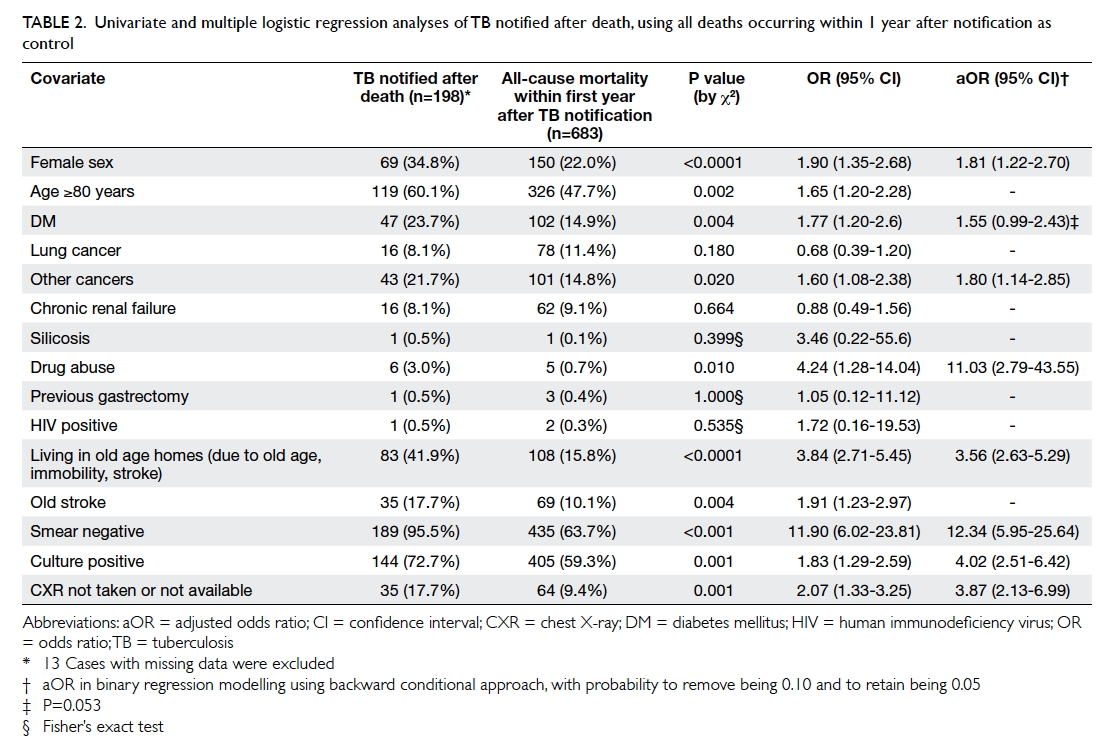
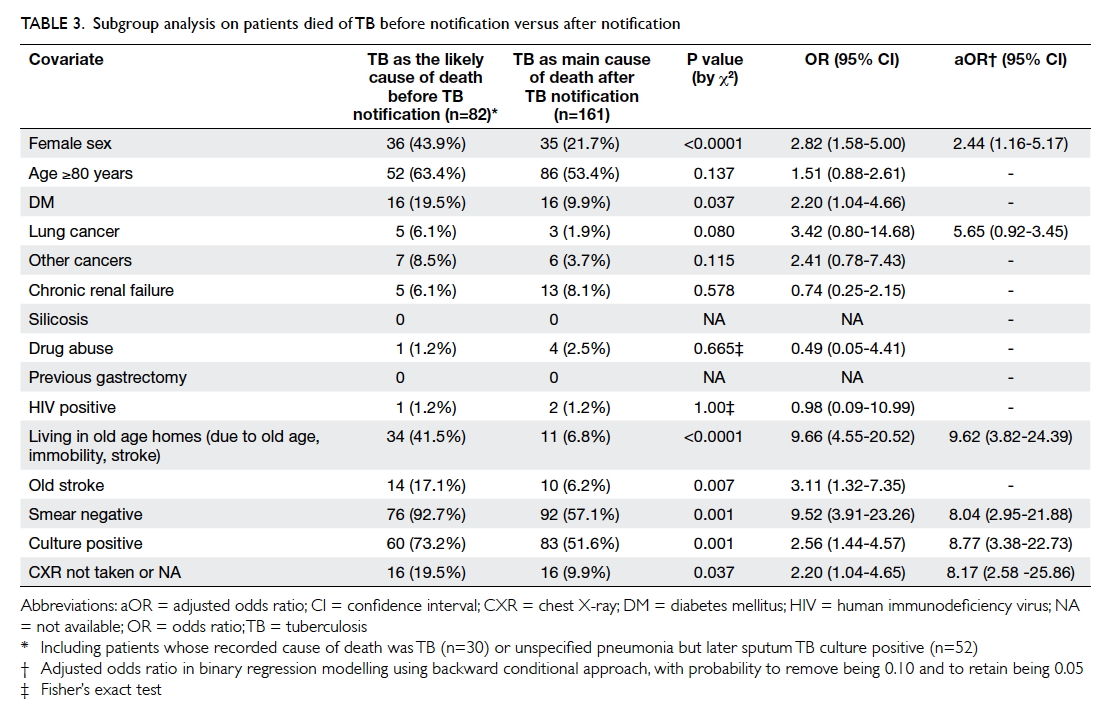
Depression and anxiety among university students in Hong Kong
Hong
Kong Med J 2018 Oct;24(5):466–72 | Epub 24 Sep 2018
DOI: 10.12809/hkmj176915
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Depression and anxiety among university students in
Hong Kong
Kevin WC Lun, CK Chan, Patricia KY Ip, Samantha YK
Ma, WW Tsai, CS Wong, Christie HT Wong, TW Wong, D Yan
Li Ka Shing Faculty of Medicine, The University of
Hong Kong, Pokfulam, Hong Kong
Corresponding author: Mr Kevin WC Lun (lunkwc@gmail.com)
Abstract
Introduction: Entry into
tertiary education is a critical juncture where adolescents proceed to
adulthood. This study aimed to determine the prevalence of depression
and anxiety, and factors associated with such symptoms, among university
undergraduate students in Hong Kong.
Methods: A cross-sectional
questionnaire study was employed. A total of 1200 undergraduate students
from eight University Grants Committee–funded universities were invited
to complete three sets of questionnaires, including the 9-item patient
health questionnaire for screening of depressive symptoms, the 7-item
generalised anxiety disorder scale for screening of anxiety symptoms,
and a socio-demographic questionnaire.
