
postpartum vaccination is arranged at the Maternal
Child Health Centre to protect the mother and her
future pregnancies. With universal immunisation
against rubella implemented in Hong Kong, the
seronegativity rate among resident women who delivered their babies in a local hospital was 8.1%,
less than half of the non-residents being 19.9%,
who were mostly Chinese from the mainland China
where there is no such immunisation programme
implemented.57
C – Congenital cytomegalovirus
Cytomegalovirus is a ubiquitous herpesvirus that
can reside in the body after a primary infection
throughout the person’s life.9 Non-primary
infection can result from reactivation of the latent
virus or reinfection by a different strain of CMV.
Approximately 1% to 7% of pregnant women acquire
primary CMV infection; of these, about 30% to 40%
transmit the infection to the fetus.58 59 Congenital
CMV infection represents the most common
congenital viral infection worldwide and is a leading
cause of hearing loss and neurological disabilities in
children.60 61
According to the Centre for Health Protection
of Hong Kong, the seroprevalence rates of CMV
antibodies in local women aged between 20 and
39 years ranged from 50% to 86% in 2015, with the
highest rate in the age-group of 35 to 39 years.62 A
local study of Chinese women in Hong Kong found
a 7.4% prevalence rate of CMV in cervical excretions
during the third trimester, which was low relative
to other Asian countries.63 A 1994 report indicated
that the seroprevalence rate of CMV in Hong Kong
increased steadily from 37% at aged 1 year to 51%
by aged 10 years and increased drastically to almost
100% by aged 21 years, implying that CMV infection
is usually acquired early in life.64
Cytomegalovirus can be transmitted through
direct or indirect contact with infectious body
fluids like saliva, urine, blood, semen, or cervical
or vaginal secretions.60 Maternal CMV infection
is mainly acquired through contact with the
urine or saliva of infected individuals or sexual
contact.60 Cytomegalovirus can be transmitted
transplacentally, resulting in congenital infection,
with the transmission rate reaching approximately
32.3% in mothers with primary infection but only
1.4% in those with non-primary infection.65 Despite
the higher rate of vertical transmission and the
fact that many women of childbearing age are
seropositive for CMV, only one-quarter of congenital
CMV infections are caused by primary maternal
infection, with the rest three-quarters resulting
from non-primary maternal infections.66 The risk of
vertical transmission is approximately 36% when the
primary maternal infection occurs during the first
trimester, whereas in the third trimester67 rise to
77.6%.
Primary CMV infection in immunocompetent
individuals is asymptomatic including 75% to 95%
of pregnant women with infection.9 68 In others, it may present as a mild mononucleosis- or flu-like
syndrome with non-specific symptoms such as
fever and fatigue.68 Nevertheless, congenital CMV
infections can have severe disabling consequences.
Congenital CMV infections from primary
maternal infections are more likely to result in
symptoms and long-term defects in neonates than
those from non-primary maternal infections.58 59 60 61 62 63 64 65 66 67 68 69
Approximately 10% of newborns congenitally
infected with CMV are symptomatic.65 The most
common manifestations are jaundice at birth,
petechiae, hepatosplenomegaly, small size for age,
and microcephaly.60 Other clinical manifestations
include premature birth, hypotonia, poor feeding,
lethargy, sensorineural hearing loss, chorioretinitis,
hydrocephalus, seizures, thrombocytopenia,
anaemia, and pneumonitis.60 69
The risk of developing central nervous system
(CNS) sequelae is higher if the congenital infection
results from primary maternal infection occurring in
the first trimester than later stage in pregnancy.70 The
development of permanent sequelae is more frequent
in symptomatic newborns, while 10% to 15% of those
who are asymptomatic at birth have developmental
abnormalities including sensorineural hearing loss,
microcephaly, motor defects, mental retardation,
chorioretinitis, and dental defects, usually appearing
before the age of 2 years.9 61 71 For instance, while
nearly 50% of symptomatic neonates develop
sensorineural hearing loss, only 7% of asymptomatic
ones develop it.72 Fetal and neonatal deaths can
also result from congenital CMV infections in
approximately 0.5% of cases.71
A history of maternal primary CMV
infection, together with ultrasonography findings
such as echogenic bowel, bilateral periventricular
cerebral calcifications, hydrocephalus,
microcephaly, fetal growth retardation,
oligohydramnios, polyhydramnios, placentomegaly,
hepatosplenomegaly, hepatic calcifications, ascites,
or hydrops, are suggestive of fetal infection.71 Fetal
CMV infection can be diagnosed by PCR for CMV
DNA and virus isolation from the amniotic fluid, but
PCR does not predict adverse fetal outcomes.68 As
for newborns, isolation of CMV in the infant’s urine
within the first 2 weeks of life is the standard for
diagnosing congenital infection.59
While no prenatal treatment has yet been shown
to reduce in-utero infections or sequelae, antiviral
treatment with ganciclovir and valganciclovir has
been proven to reduce the risk of sensorineural
hearing loss and improve neurodevelopmental
outcomes, especially when treatment is initiated
within the first month after birth in symptomatic
infants and continued for 6 months.73 Meanwhile,
more evidence is needed to justify the risks and
benefits of antiviral treatment in asymptomatic
infants to devise a suitable treatment scheme that helps to minimise the risk of development of severe
sequelae later in life.
