Hong Kong Med J 2017 Oct;23(5):503–16 | Epub 1 Sep 2017
DOI: 10.12809/hkmj166154
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Cancer screening for older people: to screen or
not to screen
Claudia KY Lai, PhD, RN1;
Ayumi Igarashi, PhD, RN2;
Natalie MY Lau, MN, BA1;
Clare TK Yu, BSc1
1 School of Nursing, The Hong Kong Polytechnic University, Hunghom, Hong Kong
2 School of Health Sciences and Nursing, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Corresponding author: Dr Claudia KY Lai (claudia.lai@polyu.edu.hk)
Abstract
In this scoping review, the evidence of the benefits
of screening older people for the five most common
types of cancer in Hong Kong, namely colorectal, lung,
breast, liver, and prostate cancers, is discussed.
Although cancer treatments can be extensive and a
good prognosis is less likely if cancer is diagnosed at
a late stage, screening programmes for older people
in primary care remain a matter of contention. The
general recommendation for the screening of older
people is to adopt an individualised approach that
takes account of not only age but also co-morbidity,
life expectancy, harms and benefits, and patient’s
preference.
Introduction
Cancer, a word that evokes fear in most people, is
the second leading cause of death worldwide; 8.8
million of deaths due to cancer are estimated to
have occurred in 2015.1 Globally, the most common
types of cancer are lung, breast, bowel, and prostate.2
In Hong Kong, the five leading types of cancer
(combining both males and females) are, in order
of incidence, colorectal, lung, breast, liver, and
prostate cancers.3 Because the incidence of cancer
increases with age, early detection can help reduce
the burden of treatment and is more likely to lead to
a better outcome if the cancer is adequately treated.
Although there are guidelines on the recommended
ages at which to begin screening for different types
of cancer, there is less guidance on the screening
needs of older adults.4
Controversies over cancer
screening for older people
Cancer screening for early detection is promoted
globally because of the link between an ageing
population and an increase in the prevalence of
cancer worldwide, on the supposition that this
will improve the prognosis of cancer patients and
may therefore be beneficial for older people.5 Of
note, 33 countries have joined the International
Cancer Screening Network and are participating
in active population-based screening programmes
for breast, colorectal, cervical, and lung cancers.6
There are practice guidelines recommending the
ages at which to start screening for various types
of cancer, but there is less information about when
to cease screening, specifically in older people.
Epidemiological studies of cancer in people aged 70
years or older are rarely reported in the literature;
even if there are such studies, only subgroup analyses
are found.7 The efficacy of screening older people for
cancer remains controversial. Even though cancer
rates have increased with age, this does not imply
that routine cancer screening is recommended or
even appropriate for older people.8
The aims of this review were to examine
the evidence regarding screening for cancer in
older people and to present an overview of the
current state of knowledge about controversies
and recommendations with regard to screening for
the top five most common kinds of cancer in older
people in Hong Kong.
Methods
A scoping review was conducted to explicate current
discussions of recommendations for screening older
adults for cancer. A scoping review was deemed to be
appropriate because it is often used to address broad
topics, the literature on which may include studies
with numerous designs. The approach described by
Arksey and O’Malley was adopted.9
The databases of MEDLINE, EMBASE, the
Web of Science, the Cochrane Library, CINAHL,
and SCOPUS were searched using the following
strategies:
• Strategy 1—(Elder*) and (Cancer screening or cancer prevention) and (Mortality or morbidity)
• Strategy 2—(Elder*) and (Cancer screening or cancer prevention) and (Effect or efficacy or effective*)
• Strategy 1—(Elder*) and (Cancer screening or cancer prevention) and (Mortality or morbidity)
• Strategy 2—(Elder*) and (Cancer screening or cancer prevention) and (Effect or efficacy or effective*)
Searches were conducted for studies in which
the population was restricted to those aged 65
years or above. The search fields included abstracts
and titles, studies written in English, and studies
published in the last 10 years only (January 2007 to
April 2017). We included only studies that evaluated
screening for five types of cancer (colorectal, lung,
breast, liver, and prostate). Studies that employed
retrospective data analysis, simulation modelling,
and observational, experimental (randomised
controlled trials [RCTs]) or uncontrolled clinical
trials were included. Systematic reviews were also
included, but discussion papers that did not describe
the process by which the database searches were
conducted were excluded. Articles that discussed
knowledge or attitudes to cancer screening and
surveillance monitoring were also excluded.
Three members of the team screened all of
the titles of the papers that were retrieved; papers
that were considered irrelevant were discarded.
Next, irrelevant papers were also excluded after the
abstract and/or the full paper had been reviewed. At
least two members independently read the full text
of all of the papers that were potentially relevant and
selected those that met the criteria for inclusion.
The final selection of papers was achieved through
a series of virtual online discussions that continued
until a consensus was reached.
Other reports relevant to the topic found
or cited in text in the reviewed papers were also
included. As a result, 13 papers were added to the 23
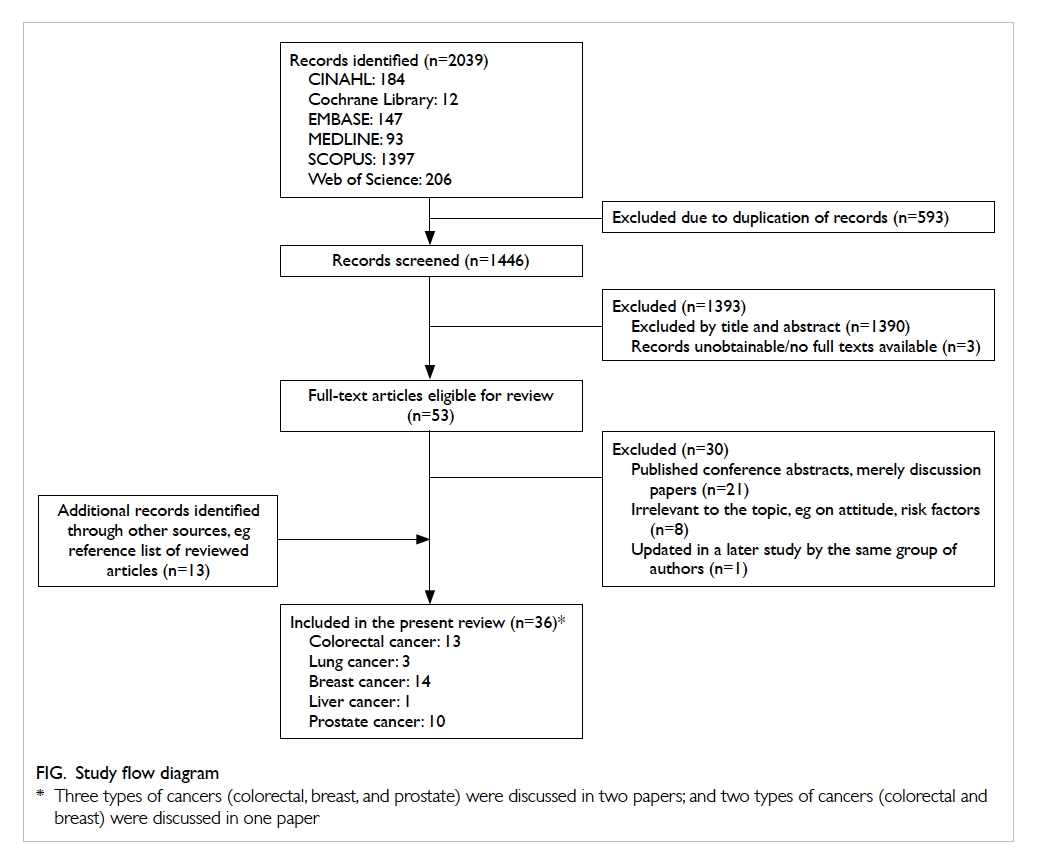
papers from the scoping search. A total of 36 papers
were included for review, with 13, 3, 14, 1, and 10
papers that concerned the screening of colorectal,
lung, breast, liver, and prostate cancers, respectively
(Fig). Three of the papers examined the evidence of
more than one type of cancer and have therefore been
included in different sections for review purposes.
