Hong Kong Med J 2018 Apr;24(2):175–81 | Epub 6 Apr 2018
DOI: 10.12809/hkmj177086
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Management of complications of ketamine abuse: 10
years’ experience in Hong Kong
YL Hong, MSc1; CH Yee, FHKAM (Surgery)2,
YH Tam, FHKAM (Surgery)1; Joseph HM Wong, FHKAM (Surgery)2;
PT Lai, BN2; CF Ng, FHKAM (Surgery)2
1 Division of Paediatric Surgery and
Paediatric Urology, Department of Surgery, The Chinese University of Hong
Kong, Shatin, Hong Kong
2 SH Ho Urology Centre, Department of
Surgery, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Ketamine is an N-methyl-d-aspartate receptor
antagonist, a dissociative anaesthetic agent and a treatment option for
major depression, treatment-resistant depression, and bipolar disorder.
Its strong psychostimulant properties and easy absorption make it a
favourable candidate for substance abuse. Ketamine entered Hong Kong as a
club drug in 2000 and the first local report of ketamine-associated
urinary cystitis was published in 2007. Ketamine-associated lower–urinary
tract symptoms include frequency, urgency, nocturia, dysuria, urge
incontinence, and occasionally painful haematuria. The exact prevalence of
ketamine-associated urinary cystitis is difficult to assess because the
abuse itself and many of the associated symptoms often go unnoticed until
a very late stage. Additionally, upper–urinary tract pathology, such as
hydronephrosis, and other complications involving neuropsychiatric,
hepatobiliary, and gastrointestinal systems have also been reported.
Gradual improvement can be expected after abstinence from ketamine use.
Sustained abstinence is the key to recovery, as relapse usually leads to
recurrence of symptoms. Both medical and surgical management can be used.
The Youth Urological Treatment Centre at the Prince of Wales Hospital,
Hong Kong, has developed a four-tier treatment protocol with initial
non-invasive investigation and management for these patients.
Multidisciplinary care is essential given the complex and diverse
psychological factors and sociological background that underlie ketamine
abuse and abstinence status.
Introduction
Ketamine is an N-methyl-d-aspartate (NMDA) receptor
antagonist, a dissociative anaesthetic agent that was first
synthesised in the United States in 1962. It has been widely used in both
human and veterinary medicine since 1971. It has also been used as a
treatment for major depression, treatment-resistant depression, and
bipolar disorder.1 However, its
strong psychostimulant properties and easy absorption make it a favourable
candidate for substance abuse. Ketamine abuse has become increasingly
common over the past two decades. It entered Hong Kong as a club drug in
2000 and was initially used as a ‘top-up’ drug to ecstasy
(3,4-methylenedioxymethamphetamine) by those aiming to elevate and
redirect the ‘high’.2 It was viewed
as a poor man’s version of cocaine, as it is available in powder form and
can be consumed by snorting. By 2002, ketamine had become the drug of
choice instead of a ‘top-up’ drug. Users self-administered ketamine to