Currently, systemic screening for CMV
primary infection is not performed in any one
country.59 Risk reduction strategies should focus
on preventing maternal primary infection during
pregnancy. Thus, proper hygiene practices (eg, hand
washing), reducing maternal exposure to body fluids
that contain the virus (eg, saliva and urine of children
with infection), and access to clean running water
are effective measures to reduce CMV infections.60
H – Congenital herpes simplex virus
Herpes simplex viruses types 1 and 2 are
herpesviruses, which can establish lifelong latency
after the primary infection.74 75 Both types of HSV can
cause genital herpes, which when acquired during
pregnancy may pose a risk of vertical transmission to
the neonate.76 Neonatal HSV infection is estimated
to occur in 1 in 3000 to 20 000 livebirths, but it is rare
in Hong Kong, with only one case of neonatal HSV
type 1 infection reported from 2008 to 2010.75 77
In Hong Kong, HSV infection tends to
be acquired early in life, with a gradual rise in
seropositivity in childhood and a drastic rise in young
adults.64 Infection with HSV occurs through direct
contact of mucosal or abraded skin surfaces with
infectious secretions or lesions, commonly found in
the oral and genital regions.9 Approximately 5% of
neonatal HSV infections occur in utero, 85% during
the peripartum period, and the remaining 10%
postnatally, through direct contact with infectious
lesions or secretions.78 Peripartum infection is
mainly caused by direct contact with infected genital
lesions in the birth canal.79 80 The risk of vertical
transmission is higher in mothers with newly
acquired genital HSV infections than those with
HSV reactivation, with 57% and 2% increases in risk,
respectively.79 80 81 The presence of maternal antibodies
to either HSV subtype seems to offer a certain
degree of protection against neonatal transmission.
Even antibodies against HSV-2 seem to have a
protective effect against neonatal transmission of
both HSV subtypes, women of all HSV serological
statuses can transmit the virus to neonates.79 Genital
infection occurring closer to term incurs a higher
risk of neonatal transmission, with 50% to 80%
risk of neonatal infections resulting from maternal
genital HSV infections close to term.79
The majority of both newly acquired or
recurrent genital HSV infections are asymptomatic
or too mild to be recognised or properly diagnosed,
such that no known history of genital HSV infection
was found in close to 80% of mothers who delivered
an infected infant.9 82
In-utero HSV infection accounts for only
5% of all neonatal HSV infections.9 The triad of
cutaneous, CNS, and ophthalmic findings are typical in this group of infants. Common cutaneous
presentations include vesicles, scarring, aplasia
cutis, and hypopigmentation/hyperpigmentation.