Results: Among the valid
responses (n=1119) analysed, 767 (68.5%) respondents indicated mild to
severe depressive symptoms, which were associated with mild to severe
anxiety symptoms. Several lifestyle and psychosocial variables,
including regular exercise, self-confidence, satisfaction with academic
performance, and optimism towards the future were inversely related with
mild to severe depressive symptoms. A total of 599 (54.4%) respondents
indicated mild to severe anxiety symptoms, which were associated with
level of academic difficulty. Satisfaction with friendship, sleep
quality, and self-confidence were inversely associated with mild to
severe anxiety symptoms.
Conclusion: More than 50% of
respondents expressed some degree of depressive and anxiety symptoms
(68.5% and 54.4%, respectively). Approximately 9% of respondents
exhibited moderately severe to severe depressive symptoms; 5.8%
exhibited severe anxiety symptoms. Respondents reporting regular
exercise, higher self-confidence, and better satisfaction with both
friendship and academic performance had fewer depressive and anxiety
symptoms.
New knowledge added by this study
- Up to 9% of university students in Hong Kong exhibit moderately severe to severe depressive symptoms.
- Up to 5.8% of university students in Hong Kong exhibit severe anxiety symptoms.
- Respondents reporting regular exercise, higher self-confidence, and better satisfaction with both friendship and academic performance had fewer depressive and anxiety symptoms.
- Health care workers and organisations such as universities should be aware of potential depression and anxiety among university undergraduate students.
- Adolescents and young adults in Hong Kong should be educated, to raise social awareness of depression and anxiety among university undergraduate students.
Introduction
Recently, an increased incidence of suicide among
students has triggered immense public concern regarding the mental health
of adolescents and young adults. In 2014, there were 52 suicides in the
age-group 15 to 24 years. The number of suicides in this age-group
increased in subsequent years to 68 in 2015 and 75 in 2016.1 A report from the Centre for Health Protection in Hong
Kong showed that, in 2012, the prevalences of mild, moderate, and severe
depressive symptoms in adolescents and children were 36.4%, 14.7%, and
4.2%, respectively.2 The Population
Health Survey 2003/2004 conducted collaboratively by the Department of
Health and the Department of Community Medicine of the University of Hong
Kong revealed that the median score for the State-Trait Anxiety Inventory
was highest in those aged 25 to 34 years, whereas the median score for the
Centre for Epidemiologic Studies Depression Scale was highest in those
aged 15 to 34 years.3 A web-based
survey targeting first-year students receiving tertiary education in Hong
Kong concluded that, in 2006, the prevalence of depression was 20.9%,
while that of anxiety was 41.2%.4
Another recent study, the Hong Kong Mental Morbidity Survey,5 revealed that the most common mental problem in Hong
Kong was mixed anxiety and depressive disorder; moreover, there was a
strong association between anxiety symptoms and depressive symptoms.
According to the Diagnostic and Statistical Manual
of Mental Disorders, published by the American Psychiatric Association,
major depressive disorder is defined as the presence of five or more of
the listed symptoms for most of the days during the same 2-week period; at
least one of the symptoms must be either depressed mood or loss of
interest or pleasure. Notably, these symptoms should reflect a change from
previous functioning. Generalised anxiety disorder (GAD) is characterised
by excessive worries that cause distress and interfere with psychosocial
functioning. These worries frequently happen without precipitants, exhibit
longer durations, and are accompanied by three or more of the six listed
additional symptoms.
Various factors associated with major depressive
disorder and GAD have been identified, including alcohol use, illicit drug
use, tobacco use, and level of physical activity.6
However, evidence for an association between academic pressure and these
two psychiatric disorders remains unknown among university undergraduate
students in Hong Kong. We aimed to provide an update regarding the
prevalence of depressive and anxiety symptoms experienced by undergraduate
students in Hong Kong, and to identify factors associated with these
symptoms.
Methods
Full-time undergraduate students of the eight
universities in Hong Kong that are funded by the University Grants
Committee (UGC) were the target participants in this study. A
cross-sectional study design was employed and the target number of
students to be enrolled from each university was 150; the total target
number of students to be recruited was 1200.
The inclusion criteria for this study were as
follows: full-time undergraduate students in the eight UGC-funded
universities who were ≥18 years, were able to understand the consent,
provide a valid oral consent, comprehend the questionnaire, and had not
previously participated in this research. Students who failed to fulfil
all inclusion criteria were excluded from the study.
Convenience sampling was employed. Questionnaires
were distributed at popular locations within all eight university campuses
on school days in September and October 2016. Participants were asked to
complete three sets of questionnaires, including the 9-item Patient Health
Questionnaire (PHQ-9) for screening of depressive symptoms, the 7-item GAD
scale (GAD-7) for screening of anxiety symptoms, and a socio-demographic
questionnaire to identify factors associated with depressive and anxiety
symptoms.7 8 When completed questionnaires were being returned,
respondents were reminded that they would not be able to withdraw from the
study after returning the questionnaires, as the questionnaires did not
contain any personal identifying information to allow individual
retrieval.
The PHQ-9 was adopted to screen for depressive
symptoms in this study. Both Chinese and English versions were provided.
The PHQ-9 has been shown to be a valid and reliable tool for assessing
depressive symptoms in the Hong Kong general population.7 8 Another study
showed that the PHQ-9 was a useful tool to detect both major depression
and subthreshold depression.9
According to the PHQ-9, a score of <5 was defined as none-minimal
depressive symptoms, score of 5 to 9 as mild, score of 10 to 14 as
moderate, score of 15 to 19 as moderately severe, and score of ≥20 as
severe.
The GAD-7 is a practical self-report anxiety
questionnaire that is generally used in out-patient and primary care
settings for referral to a psychiatrist to confirm the diagnosis of GAD.
Both Chinese and English versions were available. The adoption of GAD-7 in
this study was due to its good reliability, as well as its criteria,
construct, and factorial and procedural validity.10
11 According to the GAD-7, a score
of <5 was defined as none-minimal anxiety symptoms, score of 5 to 9 as
mild, score of 10 to 14 as moderate, and score of ≥15 as severe.
Descriptive analysis was performed to summarise the
demographics and statuses of depressive and anxiety symptoms of the
respondents. A binary logistic regression was conducted to ascertain the
effects of various covariates on the odds of exhibiting mild to severe
depressive symptoms, on the basis of PHQ-9 results. A PHQ-9 score of <5
was defined as no depressive symptoms, while a score of ≥5 was defined as
mild to severe depressive symptoms. Another binary logistic regression was
used to ascertain the effects of multiple covariates on the odds that
participants would exhibit mild to severe anxiety symptoms, on the basis
of GAD-7 results. A GAD-7 score of <5 was defined as no anxiety
symptoms, while a score of ≥5 was defined as mild to severe anxiety
symptoms. A P value of <0.05 was regarded as a significant difference.