have a ‘time-out’ or ‘sit-down-to-float’ experience.2 3 Within a
short period, the number of reported ketamine abusers in Hong Kong
increased from 1605 in 2000 to its peak of 5280 in 2009. Ketamine remained
the most popular psychotropic substance abused from 2005 to 2014 (Fig
1).4
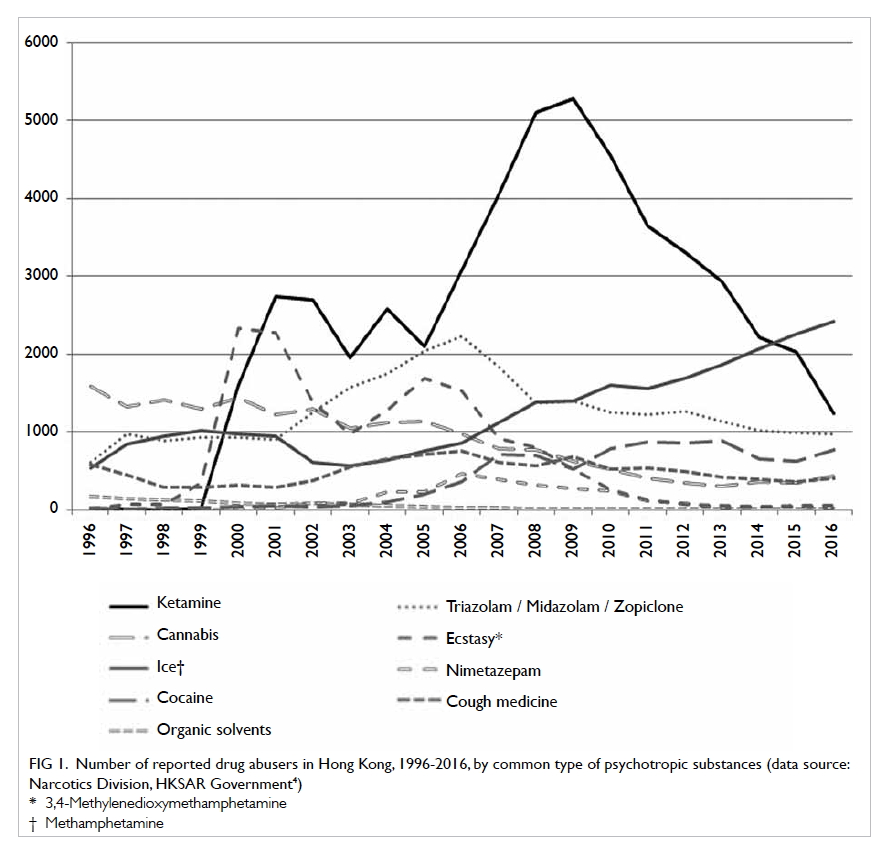
Figure 1. Number of reported drug abusers in Hong Kong, 1996-2016, by common type of psychotropic substances (data source: Narcotics Division, HKSAR Government4)
The abuse of ketamine and its popularity
nonetheless created a new medical entity. The first report of
ketamine-associated urinary cystitis in Hong Kong was published in 2007.5 In the same year, Shahani et al
reported a similar condition overseas.6
In the past decade, owing to the joint efforts of urologists, general
surgeons, physicians, psychiatrists, pathologists, and basic scientists,
we have gained a better understanding of other ketamine-associated
conditions. This understanding spans from pathology to clinical
management, from urological complications to upper-gastrointestinal (GI)
complications, and from a mouse model to humans. We have also explored
holistic ways to manage this condition in the long term, such as helping
young adults to have a fresh start while living with potentially
irreversible complications. This article reviews the evolution of local
clinical awareness and management of these complications, with a
particular focus on the work and contributions of local researchers.
Urological complications
Chu et al5
reported the first local case series of ketamine-associated bladder
dysfunction in 2007. Ketamine-associated lower–urinary tract symptoms
(LUTS) include frequency, urgency, nocturia, dysuria, urge incontinence,
and occasionally painful haematuria. The exact prevalence of
ketamine-associated urinary cystitis is difficult to assess because
ketamine abuse and many of the associated symptoms often go unnoticed
until a very late stage. A survey among 12 000 local secondary school
students revealed that 18.5% of non-psychotropic substance users had LUTS,
whereas 47.8% and 60.7% of psychotropic substance users and ketamine users
had LUTS, respectively.7
Unpublished data from the same study showed that compared with
non-psychotropic substance users, sole ketamine users were five times as
likely to have LUTS, whereas concomitant users of ketamine and
methamphetamine were eight times as likely to have LUTS.
In 2015, Yee et al8
reported the largest available cohort of both active and past ketamine
users who had ketamine-associated uropathy. Among 463 patients, ketamine
users had a significantly higher pelvic pain and urgency/frequency (PUF)
score than ex-ketamine users. The PUF score is initially used to assess
interstitial cystitis, and a higher PUF score correlates with worse
symptoms. Among active ketamine users, a higher PUF score was found to
correlate with a poorer quality of life and a smaller functional bladder
capacity.8 Achieving abstinence
from ketamine use and consuming smaller amounts of ketamine were factors
that predicted improvement in PUF score.