Recommendations for screening guidelines from
national institutes or professional associations have
also been included in each section after discussion of
the reviewed papers. In the papers being reviewed,
‘benefit’ in screening is defined as early detection,
survival, or reduced risk in mortality or co-morbidity;
‘harm’ is defined as mortality (death due
to the specific type of cancer under investigation),
false-positive and false-negative test results, or overdiagnosis.
Colorectal cancer screening
The faecal occult blood test (FOBT), barium enema,
sigmoidoscopy, and colonoscopy are used to screen
for colorectal cancer.4 10 Quarini and Gosney11
reviewed all available articles on colorectal cancer
screening in MEDLINE from 1990 to 2007. They
found limited evidence relating to the screening of
older people.11
In a recent population-based simulation
study, Meester et al12 analysed the number of deaths
from colorectal cancer that were attributable to
non-screening in the US; most such deaths were
attributable to non-screening. Similar findings
have been reported in Germany where screening
colonoscopy has been offered since 2002. Brenner et
al13 14 used simulation modelling based on Germany’s
national data and found that screening colonoscopies
have great potential in the prevention and early
detection of colorectal cancer, with a low risk of
over-diagnosis. Moreover, the majority of prevented
cases would have occurred at the age of 75 years or
older.14 Rozen et al15 also reported that those aged 75
years or older, rather than younger individuals, could
benefit from screening.
Using colorectal cancer–specific mortality
data between 1991 and 2001 obtained from the
National Center for Health Statistics database in
the US, Maheshwari et al16 compared the impact of
prematurely stopping screening with the maximal
potential benefit expected from lifelong screening. A
total of 80% of the maximal benefit from screening
was achieved by screening up to the age of 82 years.
Kahi et al17 examined the survival of older people
after colonoscopy using a retrospective cohort
analysis of those aged 75 years or above and followed
up for a median of 5.95 years. The authors reported
that colonoscopy was safe and yielded clinically
significant findings in 15% of older patients.17
van Hees et al18 used a simulation model
to determine up to what age colorectal cancer
screening should be considered in unscreened older
people with no, moderate, or severe co-morbidity.
They concluded that if the physical condition of
unscreened older people with different co-morbidity
status permits them to undergo a colonoscopy,
screening should be considered up to the ages of
86, 83, and 80 years for no, moderate, and severe
co-morbidity, respectively. van Hees’ team also
reported that fewer co-morbidities were associated
with screening at older ages.19 Lansdorp-Vogelaar et
al20 and Gross et al21 whose studies are included in
this review, also arrived at a similar conclusion.
Not all studies found positive results for
colorectal screening. A longitudinal study by
Fillenbaum et al22 observed no significant association
between cancer screening and population-level
health-related outcomes (including mortality).
Over-diagnosis and complications from
treatment are often concerns in the promotion of
screening. Although colorectal polyps are detected