Neurological findings include microcephaly,
intracranial calcifications, and hydranencephaly,
while ophthalmic manifestations typically include
chorioretinitis, microphthalmia, and optic
atrophy.9 76
Peripartum and postnatal HSV infections
share similar clinical manifestations. They can
generally be categorised into three main groups:
disease localised to the skin, eyes, or mouth (SEM
disease); disease localised to the CNS (CNS disease);
and disseminated disease.78 Approximately 45%
of all cases are categorised as SEM disease, and
these cases typically include vesicles on the skin,
keratoconjunctivitis of the eye, and infection of
the oropharynx. Approximately 30% of cases are
categorised as CNS disease, which is mainly caused
by meningoencephalitis and can present with focal
or generalised seizures, fever, lethargy, irritability,
tremors, poor oral intake, temperature instability,
bulging fontanelle, or pyramidal tract signs.9 78
Disseminated disease involving multiple visceral
organs, such as the lungs, liver, heart, and brain,
occurs in the remaining 25% of cases.78 It presents
with irritability, seizures, respiratory failure,
hepatic failure, jaundice, disseminated intravascular
coagulopathy, and shock. Disseminated disease is
associated with a mortality rate of 29%.78
Diagnosis of neonatal HSV infection is
challenging because of its non-specific presentation.
All of the following specimens should be obtained
and sent for PCR assay: surface specimens (mouth,
nasopharynx, conjunctivae, anus), skin vesicles, CSF,
and whole blood.22 Other organ involvement can be
screened by laboratory tests and imaging, such as
alanine transaminase levels as indicator of hepatic
involvement; chest X-ray for lung involvement;
and neuroimaging and electroencephalogram as
indicators of CNS disease.
Antiviral treatment with intravenous acyclovir
is recommended for neonates with HSV infection.
For infants with disseminated or CNS disease, 21
days of antiviral therapy are indicated. For those with
SEM disease, 14 days of treatment is recommended.22
The 14-day treatment is also recommended for
asymptomatic neonates born to mothers who are
infected with HSV close to term.22 The emergence
of antiviral treatment for HSV infection has
contributed to a drastic reduction in the 12-month
mortality rate of infants with disseminated HSV
disease, from 85% to 29% overall, and from 50% to
only 4% in those with CNS disease.78 80 Morbidity
rates and long-term outcomes have also been
improved with the use of antiviral agents, especially
when treatment is initiated as soon as possible with
an early diagnosis.9 78
As the majority of neonatal HSV infections
are peripartum, reducing neonates’ exposure to
active genital lesions during delivery is important
for prevention of transmission. Active genital
HSV lesions or presence of prodromal symptoms
at the time of delivery are indications for delivery