As well as bladder involvement, upper–urinary tract
pathology presenting as hydronephrosis and flank pain has also been
reported (Fig 2). A study by Yee et al9 of 572 patients with ketamine-related LUTS found that
up to 16.8% (n=96) of patients had hydronephrosis according to
ultrasonography. Hydronephrosis was frequently accompanied by ureteral
lesions, ureteral wall thickening, or vesicoureteral (vesicoureteric)
reflux. Similarly, Chu et al10
reported that 51% (30/59) of their patients had hydronephrosis according
to ultrasonography.
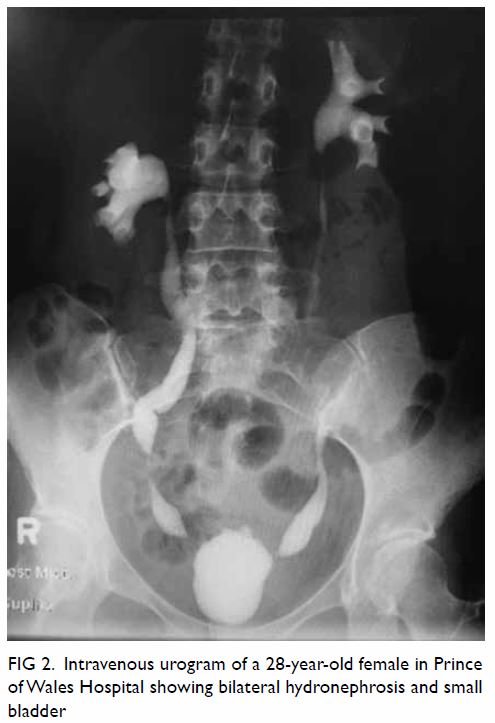
Figure 2. Intravenous urogram of a 28-year-old woman presenting to the Prince of Wales Hospital, showing bilateral hydronephrosis and small bladder
Pathophysiology
Although the exact mechanism of ketamine-associated
cystitis remains to be explored, there is evidence that ketamine
metabolites in the urine induce chemical irritation of the urothelium,
thereby causing an inflammatory response.11
Severe irritation may lead to denudation of the urothelium and consequent
transmural inflammation, loss of muscle thickness, fibrosis of the
detrusor muscle, and ultimately poor urinary bladder compliance.
Vesicoureteral reflux or urinary stasis in the ureter may occur, causing
chronic ureteral inflammation and ureteral stricture. There is also
evidence that both systemic and local inflammatory markers are elevated in
ketamine users.10 12 In addition, the NMDA antagonist properties of
ketamine may exert their effect via a central pathway.13
Neuropsychiatric complications
As ketamine is a psychostimulant, it is not
surprising that it is associated with long-term neurocognitive problems.
Chan et al14 found that when
ketamine users were compared with healthy controls, they had impaired
verbal fluency, cognitive processing speed, and verbal learning. Heavy
ketamine use correlated with deficits in verbal memory and visual
recognition memory. Liang et al15
also identified predominant verbal and visual memory impairment in
ketamine poly-drug users. Unfortunately, these deficits persisted in
ex-users. A much higher incidence of psychiatric co-morbidities, including
psychosis, depression, and anxiety, was observed among ketamine users.16
Pathophysiology
Structural brain damage associated with ketamine
abuse was supported by magnetic resonance imaging (MRI) by Wang et al.17 They were the first group to report patches of
degeneration in the superficial white matter as early as 1 year after
ketamine addiction onset. Cortical atrophy was also found in the frontal,
parietal, or occipital cortices of addicts.17
Another MRI study also provided evidence of brain damage in chronic
ketamine users. Reduced grey- and white-matter volumes were noted in the
bilateral orbitofrontal cortex, right medial prefrontal cortex, and
bilateral hippocampi. There was also significantly decreased connectivity
inside the brain in chronic ketamine abusers.18
A series of studies on the neurotoxicity of
ketamine suggested that ketamine could cause apoptosis of neuronal cells
in both in-vitro and in-vivo models. Ketamine also
potentially causes phosphorylation of tau protein, a marker of Alzheimer’s
degeneration in the brain.19 20 21 22
Hepatobiliary complications
In 2009, Wong et al23
reported ketamine-associated hepatobiliary complications for the first
time. Three ketamine abusers presented with recurrent epigastric pain and
dilated bile ducts mimicking choledochal cysts. Subsequently, more similar
cases were identified. Fusiform dilatation of the common bile duct was
also observed.24 25 26 Liver
biopsy confirmed development of active liver and/or bile duct injury. A
study of 297 chronic ketamine abusers with urinary tract dysfunction
showed that the prevalence of liver injury was 9.8%.27 These studies and reports show the possibility and
severity of damage by ketamine to the hepatobiliary and pancreatic system.