more frequently in older people, especially those
over the age of 80 years, the view is that to conduct a
colonoscopy in older people is to introduce a higher
risk for only a smaller gain in life expectancy (15%)
than would be the case with younger people.23 Cancer
screening in people older than 75 years remains
controversial because they have not been included
in RCTs on the efficacy of screening studies.10
The guidelines from the National Cancer
Institute,24 the American Cancer Society,25 and
the US Preventive Services Task Force (USPSTF)26
generally recommend that people who are at an
average risk of developing colorectal cancer or
who have a family history of colorectal cancer
or colorectal polyps begin regular screening for
colorectal cancer at the age of 50 years. Specifically,
the USPSTF recommends against screening with
colonoscopy beyond the age of 85 years.27 The
Canadian Task Force on Preventive Health Care
recommends against screening with colonoscopy
at all ages, but it does support the use of FOBT or
faecal immunochemical testing for screening every
alternate year and with sigmoidoscopy every 10
years for those aged 50 to 74 years.27
In Hong Kong, the Department of Health
launched a 3-year Colorectal Cancer Screening Pilot
Programme in 2016 to provide subsidised screening
for those born between 1946 and 1948. The Hong Kong
Anti-Cancer Society recommends that individuals
aged 50 to 75 years with an average risk of developing
colorectal cancer should consider undergoing an
annual FOBT and flexible sigmoidoscopy every 5
years or a colonoscopy every 10 years.28 Overall,
the current literature suggests that colorectal
cancer screening is beneficial, but that age as well as
morbidity and life expectancy should be considered
when determining the age at which screening should
stop. Screening colonoscopy in very elderly patients
(aged ≥80 years) should be performed only after
carefully considering the potential benefits and risks,
and the preferences of the patient. A summary of the
literature review of screening for colorectal cancer is
shown in Table 1.11 12 13 14 15 16 17 18 19 20 21 22 27
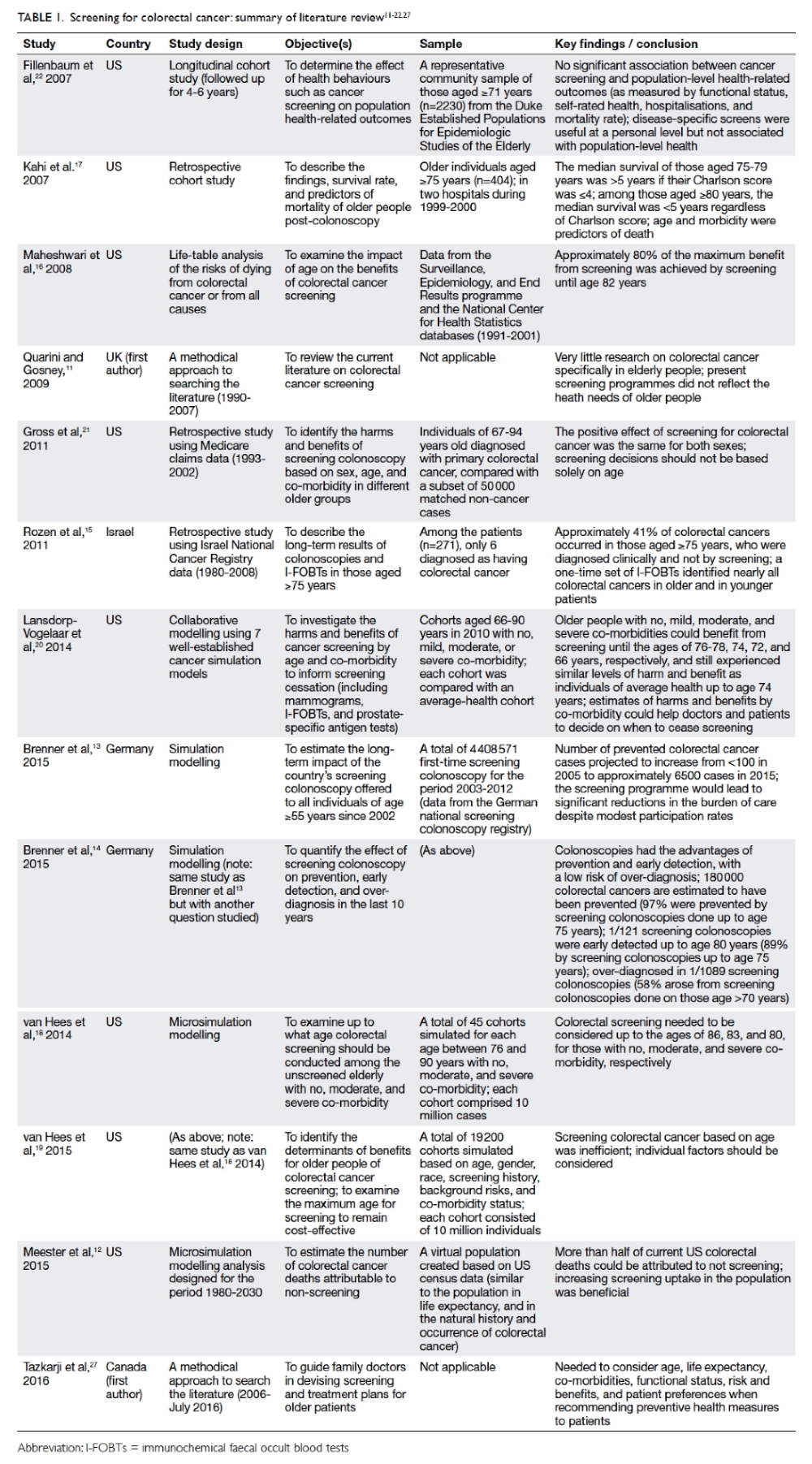
Table 1. Screening for colorectal cancer: summary of literature review11 12 13 14 15 16 17 18 19 20 21 22 27
Lung cancer screening
Chest X-rays, sputum cytology, and low-dose
computed tomography (CT) have been used to
screen for lung cancer.29 Although chest X-rays or
sputum cytology have been used to check for signs
of lung cancer, there is less evidence from RCTs to
show that using them can lead to a reduction in the
number of associated deaths.29 There is, however,
some evidence from large-scale RCTs that low-dose
CT screening can reduce lung cancer deaths.30
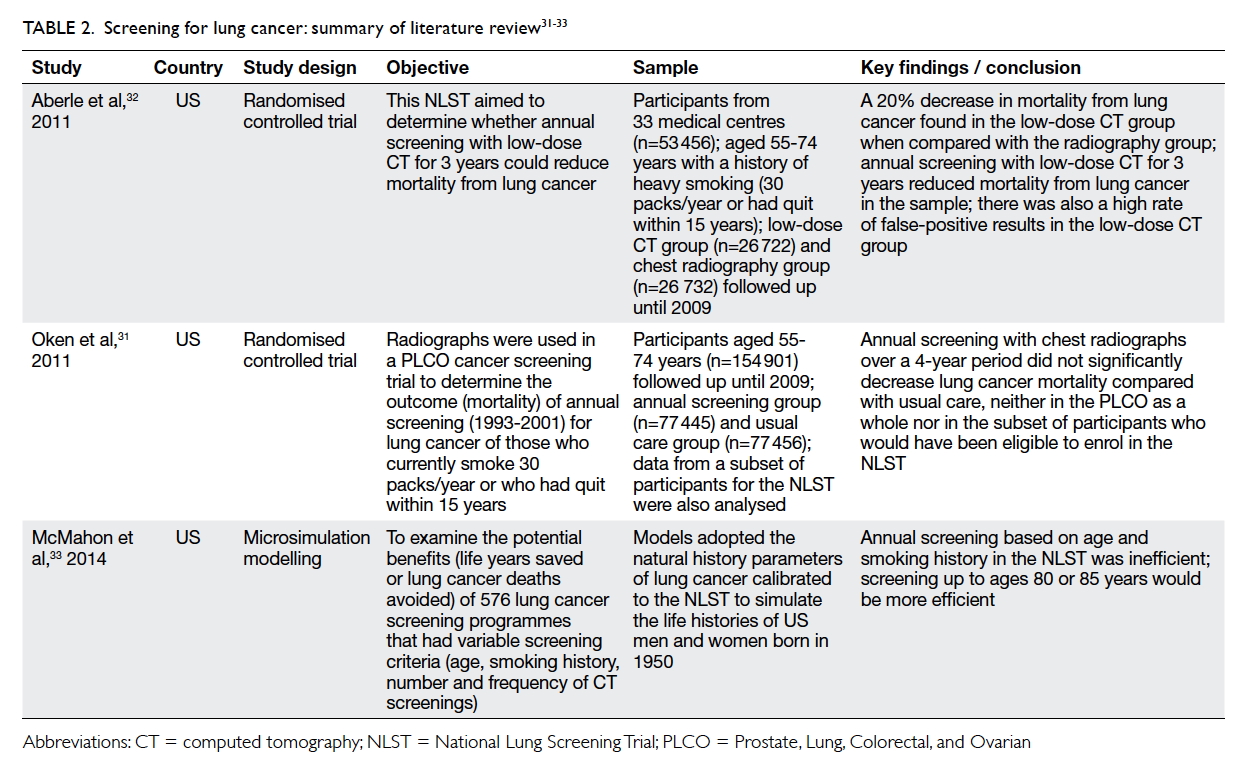
Oken et al31 compared annual chest
radiographic screening with the usual care in the
Prostrate, Lung, Colorectal, and Ovarian (PLCO)
cancer screening trial. The participants were aged 55
to 74 years and were heavy smokers. Radiographic
screening did not reduce lung cancer mortality
when compared with the usual care. Aberle et al’s
National Lung Screening Trial32 in the US examined
the effects of lung cancer screening by low-dose
CT for participants aged 55 to 74 years who were
either current or former (within the past 15 years)
heavy smokers (at least 30 packs of cigarettes/year).