Results
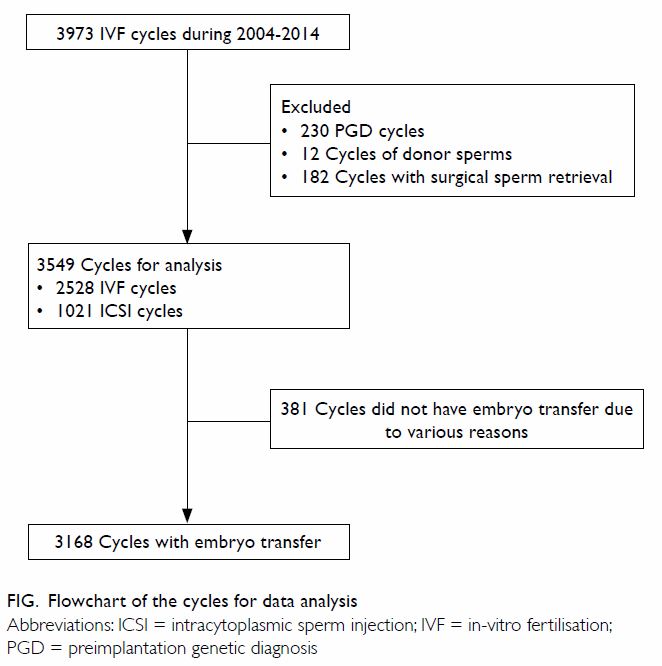
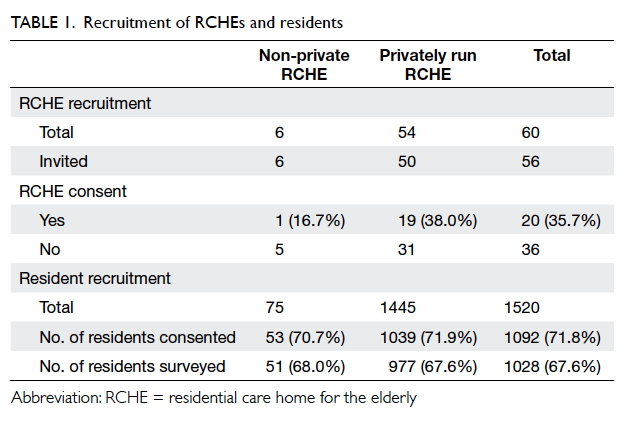
In total, 1480 undergraduate students from the
eight UGC-funded universities were approached from 6 September 2016 to 3
October 2016. From these, 1200 completed questionnaires were collected,
for a response rate of 81.1%. A total of 81 students did not meet at least
one of the inclusion criteria and their responses were subsequently
excluded from analysis (Fig). The remaining 1119 students fulfilled all
inclusion criteria and their responses were analysed by SPSS (Mac version
24; IBM Corp, Armonk [NY], United States).