Pathophysiology
The exact mechanism of ketamine-associated bile
injury is still unknown. The associated rise in C-reactive protein
suggests a possible inflammatory process in the liver parenchyma,
including or excluding the bile duct.27
Others have postulated that either central or direct action of ketamine on
the biliary smooth muscle in turn leads to the cholestasis and biliary
dilatation observed in ketamine abusers.28
Gastrointestinal complications
In addition to urological complaints, GI problems
are also frequently the symptoms for which ketamine abusers seek medical
help. A review of 233 ketamine-related visits to accident and emergency
departments found that 49 (21.0%) patients had abdominal pain, 23 (9.9%)
had nausea or vomiting, and 41 (17.6%) had abdominal tenderness.29 Gastrointestinal complaints often co-exist with and
precede the presentation of urological symptoms. Liu et al30 found that about a quarter (168; 27.5%) of 611
ketamine users who sought treatment for ketamine uropathy reported the
presence of upper-GI symptoms, whereas only 42 (5.2%) of 804 non-ketamine
users attending a general urology clinic reported similar symptoms
(P<0.001). The majority of the symptoms reported were epigastric pain
and recurrent vomiting. Nearly three-quarters of patients required
hospitalisation for acute or chronic upper-GI symptoms. With the exception
of acid reflux and perforated peptic ulcer, the prevalence of all the
above-mentioned symptoms and hospitalisation rates were statistically
significantly higher in ketamine users than in non-ketamine users. All 168
patients using ketamine had undergone oesophagogastroduodenoscopy during
which biopsies were taken. Pathological findings ranged from gastritis to
gastroduodenal erosions, peptic ulceration, and intestinal metaplasia.30
Liu et al30
also found that more than 80% of patients developed upper-GI symptoms
before urological symptoms. Patients developed upper-GI symptoms after a
mean (standard deviation) of 5.1 (3.1) years of ketamine use and developed
uropathy symptoms after another 4.4 (3.0) years of ketamine use.30 Epigastric symptoms are not common in young people,
but common in ketamine abusers. This difference may provide an opportunity
to identify hidden ketamine abuse when assessing young patients with
epigastric symptoms. The identification of ketamine use is important, as
cessation of use can greatly improve GI symptoms.31
Further referral for help and counselling may improve psychological and
physical health and promote long-term ketamine abstinence.
Pathophysiology
The exact pathophysiological mechanism by which
ketamine produces upper-GI toxicity remains unknown but there are several
postulations. First, ketamine, as an NMDA antagonist, might act on local
smooth muscle or the central nervous system, thereby affecting gastric
motility and leading to cramping pain. Second, microvascular damage by
ketamine and its metabolites, which was believed to be a possible cause of
ketamine uropathy, might also cause similar microvascular damage in the
stomach and duodenum, leading to ischaemic pain and inflammation.
Likewise, circulating ketamine might also trigger some unknown autoimmune
responses, and thus induce interstitial inflammation in the urinary and GI
tracts. Finally, as many ketamine abusers like to swallow the nasal drips
occurring from ketamine inhalation, the swallowed ketamine might also
induce direct cytotoxic injury to the vulnerable GI tract.30
Management
As in the management of other substance abuse,
abstinence is the key to success in overall management of ketamine use.