An annual low-dose CT for 3 years reduced 20% of
deaths from lung cancer when compared with chest
radiography.
Using simulation modelling, McMahon et al33
examined the potential benefits (life years saved
or lung cancer deaths avoided) of 576 lung cancer
screening programmes that included a variety of
screening criteria in terms of age, smoking history,
and the number and frequency of CT screenings.
They concluded that it would be more efficient
(measured in terms of the number of cancer deaths
compared with no screening) if screening were
extended to the age of 80 or 85 years. The potential
harm of low-dose CT, however, should also be noted,
such as false-positive and false-negative results, overdiagnosis,
exposure to radiation, and an emotional
toll on the individual concerned.
Based on the National Lung Screening Trial,32
a systematic evidence review30 and modelling
studies, the USPSTF has updated their guidelines.
They now recommend that people aged 55 to 80
years who currently smoke or who have quit smoking within
the past 15 years should undergo annual screening
with low-dose CT.34 The USPSTF also recommends
that screening be discontinued for those who have
quit smoking for 15 years or who have developed
a health problem that substantially limits their life
expectancy or their ability or willingness to undergo
curative lung surgery.30 The Hong Kong Anti-Cancer
Society does not recommend any routine screening
for the general population. Considering both life
expectancy and co-morbidity status would help
physicians to decide the necessity of screening in
different individuals.35 A summary of the literature
review of screening for lung cancer is shown in Table 2.31 32 33
Breast cancer screening
Clinical breast examinations and breast self-examinations,
mammograms, ultrasound, and
magnetic resonance imaging, are all measures to
screen for breast cancer, with the mammogram
being the most widely used test.
A systematic review by Galit et al36 suggested
that with a reasonable life expectancy and without
severe co-morbidities, women aged 75 years or
above are likely to benefit from mammography. Mo
et al37 found that mammography screening alone for
Chinese women over the age of 70 years with positive
clinical breast examination results would save on
the cost of ultrasonography without any loss in the
effectiveness of screening. When co-morbidity and
screening are considered, Lansdorp-Vogelaar et al20
showed that the benefits of a biennial mammography
existed until the median ages of 76, 74, 72, and 66
years for older women with no, mild, moderate, or
severe co-morbidity, respectively.
The EUROSCREEN Working Group reviewed
observational studies and reported that the reduction
of the breast cancer mortality rate was 38% to 48%
for women who had actually been screened, with
the rate of over-diagnosis of only 6.5%.38 39 They argued
that the current controversy is related to the use of
inappropriate methods that are incapable of revealing
the true effect of screening, and that population-based
mammography screening is of greater benefit
than harm.
Using simulation modelling, Tejada et al40
evaluated seven screening policies to determine
which combination of upper age limit and screening
interval could maximise screening benefits for
older women. Annual screening with an upper age
limit of 80 years was found to be most effective in
increasing the survival rate.40 Similarly, Sanderson et
al41 found a significant reduction in the breast cancer
mortality rate of older women who underwent an
annual mammography compared with those who
underwent a biennial or irregular mammography.
Some researchers have nonetheless taken a
contrary view. Fillenbaum et al22 found no significant
association between breast cancer screening and
health-related outcomes such as self-rated health
and mortality. Similarly, the benefits of screening
were found to be limited due to a huge number of
cases of over-diagnosis in the older population.5
Mandelblatt et al42 evaluated the effectiveness of
20 different mammography screening programmes
using six established models of cancer incidence
and mortality trends in the US. They reported that
if the age of cessation was set at after 69 years, the
reduction in mortality would be slight.
A Cochrane review examined the effect of
mammography screening in terms of mortality and
morbidity in a total sample of 600 000 women.43
Screening led to a 15% reduction in mortality but
the over-diagnosis and over-treatment rate was 30%.
The authors suggested that screening might not be
doing more good than harm.43 Of note, only one
trial in this Cochrane review included women up
to the age of 69 years. In their retrospective cohort
study, Parvinen et al44 found that a mammography
screening programme for women up to the age of 74
years effectively reduced mortality rates in the older
population, but that the reduction in rate was only
20%. Their conclusion was that the gain in benefits
may not justify the harm from screening.