by Caesarean section, which has been found to be
effective in preventing neonatal transmission.76 Oral
acyclovir or valacyclovir as antiviral suppression
therapy, initiated at 36 weeks of gestation, is
associated with reduced genital lesions and
decreased viral detection by viral culture or PCR at
the time of delivery.76 78 More studies are required to
establish its safety and effectiveness as prophylaxis
against neonatal HSV infection.
Counselling on prevention of unprotected
sexual contact during pregnancy, especially late
pregnancy, may reduce the risk of maternal HSV
infection and thus neonatal infection through
vertical transmission.80
Diagnosing TORCH infection
‘TORCH titres’ are often ordered by clinicians who suspect congenital infections; however, various
studies have shown that the diagnostic yield is very
low if they are not used appropriately.83 84 Maternal
IgG antibodies can cross the placenta and IgG antibody titres from one blood sample might not
be adequate for diagnosis. Therefore, appropriate
follow-up testing should be arranged to evaluate
the levels of IgG-specific antibody of concern, and
whenever possible, isolation of the organism should
be attempted.83 Targeted specimens and tests
rather than a TORCH screen should be sent if
the clinical suspicion for a specific congenital
infection is strong after reviewing the maternal
history and infant’s clinical features. The clinical
features, antenatal screening, diagnostic testing,
and treatment of congenital toxoplasmosis, syphilis,
rubella, CMV, and HSV are summarised in the
Table.9 10 13 15 18 19 20 22 31 36 4147 50 53 57 69 71 81 84
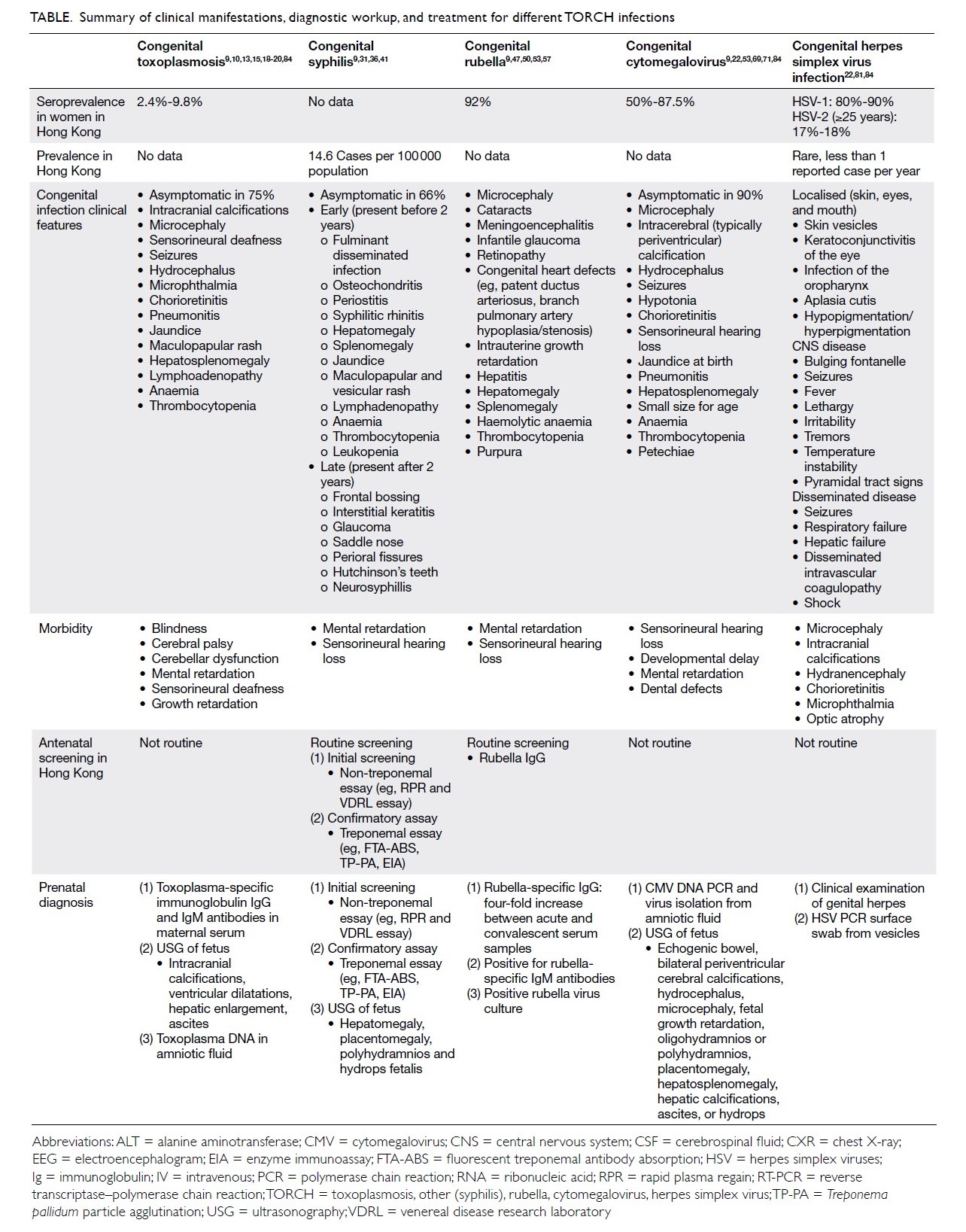
Table. Summary of clinical manifestations, diagnostic workup, and treatment for different TORCH infections
Conclusion
Despite being densely populated, Hong Kong
has relatively low rates of congenital infections.
Nevertheless, it is important to stay vigilant for
infections during pregnancy that may lead to severe
disabilities or even death of the fetus. In Hong Kong,
comprehensive antenatal services that are available to
eligible mothers have been provided free of charge by
the public sector in collaboration with the Maternal
and Child Health Centres (under the Department of
Health) and hospital Obstetrics Departments (under
the Hospital Authority). Pregnant women registered for the shared care system are entitled to receive
regular antenatal check-ups, postnatal services, and
close monitoring of the pregnancy.
Prevention is better than intervention, especially
in the context of congenital infections. Primary
prevention of maternal infection during pregnancy is
the key to preventing congenital infections. Effective
measures for primary prevention are available, and
during the first antenatal visit, screening for rubella,
syphilis, and HIV are performed. Available resources
should focus on promoting public health.