Whereas other treatment modalities may relieve symptoms and hasten the
recovery process, many ketamine abusers have complicated underlying
psychosocial problems and psychiatric co-morbidities. Long-term and
consistent support and encouragement from doctors, nurses, social workers,
family, and friends are vital for success.
Abstinence from ketamine use
Recurrence of symptoms after resuming ketamine use
highlights the importance of ketamine abstinence. Studies have shown that
abstinence leads to symptomatic improvement. Compared with active ketamine
abusers, those who had abstained for 1 year had significantly lower PUF
scores and a larger voided volume. There was a trend towards higher voided
volumes and lower PUF scores as duration of ketamine cessation increased,
although neither variable was statistically significant.32 Another follow-up study of 101 participants who had
abstained from ketamine and 218 active ketamine users showed that the
abstinence group had a statistically significantly lower PUF score, and a
higher functional bladder capacity.8
Moreover, abstinence was the only protective factor associated with fewer
symptoms, larger voided volume, and bladder capacity.33
Nonetheless, abstinence does not lead to immediate
and full recovery of symptoms. Gradual improvement can be expected but
sustained abstinence is the key to recovery. Patience and continuous
support are of paramount importance. A study showed that on admission to a
drug rehabilitation centre, 90% of 40 female ex-ketamine users still had
active urinary symptoms, with increased 24-hour urinary frequency, lower
maximum voided volume, smaller median functional bladder capacity, and
higher mean Urogenital Distress Inventory Short Form (UDI-6) and
Incontinence Impact Questionnaire Short Form (IIQ-7) scores, when compared
with age-matched controls who attended a general gynaecology clinic. After
having stopped using ketamine for 3 months or more, mean 24-hour urinary
frequency and mean UDI-6 and IIQ-7 scores decreased, and maximum voided
volume increased. These scores further improved after another 3 months,
although this group continued to perform more poorly in all aspects
compared with controls.34
Medical and surgical management
As ketamine-associated uropathy is an evolving
‘disease entity’, the exact pathophysiology remains to be elucidated. Some
of the clinical features share similarities with interstitial cystitis.
Protocols are being developed to cater to the needs of patients in Hong
Kong. A one-stop service model has been adopted by the Youth Urological
Treatment Centre at the Prince of Wales Hospital since 2011 (Fig
3). The standard treatment protocol involves four tiers of
treatment, starting with an initial non-invasive investigative approach,
including questionnaire assessment of symptoms and calculation of (1)
functional bladder capacity by measuring voided volume using uroflowmetry
and (2) residual urine using ultrasound bladder scanning. This
non-invasive investigative approach helps gain patients’ trust and improve
adherence to later follow-up.
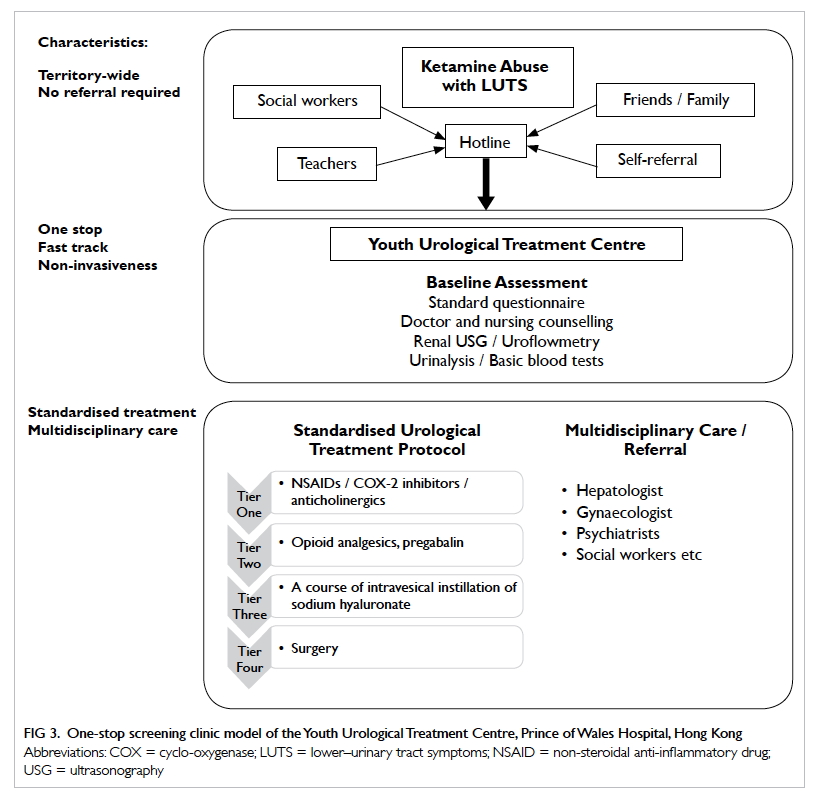
Figure 3. One-stop screening clinic model of the Youth Urological Treatment Centre, Prince of Wales Hospital, Hong Kong
First-tier treatment includes oral non-steroidal
anti-inflammatory drugs (NSAIDs) (eg, diclofenac) and anticholinergics
(eg, solifenacin) or COX-II inhibitors (eg, etoricoxib) if patients cannot
tolerate NSAIDS. Simple analgesics such as paracetamol and phenazopyridine