Mammography screening is unlikely to benefit
those with a life expectancy of less than 5 years as
reported in Tazkarji et al’s study.27 Braithwaite et al45
found that the benefits of screening decrease with
increasing age and co-morbidity. Their sample of
women aged 65 years or above without severe co-morbidity
showed only a slight improvement in life
expectancy. They, too, argued that the magnitude
of the benefit may not justify screening given the
potential harm. Citing the Canadian National
Breast Screening Study with 25 years of follow-up,
the National Cancer Institute in the US concluded
that the benefits of mammography screening are
uncertain.46
In summary, the overall effect of breast cancer
screening in older women remains a controversial
topic. Currently, the American Cancer Society
suggests that women who are at an ‘average
risk’ of developing breast cancer have an annual
mammogram starting at the age of 40 years,
with no specific age mentioned as the marker for
discontinuation, while the USPSTF now suggests
that regular screening should start from the age of
50 years and end at the age of 74 years. The Hong
Kong Breast Cancer Foundation recommends that
women over the age of 40 years consider undergoing
a mammography every 2 years.47 A summary of the
literature review of screening for breast cancer is
shown in Table 3.5 20 22 27 36 37 38 39 40 41 42 43 44 45
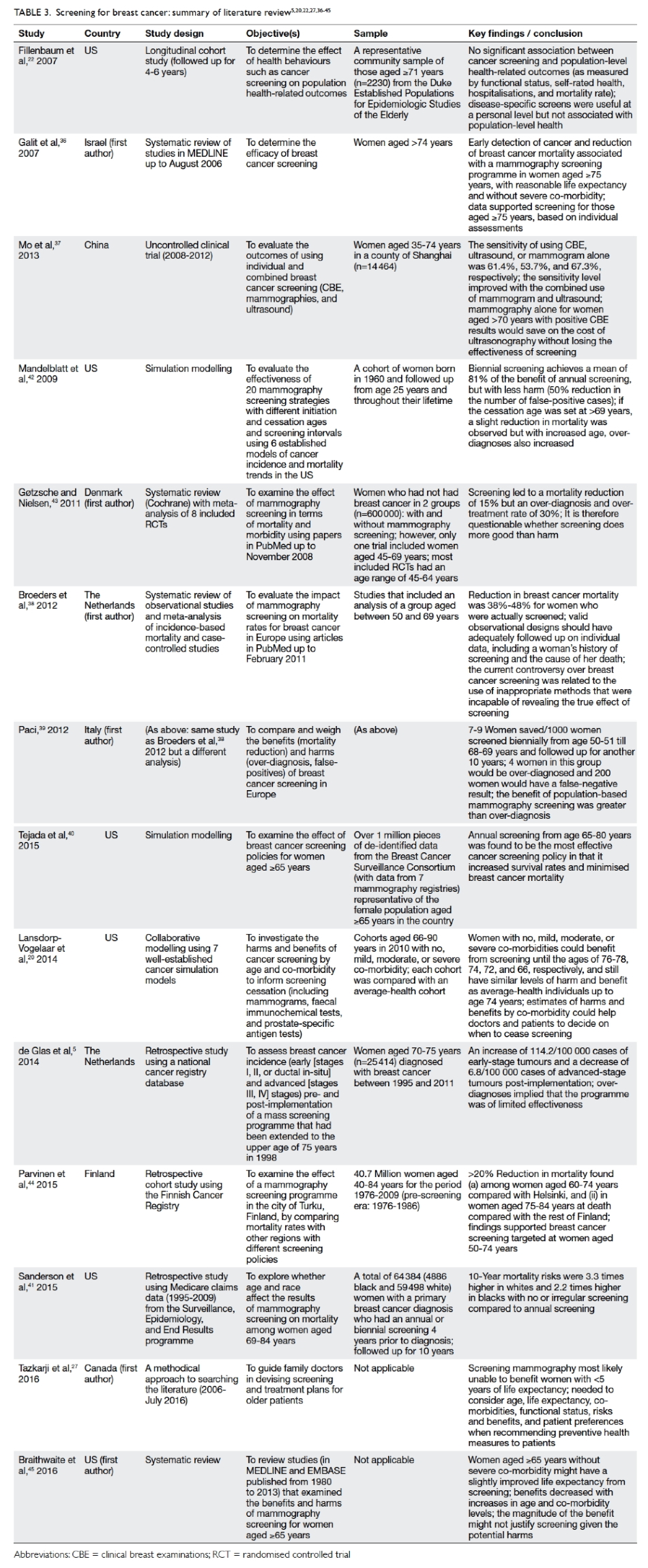
Table 3. Screening for breast cancer: summary of literature review5 20 22 27 36 37 38 39 40 41 42 43 44 45
Liver cancer screening
There are no widely recommended tests to screen
for liver cancer among the general public except for
those who are at a high risk of developing the disease.
Alpha-fetoprotein (AFP) and abdominal ultrasound
are the two most common tests in use. Nonetheless
the sensitivity and specificity levels of AFP are
unsatisfactory.48 This protein has also been shown to
be unreliable in detecting small liver cancer.
There is hardly any evidence of the benefits
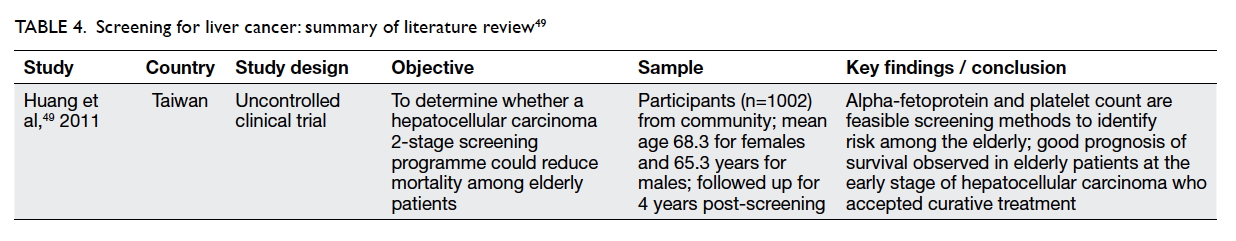
of screening older people for liver cancer. Huang
et al49 conducted a two-stage community screening
programme (first with blood tests and then by
ultrasonogram for identified high-risk cases) on a
sample of 1002 people with a mean age of 68.3 years
for women and men in an area where the hepatitis C
virus is endemic. They observed that older patients
who had early-stage hepatocellular carcinoma and
who were being treated had a good prognosis for
survival.
Currently, the American Cancer Society offers
no recommendations for liver cancer screening
in the general population. Cancer Research UK
recommends a liver screening test only for high-risk
individuals.50 Similarly, the Hong Kong Department
of Health does not recommend routine cancer
screening for people at ‘average risk’—only for
those at high risk, such as carriers of the hepatitis
B and/or C viruses and those with cirrhosis.51 In
summary, screening tests for liver cancer should
not be performed in a routine manner but should
be recommended only for people who are at a high
risk of developing the disease. A summary of the
literature review of screening for liver cancer is
shown in Table 4.49
Prostate cancer screening
Prostate-specific antigen (PSA) testing, digital
rectal examination, and prostate biopsy are the
three main approaches usually used in combination
with screening prostate cancer.4 There is no single
effective and reliable test to screen for early prostate
cancer in healthy men.50 It is not uncommon for men
to have some cancer cells in their prostate by the age
of 80 years although only one in 25 will actually die
from prostate cancer.50 To date, the debate over PSA
screening remains heated even though screening
was first introduced in the late 1980s.