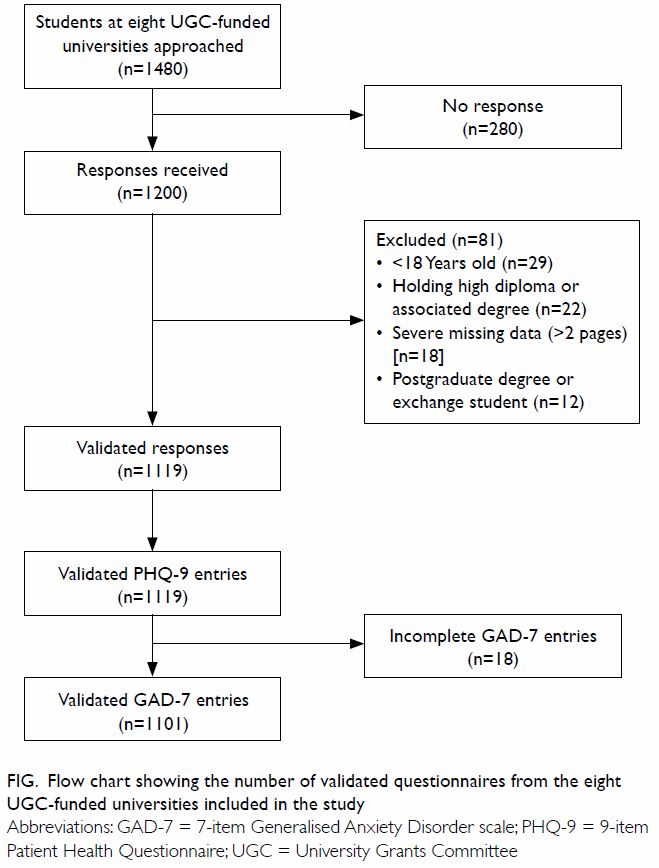
Figure. Flow chart showing the number of validated questionnaires from the eight UGC-funded universities included in the study
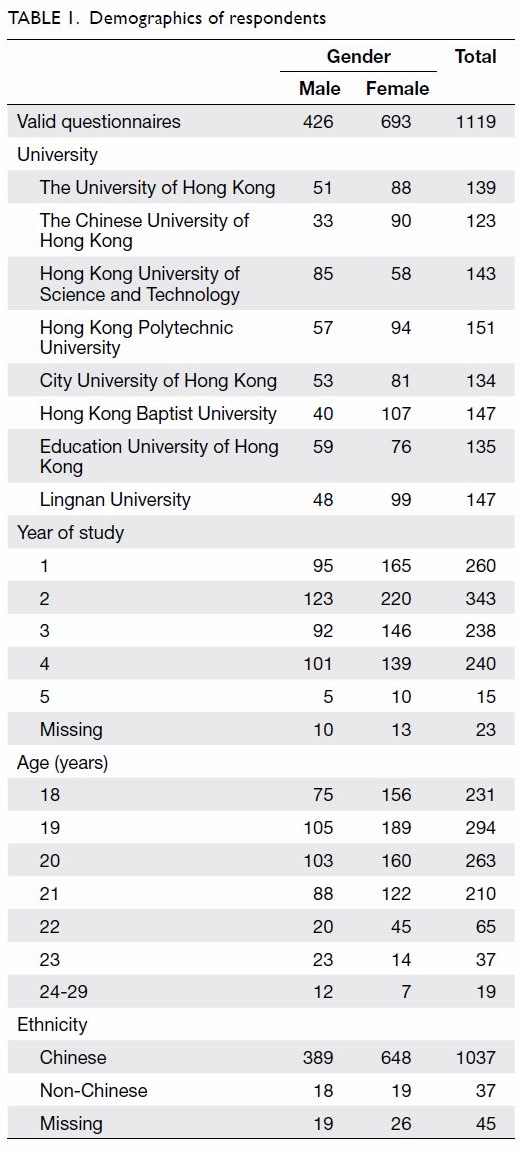
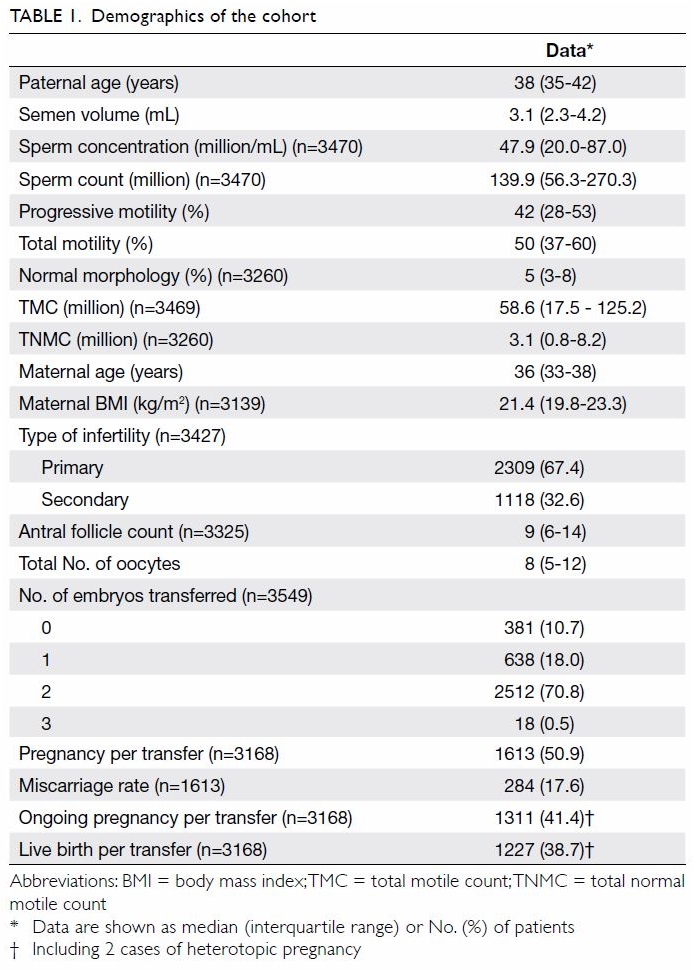
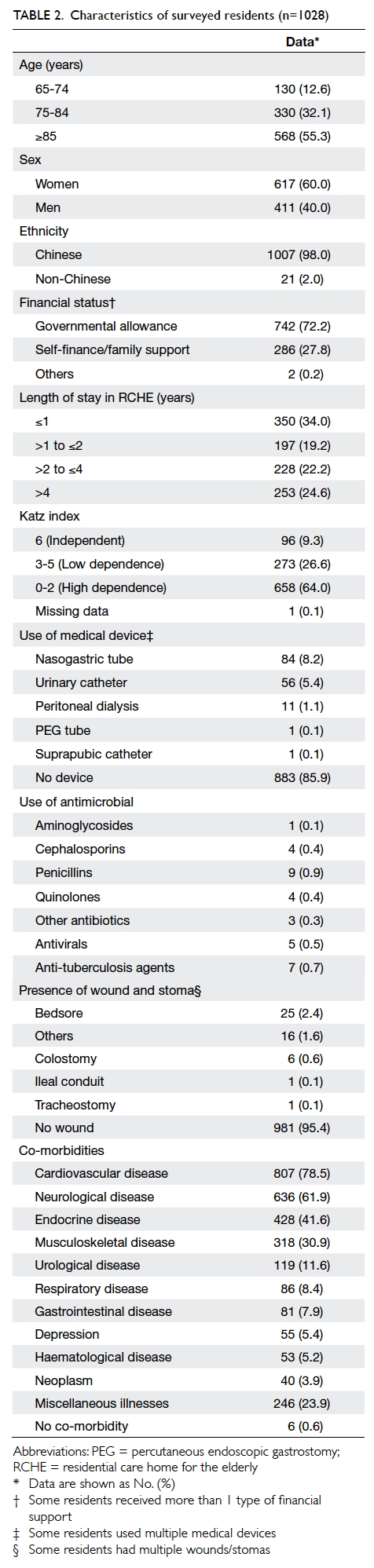
The mean (standard deviation) age of the
respondents was 19.81 (1.48) years (range, 18-29 years). Among the
respondents, 426 (38.1%) were male and 693 (61.9%) were female. Most
respondents were Chinese (92.7%). The respondents’ demographics are shown
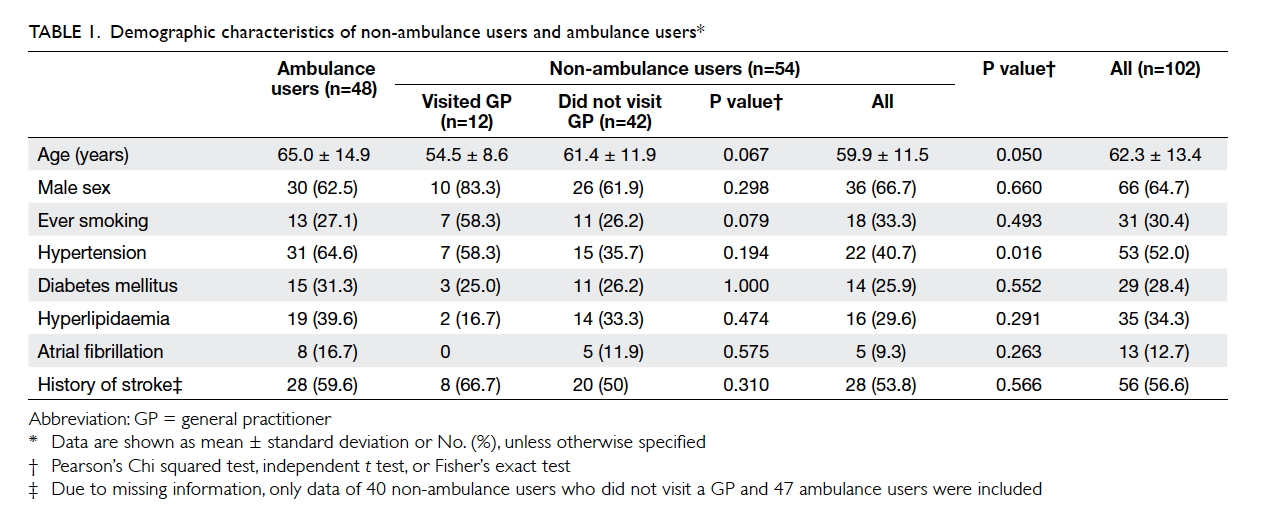
in Table 1.