are used for pain control. The Youth Urological Treatment Centre has
reported the largest series of patients with ketamine-associated uropathy
and their corresponding outcomes. Of 290 patients with ketamine cystitis
who received first-line treatment, 202 (69.7%) reported symptom
improvement and a reduction in PUF scores. Functional bladder capacity was
also shown to have improved.8
The opioid group of analgesics and pregabalin are
used in the next tier of pain-control treatment when first-tier treatment
is insufficient for symptom relief. Sixty-two patients received
second-line treatment and 42 (67.7%) responded to treatment.8
Third-tier treatment consists of a course of
intravesical instillation of sodium hyaluronate (6-weekly instillations
followed by 2-monthly instillations) attempting to repair the
glycosaminoglycan layer. The drug is given to patients whose symptoms
remain uncontrolled after second-tier treatment. Seventeen patients in the
cohort received the third-tier treatment and eight completed the course.
Significant improvement in voided volume was noted and five were able to
reduce their oral medication usage after treatment. No significant adverse
effects were reported.8
Unfortunately, for a proportion of patients with
extremely refractory symptoms, surgery becomes the fourth-tier treatment
of choice. In the Youth Urological Treatment Centre series, one patient in
the cohort required hydrodistension and another underwent robotic-assisted
laparoscopic augmentation cystoplasty. The patient with hydrodistension
experienced a recurrence of symptoms post-treatment.8 Ng et al35
reported on four patients who underwent augmentation cystoplasty. Although
they showed initial improvement, all patients relapsed and resumed
ketamine use postoperatively. Three of the patients showed a further
deterioration in renal function, secondary to new-onset ureteral
strictures and/or sepsis. Therefore, patient selection, education, close
follow-up, and support are vital to the success of augmentation
cystoplasty.35
Multidisciplinary care
Given the complex and diverse psychological factors
and sociological background contributing to an individual’s decision to
abuse ketamine or achieve abstinence, joint multidisciplinary efforts are
required to help affected young adults. Doctors, social workers, teachers,
psychiatrists, psychologists, nurses, and patients’ families all need to
support them on their long road to recovery, to help them rehabilitate
physically and achieve sustained abstinence from ketamine.33 36 37
Conclusion
Since the initial discovery of ketamine-associated
uropathy, the impact of this disease entity has become more prominent in
Asian countries. Thanks to the joint efforts of urologists,
gynaecologists, surgeons, psychiatrists, pathologists, and social workers,
as well as the support of local government, the extent of medical
complications has been revealed to also involve the brain, liver, and GI
system. Many ketamine abusers are ‘hidden’ and can use ketamine stealthily
at home for years without their family noticing. Clinicians must take the
opportunity to identify hidden abusers when they consult for non-specific
symptoms such as epigastric pain and LUTS. Doing so will not only enable
early diagnosis of ketamine-associated uropathy, but it will also help
provide appropriate medical treatment in a timely manner. In addition to
medical therapy, referral for appropriate psychosocial support is
essential to sustain abstinence and manage underlying psychosocial
problems.