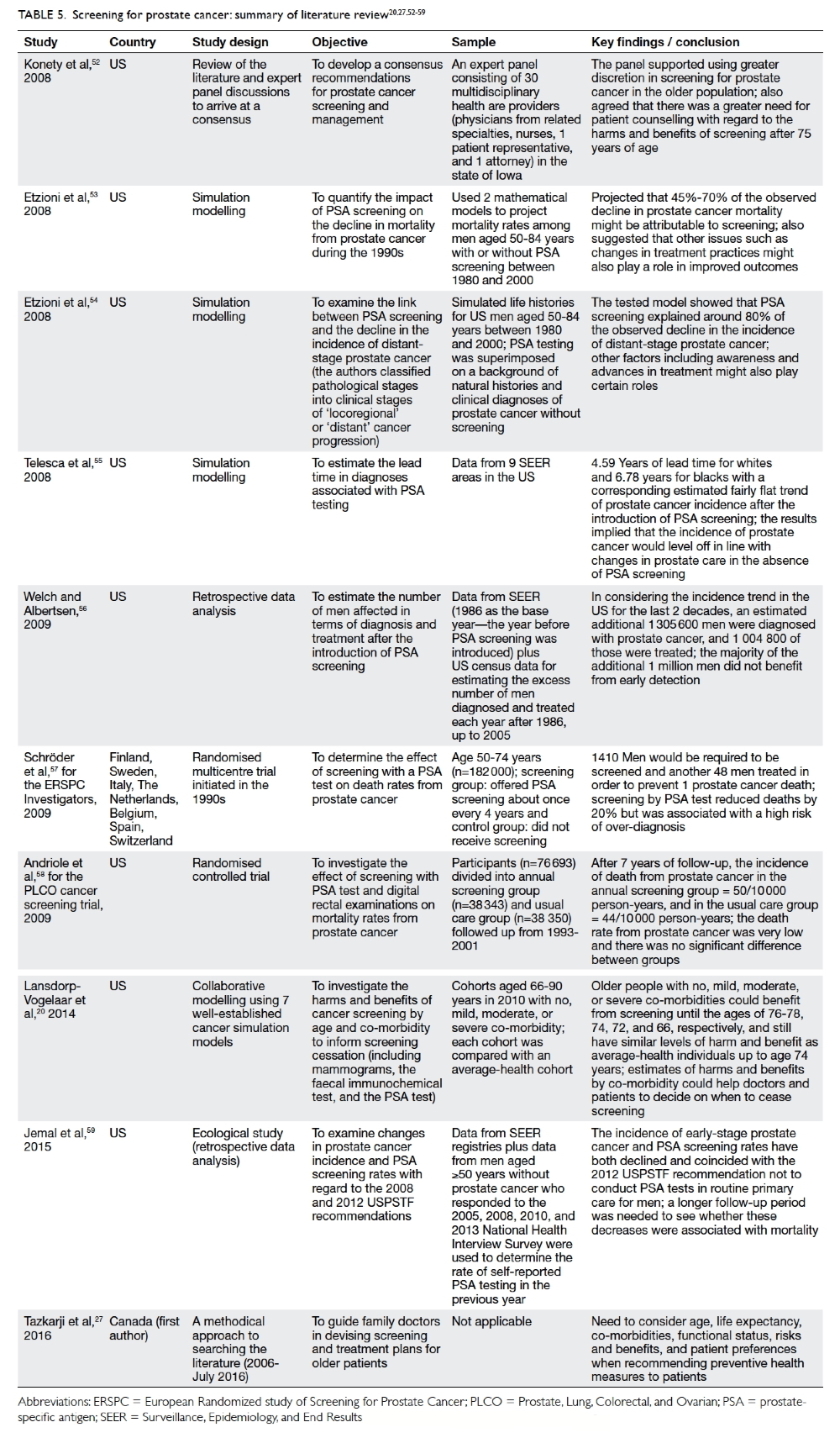
Konety et al52 reported the work of a 30-member panel of US experts who recommended that
the initiation of screening for prostate cancer in men
older than 75 years should be undertaken only after
careful consideration, and that age-normed PSA
values should be used to determine ‘normal’ levels.
Using mathematical modelling, Etzioni et al53
found that by 2000, 45% to 70% of the observed
decline in prostate cancer mortality could be plausibly
attributed to screening. They concluded that PSA
screening might account for much of the observed
drop in prostate cancer mortality. Etzioni et al54 also
studied the link between PSA screening and the
decline in the incidence of late-stage prostate cancer.
Their tested model showed that screening explained
about 80% of the observed decline in the incidence
of distant-stage (as opposed to locoregional stage)
prostate cancer. Nonetheless the team suggested
that other factors such as awareness and advances in
treatment might also play certain roles.
Telesca et al55 used data derived from the
Surveillance, Epidemiology, and End Results (SEER)
registry of the National Cancer Institute in the US
to examine the increase and subsequent decline in
the incidence of prostate cancer after the adoption
of PSA screening, and arrived at the opposite
conclusion. They maintained that the disease would
not have continued to increase in incidence in the
absence of PSA screening. Also using the SEER data,
Welch and Albertsen56 used age-specific population
estimates from the US Census data to determine the
excess or deficit in the number of men diagnosed
and treated each year after 1986. Since 1986, an
estimated additional 1.3 million men were diagnosed
and more than 1 million of them were treated. They
concluded that most of the excess incidence must be
the result of over-diagnosis.
Two large-scale RCTs published in 2009—the European Randomized Study of Screening for
Prostate Cancer57 and the US PLCO cancer screening
trial58—produced conflicting results on screening for
prostate cancer with PSA testing, providing fuel for
further debate.
Jemal et al59 conducted an interesting study
that examined changes in the incidence of stage-specific
prostate cancer and PSA screening rates
for the period 2005 to 2012 using the US National
Cancer Institute database. Both the incidence
of early-stage prostate cancer and rates of PSA
screening had declined, coinciding with the 2012
USPSTF recommendation to omit PSA screening
from routine primary care. Nonetheless, the authors
also recommended a longer follow-up period to
ascertain whether these decreases were indeed
associated with mortality trends. Thus, whether
reduced screening would lead to reductions in
over-diagnoses or to missed opportunities for early
detection remains an open question.
The American Cancer Society states that a
screening test should not be offered to men who do
not have any symptoms of prostate cancer and who
have a life expectancy of about 10 more years or less,
because of its slow-growing prognosis. The USPSTF
and the American Academy of Family Physicians do
not recommend the use of the PSA test to screen for
prostate cancer, as there is little evidence to show
that the benefits outweigh the harm. They argue
against screening for men 75 years of age and older
because of a lack of evidence to support screening.
In brief, views about the desirability of prostate
cancer screening are polarised, and much confusion
over the issue remains.60 Observational evidence to
date has not always supported the efficacy of PSA
screening in reducing mortality; rather, a growing
body of observational evidence points to the overdiagnosis
and over-treatment of prostate cancer
triggered by PSA testing.61 Randomised trials have
produced conflicting results. Thus, the efficacy of
prostate cancer screening for old men remains a
point of contention. A summary of the literature
review of screening for prostate cancer is shown in
Table 5.20 27 52 53 54 55 56 57 58 59
To screen or not to screen older
people
To screen or not to screen older people for common
types of cancer remains controversial, especially for
people over the age of 75 years. Screening may reduce
the risk that individuals will develop a condition
or its complications, but it may not guarantee
protection. Most of the papers and guidelines
suggested that screening has to be individualised for
this particular age-group. Even though the risk of
cancer increases with age, it should not be the only
factor taken into account when making decisions
about screening. Routine cancer screening does
not benefit those with a limited life expectancy.62
Estimating life expectancy will help guide decision-making
for preventive screening and treatment
plans.27 Because life expectancy varies in relation to
co-morbidity status, taking co-morbidity–adjusted
life expectancy into consideration may be helpful to
physicians.35
In any screening programme, there is an
irreducible minimum of false-positive and false-negative
results.23 The feelings and overall health
status of the patient also need to be considered.