Author contributions
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Concept or design: KL Hon.
Acquisition of data: KKY Leung, A Yeung.
Analysis or interpretation of data: KKY Leung, A Yeung.
Drafting of the article: KL Hon, KKY Leung, A Yeung.
Critical revision for important intellectual content: KL Hon, KKY Leung, AKC Leung, E Man.
Acquisition of data: KKY Leung, A Yeung.
Analysis or interpretation of data: KKY Leung, A Yeung.
Drafting of the article: KL Hon, KKY Leung, A Yeung.
Critical revision for important intellectual content: KL Hon, KKY Leung, AKC Leung, E Man.
Conflicts of interest
As an editor of the journal, KL Hon was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. DeVore NE, Jackson VM, Piening SL. TORCH infections. Am J Nurs 1983;83:1660-5. Crossref
2. Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol 2015;42:77-103. Crossref
3. Stegmann BJ, Carey JC. TORCH infections. Toxoplasmosis,
other (syphilis, varicella-zoster, parvovirus B19), rubella,
cytomegalovirus (CMV), and herpes infections. Curr
Womens Health Rep 2002;2:253-8.
4. Schwartz DA. The origins and emergence of Zika virus,
the newest torch infection: what’s old is new again. Arch
Pathol Lab Med 2017;141:18-24. Crossref
5. Nahmias AJ, Walls KW, Stewart, JA, Herrmann KL, Flynt
WJ Jr. The ToRCH complex-perinatal infections associated
with toxoplasma and rubella, cytomegol- and herpes
simplex viruses. Pediatr Res 1971;5:405-6. Crossref
6. Fuerst HT. Flame or bird? Pediatrics 1975;56:107. Crossref
7. Brumback RA. TORCHES. Pediatrics 1976;58:916.
8. World Health Organization. Global validation of
elimination of mother-to child transmission (EMTCT) of
HIV and syphilis. 2019. Available from: https://www.who.
int/reproductivehealth/congenital-syphilis/emtc-gvac/
en/. Accessed 3 Oct 2019.
9. Wilson CB, Nizet V, Maldonado Y, Remington JS, Klein JO.
Remington and Klein’s Infectious Diseases of the Fetus and
Newborn Infant. 8th ed. Philadelphia: Elsevier Saunders;
2016: 513, 520-5, 675, 677-9, 685-90, 693-4, 729, 732.
10. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965-76. Crossref
11. McAuley JB. Congenital toxoplasmosis. J Pediatric Infect
Dis Soc 2014;3 Suppl 1:S30-5. Crossref
12. Dunn D, Wallon M, Peyron F, Petersen E, Peckham C,
Gilbert R. Mother-to-child transmission of toxoplasmosis:
risk estimates for clinical counselling. Lancet
1999;353:1829-33. Crossref
13. Ko RC, Wong FW, Todd D, Lam KC. Prevalence of
Toxoplasma gondii antibodies in the Chinese population of
Hong Kong. Trans R Soc Trop Med Hyg 1980;74:351-4. Crossref
14. Torgerson PR, Mastroiacovo P. The global burden of
congenital toxoplasmosis: a systematic review. Bull World
Health Organ 2013;91:501-8. Crossref
15. Serranti D, Buonsenso D, Valentini P. Congenital
toxoplasmosis treatment. Eur Rev Med Pharmacol Sci
2011;15:193-8.
16. Tamma P. Toxoplasmosis. Pediatr Rev 2007;28:470-1. Crossref
17. Wilson CB, Remington JS, Stagno S, Reynolds DW.
Development of adverse sequelae in children born with
subclinical congenital Toxoplasma infection. Pediatrics
1980;66:767-74.
18. Sever JL, Ellenberg JH, Ley AC, et al. Toxoplasmosis:
maternal and pediatric findings in 23,000 pregnancies.
Pediatrics 1988;82:181-92.
19. Gay-Andrieu F, Marty P, Pialat J, Sournies G, Drier de
Laforte T, Peyron F. Fetal toxoplasmosis and negative
amniocentesis: necessity of an ultrasound follow-up.
Prenat Diagn 2003;23:558-60. Crossref
20. Cortina-Borja M, Tan HK, Wallon M, et al. Prenatal
treatment for serious neurological sequelae of congenital
toxoplasmosis: an observational prospective cohort study.