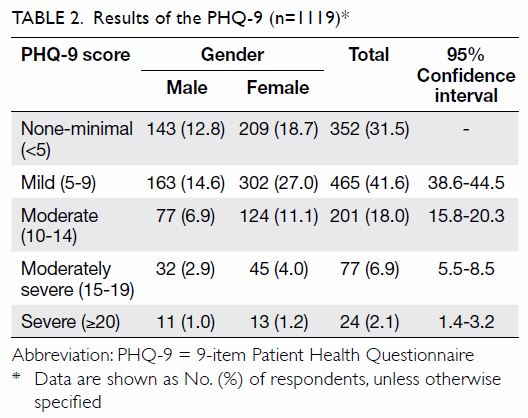
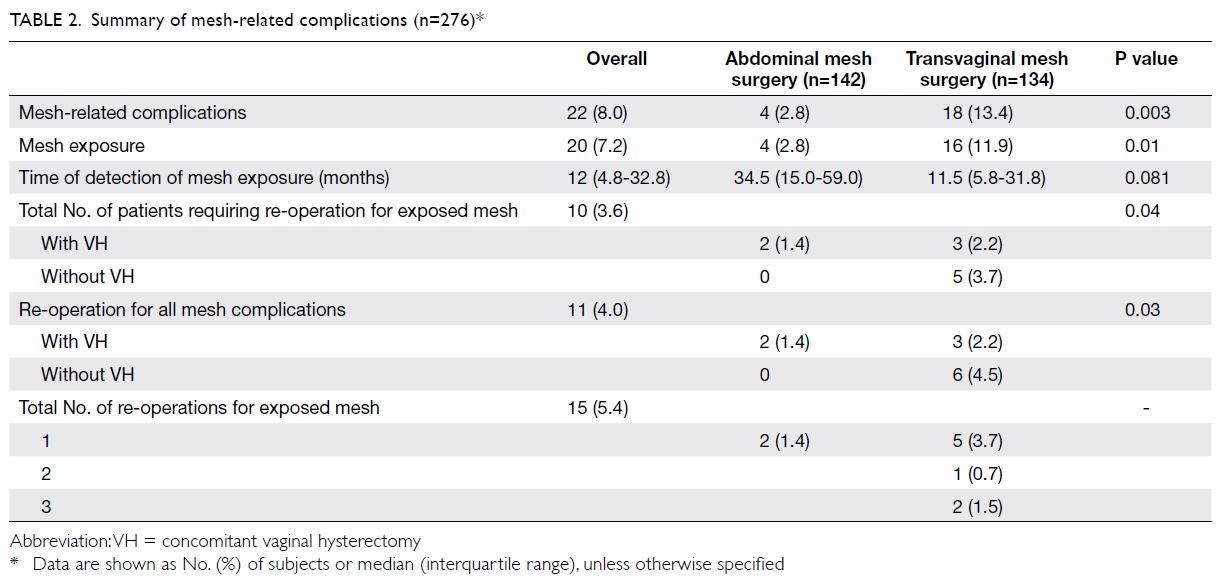
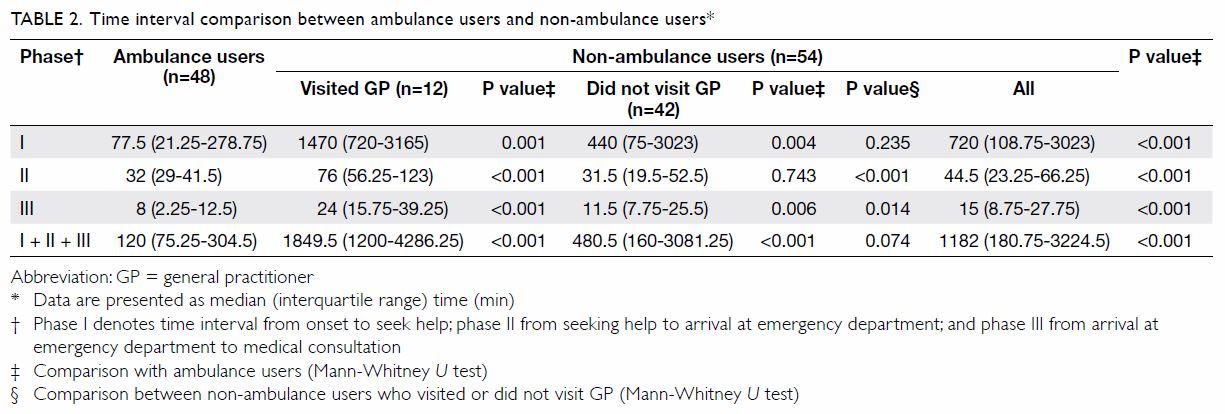
Among the 1119 valid questionnaires analysed, 767
(68.5%) were found to have mild to severe depressive symptoms (Table
2).
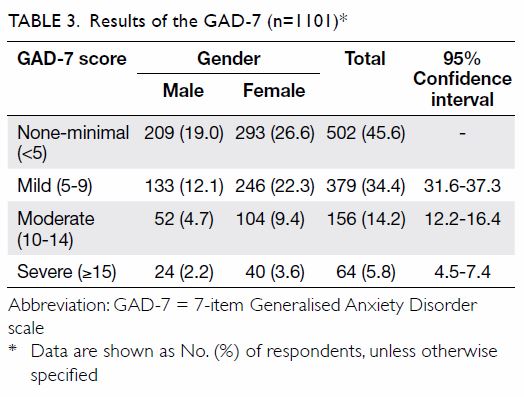
For screening of anxiety symptoms, 18 of the 1119
respondents were excluded because they did not complete the GAD-7
questionnaires; therefore, results are based on the 1101 valid responses.