It may be appropriate to screen patients with a
life expectancy sufficiently long to experience
the potential benefits of screening. Personalised
consideration might benefit older people if the
positive impacts can outweigh the negative, even for
the oldest-old.
Conclusion
Cancer is common in the older population and,
for them, the benefits of screening for common
types of cancer remain controversial. The evidence
is strongest for the efficacy of colorectal cancer
screening, even for older people aged 75 years
and beyond. Low-dose CT for screening for lung
cancer has benefits for heavy smokers. Liver cancer
screening is recommended only for those at high risk
of developing the disease. The evidence for screening
older people for breast cancer is conflicting; as
is the evidence for the effectiveness of PSA tests
for screening for prostate cancer. Although some
screening tests can bring certain benefits, other
factors related to advancing age may be present, such
as co-morbidities that may cause harm and would
eventually outweigh the benefits of cancer screening.
More research is indeed needed to understand the
relationship between cancer and ageing, and also
the risks and benefits of cancer screening for older
people, to ultimately promote good health and
functional longevity.
References
1. World Health Organization. Cancer fact sheet. February
2017. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 15 Jun 2017.
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer
incidence and mortality worldwide: sources, methods
and major patterns in GLOBOCAN 2012. Int J Cancer
2015;136:E359-86. Crossref
3. Ngan KC. Overview of Hong Kong cancer statistics of 2014. November 2016. Available from: http://www3.ha.org.hk/cancereg/pdf/overview/Summary%20of%20CanStat%202014.pdf. Accessed 15 Jun 2017.
4. Albert RH, Clark MM. Cancer screening in the older
patient. Am Fam Physician 2008;78:1369-74.
5. de Glas NA, de Craen AJ, Bastiaannet E, et al. Effect of
implementation of the mass breast cancer screening
programme in older women in the Netherlands: population
based study. BMJ 2014;349:g5410. Crossref
6. National Cancer Institute. International cancer screening
network. 2014. Available from: http://appliedresearch.cancer.gov/icsn/. Accessed 2 Oct 2016.
7. Turkoz FP, Tokluoglu S, Durnali AG, et al. Cancer
evaluation in geriatric population: a single institution
experience. Int J Hematol Oncol 2013;23:28-33. Crossref
8. Craft M. Cancer screening in the older adult: issues and
concerns. Nurs Clin North Am 2014;49:251-61. Crossref
9. Arksey H, O’Malley L. Scoping studies: towards a
methodological framework. Int J Soc Res Methodol
2005;8:19-32. Crossref
10. Wilson JA. Colon cancer screening in the elderly: when do
we stop? Trans Am Clin Climatol Assoc 2010;121:94-103.
11. Quarini C, Gosney M. Review of the evidence for a
colorectal cancer screening programme in elderly people.
Age Ageing 2009;38:503-8. Crossref
12. Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al.
Colorectal cancer deaths attributable to nonuse of screening
in the United States. Ann Epidemiol 2015;25:208-13. Crossref
13. Brenner H, Altenhofen L, Stock C, Hoffmeister M.
Expected long-term impact of the German screening
colonoscopy programme on colorectal cancer prevention:
analyses based on 4,407,971 screening colonoscopies. Eur J
Cancer 2015;51:1346-53. Crossref
14. Brenner H, Altenhofen L, Stock C, Hoffmeister M.
Prevention, early detection, and overdiagnosis of
colorectal cancer within 10 years of screening colonoscopy
in Germany. Clin Gastroenterol Hepatol 2015;13:717-23. Crossref
15. Rozen P, Shabtai EI, Liphshitz I, Barchana M. Risk
for colorectal cancer in elderly persons and possible
methodologies for their screening. Eur J Gastroenterol
Hepatol 2011;23:431-7. Crossref
16. Maheshwari S, Patel T, Patel P. Screening for colorectal
cancer in elderly persons: who should we screen and when
can we stop? J Aging Health 2008;20:126-39. Crossref
17. Kahi CJ, Azzouz F, Juliar BE, Imperiale TF. Survival of
elderly persons undergoing colonoscopy: implications for
colorectal cancer screening and surveillance. Gastrointest
Endosc 2007;66:544-50. Crossref
18. van Hees F, Habbema JD, Meester RG, Lansdorp-Vogelaar
I, Van Ballegooijen M, Zauber AG. Should colorectal
cancer screening be considered in elderly persons without
previous screening? A cost-effectiveness analysis. Ann
Intern Med 2014;160:750-9. Crossref
19. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al.
Personalizing colonoscopy screening for elderly individuals
based on screening history, cancer risk, and comorbidity
status could increase cost effectiveness. Gastroenterology
2015;149:1425-37. Crossref
20. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al.
Personalizing age of cancer screening cessation based
on comorbid conditions: model estimates of harms and
benefits. Ann Intern Med 2014;161:104-12. Crossref
21. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact
of screening colonoscopy on mortality in the medicare
population. J Gen Intern Med 2011;26:1441-9. Crossref
22. Fillenbaum GG, Burchett BM, Kuchibhatla MN, Cohen HJ,
Blazer DG. Effect of cancer screening and desirable health
behaviors on functional status, self-rated health, health
service use and mortality. J Am Geriatr Soc 2007;55:66-74. Crossref
23. Pasetto LM, Monfardini S. Colorectal cancer screening in
elderly patients: when should be more useful? Cancer Treat
Rev 2007;33:528-32. Crossref
24. National Cancer Institute. Tests to detect colorectal cancer
and polyps. 2016. Available from: http://www.cancer.gov/types/colorectal/screening-fact-sheet. Accessed 2 Oct
2016.
25. American Cancer Society. Guidelines for the early detection
of cancer. 2016. Available from: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed 2 Oct 2016.
26. US Preventive Services Task Force. Colorectal
cancer: screening. 2016. Available from: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2?
ds=1&s=Colorectal%20Cancer:%20Screening. Accessed 2 Oct 2016.
27. Tazkarji B, Lam R, Lee S, Meiyappan S. Approach
to preventive care in the elderly. Can Fam Physician
2016;62:717-21.