PLoS Med 2010;7(10). pii: e1000351. Crossref
21. Pomares C, Montoya JG. Laboratory diagnosis of congenital
toxoplasmosis. J Clin Microbiol 2016;54:2448-54. Crossref
22. Kimberlin DW, Brady MT, Jackson MA, editors. Red Book
2018: Report of the Committee on Infectious Diseases.
31st ed. Itasca; American Academy of Pediatrics; 2018:
310-7, 437-48, 459-76, 773-88, 809-19, 8698.
23. Chik TS, Tsang OT. Toxoplasmosis. HIV manual. 4th ed.
2019. Available from: https://hivmanual.hk/d27/. Accessed
7 Oct 2019.
24. Cook AJ, Gilbert RE, Buffolano W, et al. Sources of
toxoplasma infection in pregnant women: European
multicentre case-control study. European Research
Network on Congenital Toxoplasmosis. BMJ 2000;321:142-
7. Crossref
25. Halonen SK, Weiss LM. Toxoplasmosis. Handb Clin
Neurol 2013;114:125-45. Crossref
26. Jones JL, Krueger A, Schulkin J, Schantz PM. Toxoplasmosis
prevention and testing in pregnancy, survey of obstetriciangyn-aecologists.
Zoonoses Public Health 2010;57:27-33. Crossref
27. Sagel U, Krämer A. Screening of maternal toxoplasmosis in
pregnancy: laboratory diagnostics from the perspective of
public health requirements. J Bacteriol Parasitol 2013;S5.
28. Cornu C, Bissery A, Malbos C, et al. Factors affecting
the adherence to an antenatal screening programme: an
experience with toxoplasmosis screening in France. Euro
Surveill 2009;14:21-5.
29. Paquet C, Yudin MH; Society of Obstetricians and
Gynaecologists of Canada. Toxoplasmosis in pregnancy:
prevention, screening, and treatment [in English, French].
J Obstet Gynaecol Can 2013;35:78-81. Crossref
30. World Health Organization. The global elimination of congenital syphilis: rationale and strategy for
action. 2017. Available from: https://apps.who.int/iris/
handle/10665/43782. Accessed 8 Oct 2019.
31. Special Preventive Programme, Centre for Health
Protection, Department of Health, Hong Kong SAR
Government. Hong Kong STD/AIDS update—a quarterly
surveillance report. Vol 23 No 4, 2017. Available from:
https://www.chp.gov.hk/files/pdf/std17q4.pdf. Accessed 8
Oct 2019.
32. Centers for Disease Control and Prevention, US
Government. Sexually transmitted disease surveillance.
Primary and secondary syphilis—rates of reported cases
by state, United States and outlying areas, 2017. Available
from: https://www.cdc.gov/std/stats17/figures/37.htm.
Accessed 3 Oct 2019.
33. Fiumara NJ. Syphilis in newborn children. Clin Obstet
Gynecol 1975;18:183-9. Crossref
34. Jenson HB. Congenital syphilis. Semin Pediatr Infect Dis
1999;10:183-94. Crossref
35. Herremans T, Kortbeek L, Notermans DW. A review of
diagnostic tests for congenital syphilis in newborns. Eur J
Clin Microbiol Infect Dis 2010;29:495-501. Crossref
36. De Santis M, De Luca C, Mappa I, et al. Syphilis infection
during pregnancy: fetal risks and clinical management.
Infect Dis Obstet Gynecol 2012;2012:430585. Crossref
37. Leung AK, Leong KF, Lam JM. A case of congenital syphilis
presenting with unusual skin eruptions. Case Rep Pediatr
2018;2018:1761454. Crossref
38. Lago EG, Vaccari A, Fiori RM. Clinical features and follow-up
of congenital syphilis. Sex Transm Dis 2013;40:85-94. Crossref
39. Woods CR. Syphilis in children: congenital and acquired.
Semin Pediatr Infect Dis 2005;16:245-57. Crossref
40. Mannelli L, Perez FA, Parisi MT, Giacani L. A case of
congenital syphilis. Emerg Radiol 2013;20:337-9. Crossref
41. Public Health Agency of Canada. Government of Canada.
Section 5-10: Canadian guidelines on sexually transmitted
infections—management and treatment of specific
infections—syphilis. 2019. Available from: https://www.
canada.ca/en/public-health/services/infectious-diseases/
sexual-health-sexually-transmitted-infections/canadianguidelines/
sexually-transmitted-infections/canadianguidelines-
sexually-transmitted-infections-27.html.