A total of 599 (54.4%) respondents were found to have mild to severe
anxiety symptoms. A higher prevalence of anxiety was observed in females
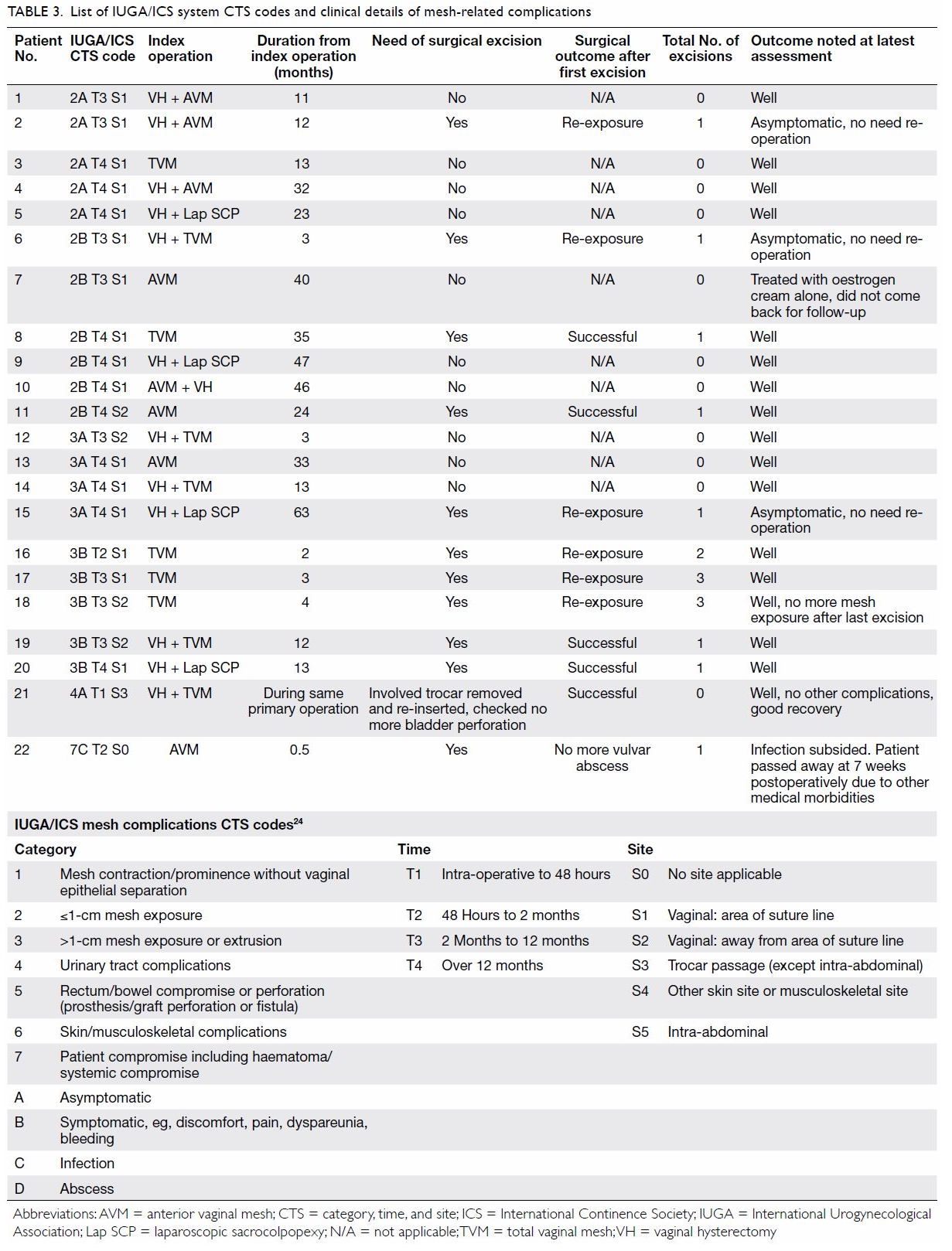
than males in all three categories of severity (Table 3).
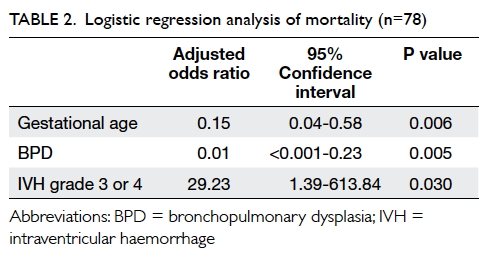
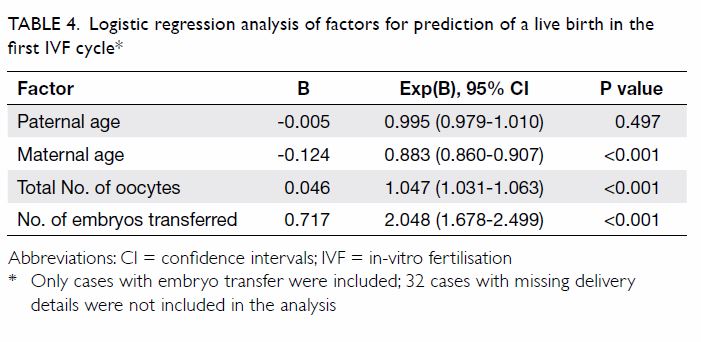
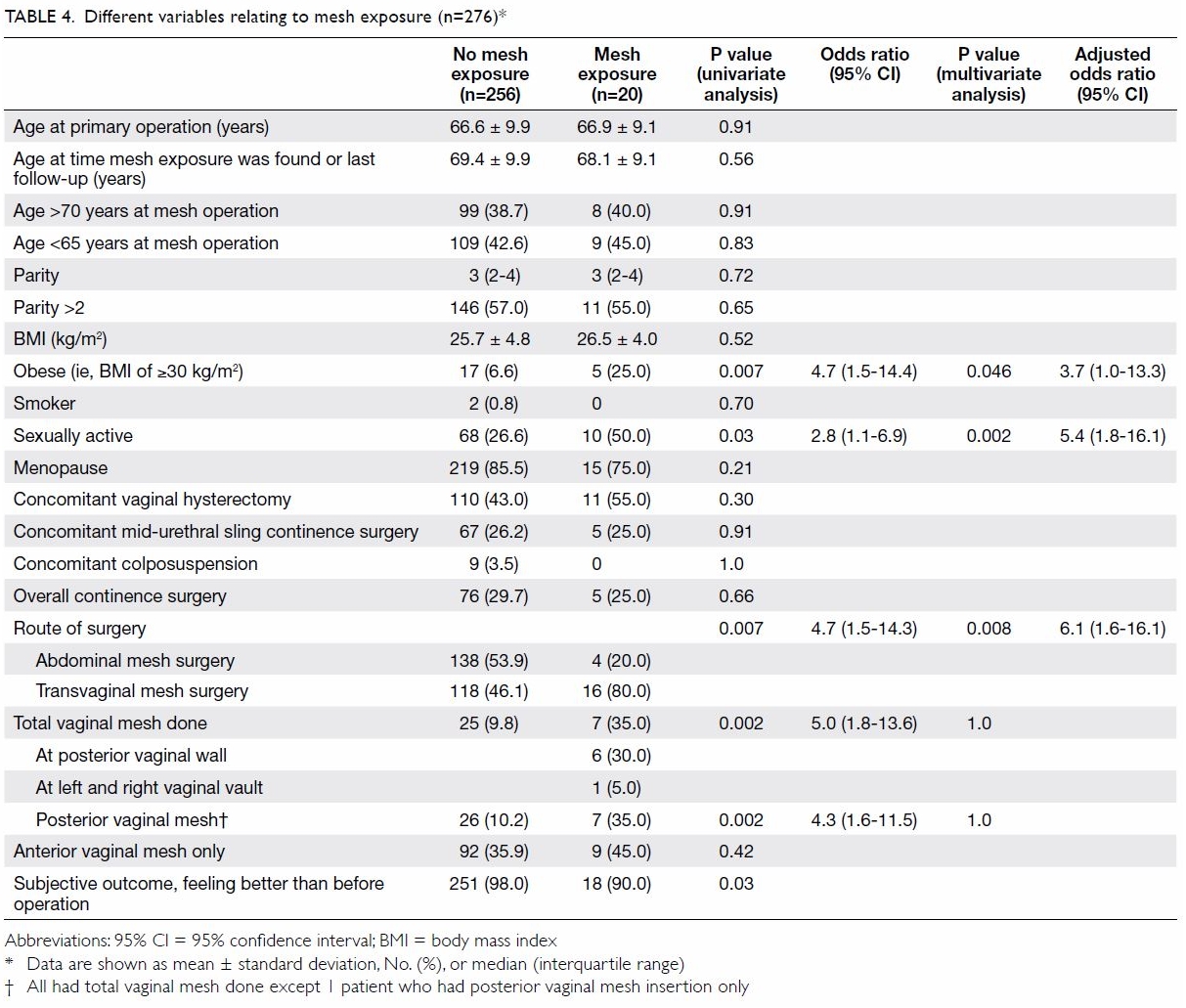
The binary logistic regression model for the
effects of multiple covariates on the odds of having mild to severe
depressive symptoms, on the basis of the PHQ-9 results, was statistically
significant with χ2=375.006, P<0.001, with 18 degrees of freedom (Table
4). The model explained 43.1% of the variance in depression
severity, as shown by Nagelkerke R2, and correctly classified
79.6% of the cases. Mild to severe anxiety symptoms, as screened by GAD-7,
were significantly associated with mild to severe depressive symptoms
(P<0.001). In contrast, several lifestyle and psychosocial variables,
including regular exercise (P<0.001), self-confidence (P=0.01),
satisfaction with academic performance (P=0.019), and optimism towards the