Acknowledgement
The Youth Urological Treatment Centre was developed
by joint efforts of The Chinese University of Hong Kong and the Hong Kong
Hospital Authority, with generous support from the Beat Drugs Fund of the
Narcotics Division, Security Bureau, Government of the Hong Kong Special
Administrative Region.
Declaration
The authors have no conflicts of interest to
disclose.
References
1. Hasselmann HW. Ketamine as
antidepressant? Current state and future perspectives. Curr Neuropharmacol
2014;12:57-70. Crossref
2. Joe Laidler KA. The rise of club drugs
in a heroin society: the case of Hong Kong. Subst Use Misuse
2005;40:1257-78. Crossref
3. Joe-Laidler K, Hunt G. Sit down to
float: the cultural meaning of ketamine use in Hong Kong. Addict Res
Theory 2008;16:259-71. Crossref
4. Narcotics Division, Security Bureau,
HKSAR Government. Statistical tables customization. Available from:
http://cs-crda.nd.gov.hk/en/enquiry.php. Accessed 22 Sep 2017.
5. Chu PS, Kwok SC, Lam KM, et al. ‘Street
ketamine’–associated bladder dysfunction: a report of ten cases. Hong Kong
Med J 2007;13:311-3.
6. Shahani R, Streutker C, Dickson B, et
al. Ketamine-associated ulcerative cystitis: a new clinical entity.
Urology 2007;69:810-2. Crossref
7. Tam YH, Ng CF, Wong YS, et al.
Population-based survey of the prevalence of lower urinary tract symptoms
in adolescents with and without psychotropic substance abuse. Hong Kong
Med J 2016;22:454-63. Crossref
8. Yee CH, Lai PT, Lee WM, et al. Clinical
outcome of a prospective case series of patients with ketamine cystitis
who underwent standardized treatment protocol. Urology 2015;86:236-43. Crossref
9. Yee CH, Teoh YC, Lai PT, et al. The risk
of upper urinary tract involvement in patients with ketamine-associated
uropathy. Int Neurourol J 2017;21:128-32. Crossref
10. Chu PS, Ma WK, Wong SC, et al. The
destruction of the lower urinary tract by ketamine abuse: a new syndrome?
BJU Int 2008;102:1616-22. Crossref
11. Chu PS, Ng CF, Ma WK. Ketamine
uropathy: Hong Kong experience. In: Yew DT, editor. Ketamine: Use and
Abuse. CRC Press; 2015: 207-26.
12. Division of Urology, Department of
Surgery, Princess Margaret Hospital and Tuen Mun Hospital. Research on
Urological Sequelae of Ketamine Abuse. Available from:
http://www.nd.gov.hk/pdf/20110307_beat_drug_fund_report.pdf. Accessed 22
Sep 2017.
13. Jankovic SM, Jankovic SV, Stojadinovic
D, et al. Effect of exogenous glutamate and N-methyl-D-aspartic acid on
spontaneous activity of isolated human ureter. Int J Urol 2007;14:833-7. Crossref
14. Chan KW, Lee TM, Siu AM, et al.
Effects of chronic ketamine use on frontal and medial temporal cognition.
Addict Behav 2013;38:2128-32. Crossref
15. Liang HJ, Lau CG, Tang A, et al.
Cognitive impairments in poly-drug ketamine users. Addict Behav
2013;38:2661-6. Crossref
16. Tang WK, Morgan CJ, Lau GC, et al.
Psychiatric morbidity in ketamine users attending counselling and youth
outreach services. Subst Abus 2015;36:67-74. Crossref
17. Wang C, Zheng D, Xu J, et al. Brain
damages in ketamine addicts as revealed by magnetic resonance imaging.
Front Neuroanat 2013;7:23. Crossref
18. Narcotics Division, Security Bureau,
HKSAR Government. Evidence of Brain Damage in Chronic Ketamine Users—a
Brain Imaging Study. Available from:
http://www.nd.gov.hk/pdf/Evidence-of-Brain-Damage-in-Chronic-Ketamine-Users_Final-report.pdf.