Accessed 3 Oct 2019.
42. Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel
GD Jr. Efficacy of treatment for syphilis in pregnancy.
Obstet Gynecol 1999;93:5-8. Crossref
43. Sheffield JS, Sánchez PJ, Morris G, et al. Congenital syphilis
after maternal treatment for syphilis during pregnancy.
Am J Obstet Gynecol 2002;186:569-73. Crossref
44. Duthie SJ, King PA, Yung GL, Ma HK. Routine serological
screening for syphilis during pregnancy—disposable
anachronism or fundamental necessity? Aust N Z J Obstet
Gynaecol 1990;30:29-31. Crossref
45. Ho FW. Update on rubella in Hong Kong. Commun Dis
Watch 2017;14:66-8.
46. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Number of notifiable infectious
diseases by month. 2019. Available from: https://www.chp.
gov.hk/en/static/24012.html. Accessed 3 Oct 2019.
47. Leung AK, Hon KL, Leong KF. Rubella (German measles)
revisited. Hong Kong Med J 2019;25:134-41. Crossref
48. Miller E, Cradock-Watson JE, Pollock TM. Consequences
of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982;2:781-4. Crossref
49. Dontigny L, Arsenault MY, Martel MJ; Clinical Practice
Obstetrics Committee. Rubella in pregnancy. J Obstet
Gynaecol Can 2008;30:152-8. Crossref
50. Reef SE, Plotkin S, Cordero JF, et al. Preparing for
elimination of congenital rubella syndrome (CRS):
summary of a workshop on CRS elimination in the United
States. Clin Infect Dis 2000;31:85-95. Crossref
51. Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L.
Measles, mumps, and rubella—vaccine use and strategies
for elimination of measles, rubella, and congenital rubella
syndrome and control of mumps: recommendations of the
Advisory Committee on Immunization Practices (ACIP).
MMWR Recomm Rep 1998;47:1-57.
52. Rubella vaccines: WHO position paper [editorial] [in
English, French]. Wkly Epidemiol Rec 2011;86:301-16.
53. World Health Organization. Manual for the laboratory
diagnosis of measles and rubella virus infection Second
edition. 2017. Available from: https://www.who.int/ihr/
elibrary/manual_diagn_lab_mea_rub_en.pdf. Accessed 8
Oct 2019.
54. Bart SW, Stetler HC, Preblud SR, et al. Fetal risk associated
with rubella vaccine: an update. Rev Infect Dis 1985;7
Suppl 1:S95-102. Crossref
55. Centers for Disease Control (CDC). Rubella vaccination
during pregnancy—United States, 1971-1988. MMWR
Morb Mortal Wkly Rep 1989;38:289-93.
56. Food and Health Bureau, Hong Kong SAR Government.
Hong Kong reference framework for preventive care
for children in primary care settings. 2012. Available
from: https://www.fhb.gov.hk/pho/english/health_
professionals/professionals_preventive_children_pdf.
html. Accessed 10 Oct 2019.
57. Lao TT, Suen SS, Leung TY, Sahota DS, Lau TK.
Universal rubella vaccination programme and maternal
rubella immune status: a tale of two systems. Vaccine
2010;28:2227-30. Crossref
58. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ.
Intrauterine transmission of cytomegalovirus to infants
of women with preconceptional immunity. N Engl J Med
2001;344:1366-71. Crossref
59. Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini
MP. Update on the prevention, diagnosis and management
of cytomegalovirus infection during pregnancy. Clin
Microbiol Infect 2011;17:1285-93. Crossref
60. Cannon MJ. Congenital cytomegalovirus (CMV)
epidemiology and awareness. J Clin Virol 2009;46 Suppl
4:S6-10. Crossref
61. Boppana SB, Ross SA, Fowler KB. Congenital
cytomegalovirus infection: clinical outcome. Clin Infect
Dis 2013;57 Suppl 4:S178-81. Crossref
62. Centre for Health Protection, Department of Health,
Hong Kong SAR Government. Seroprevalence rates of
cytomegalovirus antibodies. 2018. Available from: https://
www.chp.gov.hk/en/statistics/data/10/641/701/6445.html.