28. Hong Kong Anti-Cancer Society. Cancer screening,
early detection and prevention guidelines for health
professionals. 2011. Available from: https://www.hkacs.org.hk/ufiles/CancerScreeningprofessionals_2ndedition_1.pdf. Accessed 2 Oct 2016.
29. Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB.
Screening for lung cancer: Diagnosis and management of
lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest
2013;143(5 Suppl): e78S-92S.
30. Humphrey LL, Deffebach M, Pappas M, et al. Screening
for lung cancer with low-dose computed tomography: a
systematic review to update the US Preventive Services Task
Force recommendation. Ann Intern Med 2013;159:411-20. Crossref
31. Oken MM, Hocking WG, Kvale PA, et al. Screening by
chest radiograph and lung cancer mortality: the Prostate,
Lung, Colorectal, and Ovarian (PLCO) randomized trial.
JAMA 2011;306:1865-73. Crossref
32. National Lung Screening Trial Research Team; Aberle DR,
Adams AM, Berg CD, et al. Reduced lung-cancer mortality
with low-dose computed tomographic screening. N Engl J
Med 2011;365:395-409. Crossref
33. McMahon PM, Meza R, Plevritis SK, et al. Comparing
benefits from many possible computed tomography
lung cancer screening programs: extrapolating from the
national lung screening trial using comparative modeling.
PLoS One 2014;9:e99978. Crossref
34. Moyer VA, US Preventive Services Task Force. Screening
for lung cancer: U.S. Preventive Services Task Force
recommendation statement. Ann Intern Med 2014;160:330-8. Crossref
35. Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted
life expectancy: a new tool to inform
recommendations for optimal screening strategies. Ann
Intern Med 2013;159:667-76. Crossref
36. Galit W, Green MS, Lital KB. Routine screening
mammography in women older than 74 years: a review of
the available data. Maturitas 2007;57:109-19. Crossref
37. Mo M, Liu GY, Zheng Y, et al. Performance of breast cancer
screening methods and modality among Chinese women:
a report from a society-based breast screening program
(SBSP) in Shanghai. Springerplus 2013;2:276. Crossref
38. Broeders M, Moss S, Nyström L, et al. The impact of
mammographic screening on breast cancer mortality in
Europe: a review of observational studies. J Med Screen
2012;19 Suppl 1:14-25. Crossref
39. Paci E; EUROSCREEN Working Group. Summary of the
evidence of breast cancer service screening outcomes in
Europe and first estimate of the benefit and harm balance
sheet. J Med Screen 2012;19 Suppl 1:5-13. Crossref
40. Tejada JJ, Ivy JS, Wilson JR, Ballan MJ, Diehl KM, Yankaskas
BC. Combined DES/SD model of breast cancer screening
for older women, I: natural-history simulation. IIE Trans
2015;47:600-19. Crossref
41. Sanderson M, Levine RS, Fadden MK, et al. Mammography
screening among the elderly: a research challenge. Am J
Med 2015;128:1362.e7-14. Crossref
42. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of
mammography screening under different screening
schedules: model estimates of potential benefits and
harms. Ann Intern Med 2009;151:738-47. Crossref
43. Gøtzsche PC, Nielsen M. Screening for breast cancer
with mammography. Cochrane Database Syst Rev
2011;(1):CD001877. Crossref
44. Parvinen I, Heinävaara S, Anttila A, Helenius H, Klemi P,
Pylkkänen L. Mammography screening in three Finnish
residential areas: comprehensive population-based study
of breast cancer incidence and incidence-based mortality
1976-2009. Br J Cancer 2015;112:918-24. Crossref
45. Braithwaite D, Walter LC, Izano M, Kerlikowske K. Benefits
and harms of screening mammography by comorbidity
and age: a qualitative synthesis of observational studies and
decision analyses. J Gen Intern Med 2016;31:561-72. Crossref
46. National Cancer Institute. Breast cancer screening
(PDQ)—health professional version. June 2017. Available
from: https://www.cancer.gov/types/breast/hp/breast-screening-pdq/. Accessed 21 Jun 2017.
47. Hong Kong Breast Cancer Foundation. Breast self-examination.
Available from: https://www.hkbcf.org/content.php?cid=20&tid=3&lang=eng. Accessed 29 Aug
2017.
48. Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular
carcinoma. Mol Clin Oncol 2013;1:593-8. Crossref
49. Huang YC, Huang CF, Chang KC, et al. Community-based
screening for hepatocellular carcinoma in elderly residents
in a hepatitis B- and C-endemic area. J Gastroenterol
Hepatol 2011;26:129-34. Crossref
50. Cancer Research UK. Liver cancer. 2016. Available from:
http://www.cancerresearchuk.org/about-cancer/liver-cancer/getting-diagnosed/screening. Accessed 15 Jun
2017.
51. Department of Health. Liver cancer: prevention and
screening. Available from: http://www.chp.gov.hk/files/pdf/7_liver_cancer_prevention_and_screening_eng.pdf.
Accessed 29 Aug 2017.
52. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and
management of prostate cancer in elderly men: the Iowa
Prostate Cancer Consensus. Urology 2008;71:511-4. Crossref
53. Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the
role of PSA screening in the US prostate cancer mortality
decline. Cancer Causes Control 2008;19:175-81. Crossref
54. Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA
screening on the incidence of advanced stage prostate
cancer in the United States: a surveillance modeling
approach. Med Decis Making 2008;28:323-31. Crossref
55. Telesca D, Etzioni R, Gulati R. Estimating lead time and
overdiagnosis associated with PSA screening from prostate
cancer incidence trends. Biometrics 2008;64:10-9. Crossref
56. Welch HG, Albertsen PC. Prostate cancer diagnosis and
treatment after the introduction of prostate-specific antigen
screening: 1986-2005. J Natl Cancer Inst 2009;101:1325-9. Crossref
57. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and
prostate-cancer mortality in a randomized European study.
N Engl J Med 2009;360:1320-8. Crossref
58. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Mortality
results from a randomized prostate-cancer screening trial.
N Engl J Med 2009;360:1310-9. Crossref
59. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence
and PSA testing patterns in relation to USPSTF screening
recommendations. JAMA 2015;314:2054-61. Crossref
60. Stricker PD, Frydenberg M, Kneebone A, Chopra S.
Informed prostate cancer risk-adjusted testing: a new
paradigm. BJU Int 2012;110 Suppl 4:30-4. Crossref
61. Croswell JM, Kramer BS, Crawford ED. Screening for
prostate cancer with PSA testing: current status and future
directions. Oncology (Williston Park) 2011;25:452-60,463.
62. Royce TJ, Hendrix LH, Stokes WA, Allen IM, Chen RC.
Cancer screening rates in individuals with different life
expectancies. JAMA Intern Med 2014;174:1558-65. Crossref