future (P<0.001) were inversely related to mild to severe depressive
symptoms.
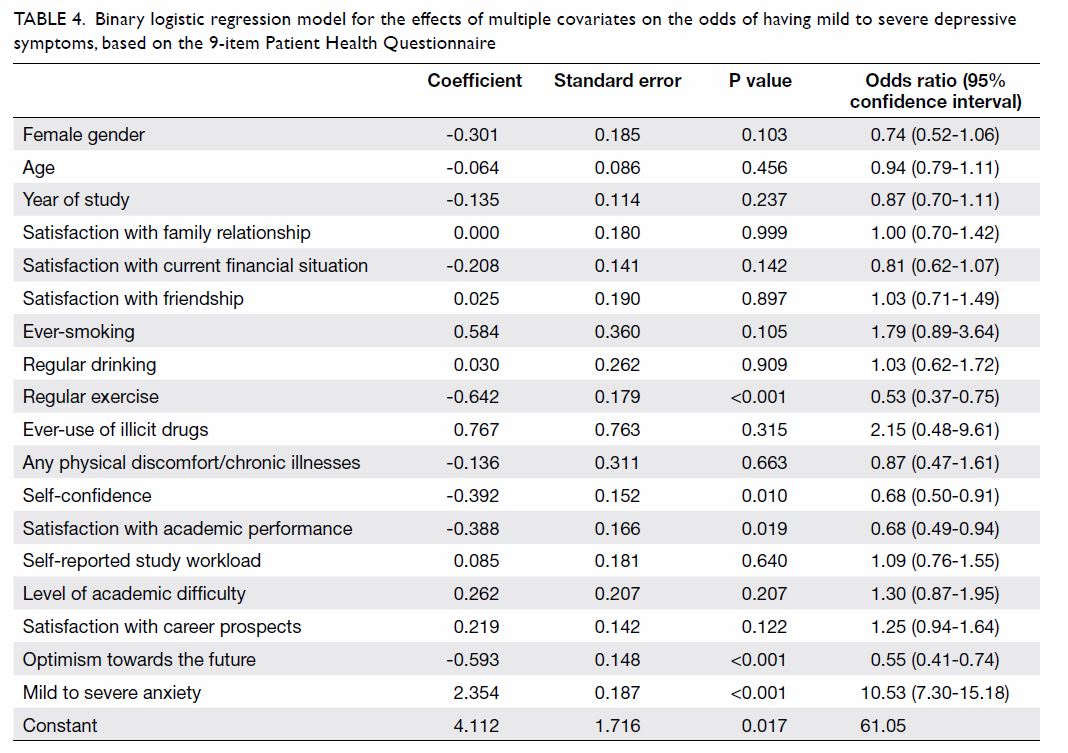
Table 4. Binary logistic regression model for the effects of multiple covariates on the odds of having mild to severe depressive symptoms, based on the 9-item Patient Health Questionnaire
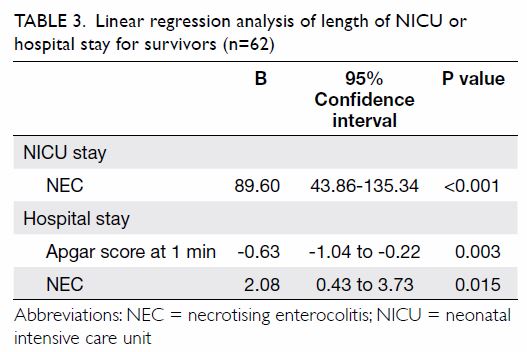
Another binary logistic regression model for the
effects of multiple covariates on the odds of having mild to severe
anxiety symptoms, on the basis of the GAD-7 results, was statistically
significant with χ2=374.842, P<0.001, with 20 degrees of
freedom (Table 5). The model explained 41.3% of the variance
in depression severity, as shown by Nagelkerke R2, and
correctly classified 76.9% of the cases. Mild to severe anxiety symptoms
were associated with level of academic difficulty (P=0.028) and mild to
severe depressive symptoms, as screened by PHQ-9 (P<0.001). Certain
lifestyle and psychosocial variables, including satisfaction with
friendship (P=0.001), sleep quality (P=0.003), and self-confidence
(P=0.003) were inversely associated with mild to severe anxiety symptoms.