Accessed 5 Oct 2019.
63. Leung AK, Loong EP, Chan RC, Murray HG, Chang
AM. Prevalence of cytomegalovirus cervical excretion in
pregnant women in Hong Kong. Asia Oceania J Obstet
Gynaecol 1989;15:77-8. Crossref
64. Kangro HO, Osman HK, Lau YL, Heath RB, Yeung CY, Ng
MH. Seroprevalence of antibodies to human herpesviruses
in England and Hong Kong. J Med Virol 1994;43:91-6. Crossref
65. Kenneson A, Cannon MJ. Review and meta-analysis of
the epidemiology of congenital cytomegalovirus (CMV)
infection. Rev Med Virol 2007;17:253-76. Crossref
66. Wang C, Zhang X, Bialek S, Cannon MJ. Attribution
of congenital cytomegalovirus infection to primary
versus non-primary maternal infection. Clin Infect Dis
2011;52:e11-3. Crossref
67. Bodéus M, Hubinont C, Goubau P. Increased risk of
cytomegalovirus transmission in utero during late
gestation. Obstet Gynecol 1999;93(5 Pt 1):658-60. Crossref
68. Nigro G. Maternal-fetal cytomegalovirus infection:
from diagnosis to therapy. J Matern Fetal Neonatal Med
2009;22:169-74. Crossref
69. Leung AK, Sauve RS, Davies HD. Congenital
cytomegalovirus infection. J Natl Med Assoc 2003;95:213-
8.
70. Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S.
Congenital cytomegalovirus infection following first
trimester maternal infection: symptoms at birth and
outcome. J Clin Virol 2006;35:216-20. Crossref
71. Dollard SC, Grosse SD, Ross DS. New estimates of the
prevalence of neurological and sensory sequelae and
mortality associated with congenital cytomegalovirus
infection. Rev Med Virol 2007;17:355-63. Crossref
72. Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ,
Pass RF. Longitudinal investigation of hearing disorders
in children with congenital cytomegalovirus. J Am Acad
Audiol 2000;11:283-90.
73. Demmler-Harrison GJ. Congenital cytomegalovirus
infection: management and outcome. Available from: https://www.uptodate.com/contents/congenital-cytomegalovirus-infection-management-and-outcome.
Accessed 10 Oct 2019.
74. Leung AK, Barankin B. Herpes labialis: an update. Recent
Pat Inflamm Allergy Drug Discov 2017;11:107-13. Crossref
75. Hon KL, Leung TF, Cheung HM, Chan PK. Neonatal
herpes: what lessons to learn. Hong Kong Med J 2012;18:60-
2.
76. James SH, Sheffield JS, Kimberlin DW. Mother-to-child
transmission of herpes simplex virus. J Pediatric Infect Dis
Soc 2014;3 Suppl 1:S19-23. Crossref
77. Sauerbrei A. Genital herpes. In: Diagnostics to
Pathogenomics of Sexually Transmitted Infections. West
Sussex; John Wiley & Sons Ltd: 2018: 83-99. Crossref
78. Kimberlin DW. Herpes simplex virus infections of the
newborn. Semin Perinatol 2007;31:19-25. Crossref
79. Brown ZA, Selke S, Zeh J, et al. The acquisition of genital
herpes during pregnancy. N Engl J Med 1997;337:509-15. Crossref
80. Corey L, Wald A. Maternal and neonatal herpes simplex
virus infections. N Engl J Med 2009;361:1376-85. Crossref
81. Lee NL, Tse IC. V. Major opportunistic infections. Herpes
simplex and zoster. 2019. Available from: https://www.aids.
gov.hk/pdf/g190htm/25.htm. Accessed 1 Jan 2020.
82. Whitley RJ, Corey L, Arvin A, et al. Changing patterns of
herpes simplex virus infection in neonates. J Infect Dis
1988;158:109-16. Crossref
83. Leland D, French ML, Kleiman MB, Schreiner RL. The use
of TORCH titers. Pediatrics 1983;72:41-3.
84. Lim WL, Wong DA. TORCH screening: time for abolition?
J Hong Kong Med Assoc 1994;46:306.