Accessed 22 Sep 2017.
19. Yeung LY, Wai MS, Fan M, et al.
Hyperphosphorylated tau in the brains of mice and monkeys with long-term
administration of ketamine. Toxicol Lett 2010;193:189-93. Crossref
20. Narcotics Division, Security Bureau,
HKSAR Government. Long-term Ketamine Abuse and Apoptosis in Cynomolgus
Monkeys and Mice. Available from:
http://www.nd.gov.hk/pdf/long_term_ketamine_abuse_apoptosis_in_cynomologus_monkeys_and_mice/final_report_with_all_attachments.pdf.
Accessed 22 Sep 2017.
21. Tan S, Rudd JA, Yew DT. Gene
expression changes in GABA(A) receptors and cognition following chronic
ketamine administration in mice. PLoS ONE 2011;6:e21328. Crossref
22. Mak YT, Lam WP, Lü L, et al. The toxic
effect of ketamine on SH-SY5Y neuroblastoma cell line and human neuron.
Microsc Res Tech 2010;73:195-201.
23. Wong SW, Lee KF, Wong J, et al.
Dilated common bile ducts mimicking choledochal cysts in ketamine abusers.
Hong Kong Med J 2009;15:53-6.
24. Ng SH, Lee HK, Chan YC, et al. Dilated
common bile ducts in ketamine abusers. Hong Kong Med J 2009;15:157.
25. Cheung TT, Poon RT, Chan AC, et al.
Education and imaging. Hepatobiliary and pancreatic: cholangiopathy in
ketamine user—an emerging new condition. J Gastroenterol Hepatol
2014;29:1663. Crossref
26. Lui KL, Lee WK, Li MK.
Ketamine-induced cholangiopathy. Hong Kong Med J 2014;20:78.e1-2. Crossref
27. Wong GL, Tam YH, Ng CF, et al. Liver
injury is common among chronic abusers of ketamine. Clin Gastroenterol
Hepatol 2014;12:1759-62.e1. Crossref
28. Lo RS, Krishnamoorthy R, Freeman JG,
et al. Cholestasis and biliary dilatation associated with chronic ketamine
abuse: a case series. Singapore Med J 2011;52:e52-5.
29. Ng SH, Tse ML, Ng HW, et al. Emergency
department presentation of ketamine abusers in Hong Kong: a review of 233
cases. Hong Kong Med J 2010;16:6-11.
30. Liu SY, Ng SK, Tam YH, et al. Clinical
pattern and prevalence of upper gastrointestinal toxicity in patients
abusing ketamine. J Dig Dis 2017;18:504-10. Crossref
31. Poon TL, Wong KF, Chan MY, et al.
Upper gastrointestinal problems in inhalational ketamine abusers. J Dig
Dis 2010;11:106-10. Crossref
32. Mak SK, Chan MT, Bower WF, et al.
Lower urinary tract changes in young adults using ketamine. J Urol
2011;186:610-4. Crossref
33. Tam YH, Ng CF, Pang KK, et al.
One-stop clinic for ketamine-associated uropathy: report on service
delivery model, patients’ characteristics and non-invasive investigations
at baseline by a cross-sectional study in a prospective cohort of 318
teenagers and young adults. BJU Int 2014;114:754-60. Crossref
34. Cheung RY, Chan SS, Lee JH, et al.
Urinary symptoms and impaired quality of life in female ketamine users:
persistence after cessation of use. Hong Kong Med J 2011;17:267-73.
35. Ng CF, Chiu PK, Li ML, et al. Clinical
outcomes of augmentation cystoplasty in patients suffering from
ketamine-related bladder contractures. Int Urol Nephrol 2013;45:1245-51. Crossref
36. Ng CF. Editorial comment to possible
pathophysiology of ketamine-related cystitis and associated treatment
strategies. Int J Urol 2015;22:826. Crossref
37. Chu PS, Dasgupta P. Lessons learned
from Asian Urology. BJU Int 2014;114:633. Crossref