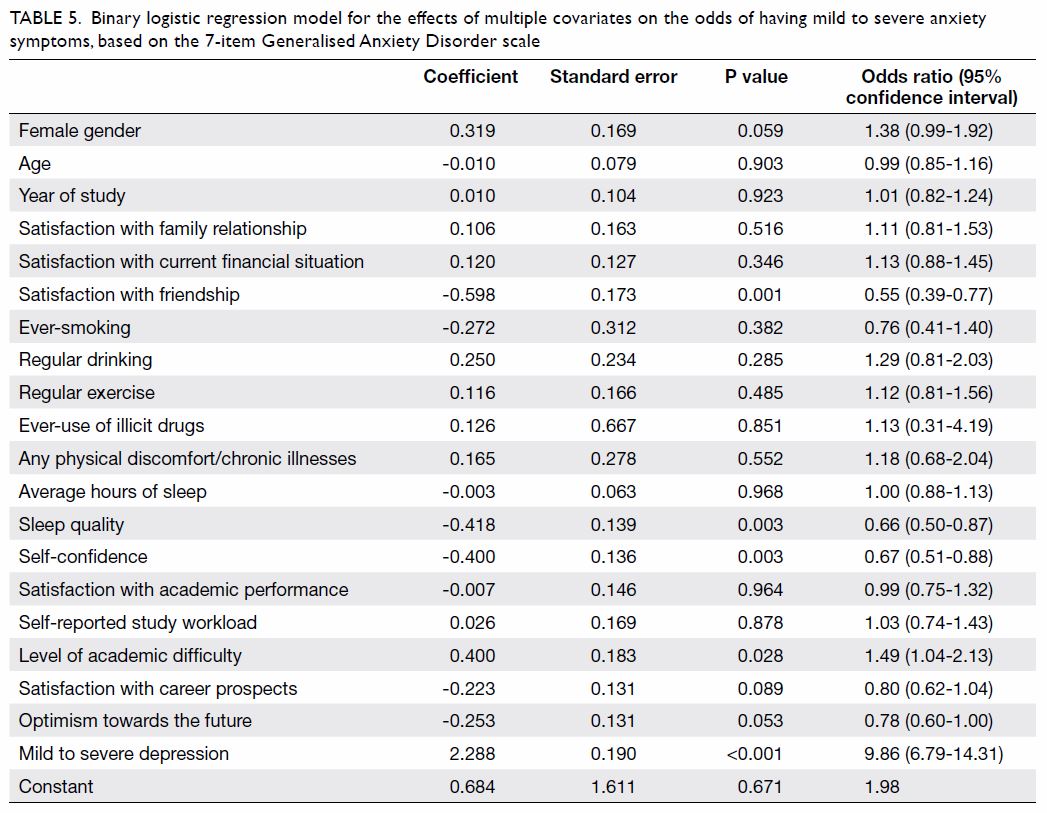
Table 5. Binary logistic regression model for the effects of multiple covariates on the odds of having mild to severe anxiety symptoms, based on the 7-item Generalised Anxiety Disorder scale
Discussion
The results revealed that 68.5% of respondents had
mild to severe depressive symptoms and 54.4% had mild to severe anxiety
symptoms. These rates are higher than and comparable to the results of a
similar survey conducted by the University of Hong Kong 10 years ago,
respectively.4 This could be
attributed to the increasing academic pressure after major reforms of the
education system in Hong Kong,12
uncertainties in career prospects due to fluctuations in the
socio-political environment, and the more prevalent use of social media.13
This study revealed that mild to severe depressive
symptoms, as screened by PHQ-9, were associated with mild to severe
anxiety symptoms, as screened by GAD-7. This is consistent with previous
studies, including a study in patients with depression showing that 85% of
those with major depression were also diagnosed with generalised anxiety.14 Sharing common risk factors and
symptoms may explain their co-existence.15
16 Another study has shown that
anxiety is more frequently an antecedent of depression, but the reverse
relationship was not observed.17
This could be explained by the withdrawal and submissive techniques
adopted by individuals with anxiety, in response to social exclusion.18 However, a definite causal relationship remains
unclear.
Regular exercise could decrease the occurrence of
depression via both physiological and psychological mechanisms. Exercise
exerts an excitatory effect on the monoamine and endorphin
neurotransmitter systems; notably, monoamines are depleted in patients
with depression.19
Psychologically, exercise is reported to improve self-esteem and
self-perception through self-actualisation and gaining pleasure from an
expanded social circle.20
Psychosocial factors, including higher
self-confidence, satisfaction with academic performance, and optimism
towards the future are inversely related to depressive symptoms. These
could be the presentation or consequences of depression; these factors may
have protective effects against developing depression.21 Optimism towards the future, apart from personal
factors, is also related to community factors. Risk factors include
community disadvantage, safety, and discrimination; an important
protective factor is community connectedness. These factors were
associated with depressive symptoms.22
This implies that, in addition to promoting personal mental health,
community-level risk and protective factors that affect individuals’
optimism towards future are sufficiently important to receive broader
attention.
For the analysis of anxiety, the level of academic
difficulty was associated with mild to severe anxiety symptoms. A higher
level of academic difficulty may create greater stress and anxiety for
students; subsequent unsatisfactory academic results may complete the
vicious cycle. Several tips were suggested by the Anxiety and Depression
Association of America (ADAA) regarding test anxiety reduction.23 Female gender was not associated with mild to severe
anxiety symptoms, which conflicts with previous studies.15 No clear causality was identified, but some potential
aetiologies include higher stress levels from academic and interpersonal
issues.
Satisfaction with friendship, sleep quality, and
self-confidence were three factors inversely associated with mild to
severe anxiety symptoms. Having good interpersonal relationships with
peers could be a protective factor against anxiety; however, symptoms of
anxiety may also hinder friendship, thus explaining the inverse
relationship. Insomnia is a symptom of anxiety. According to a survey
conducted by ADAA, worrying about falling asleep at night was identified
as a cause for an increased level of anxiety.24
Recommendations that could help in falling asleep and achieving better
sleep quality include maintaining 7 to 9 hours of uninterrupted sleep,
establishing a regular bedtime routine, and avoiding electronic gadgets,
coffee, and tea, as well as designing a relaxing environment for sleeping.24 Lack of self-confidence could be
a cause of anxiety, but anxiety symptoms could also diminish one’s
self-confidence. A bilateral association is possible.
There were several limitations in our study.
Firstly, as with all cross-sectional studies, it was not possible to
establish causality between the identified factors and symptoms of
depression and anxiety. Hence, the identified factors are regarded as
associated factors, which could be either the causes or the results of
depression or anxiety. To further explore the relationships between these
factors and symptoms of depression and anxiety, we reviewed the available
literature to provide supporting evidence. Secondly, convenience sampling
was adopted, but there could be self-selection bias when inviting students
to complete the questionnaires, limited by the locations and times at
which questionnaire distribution was conducted. The questionnaires were
distributed in the open areas of the eight UGC-funded universities in Hong
Kong. Withdrawn university students affected by depression or anxiety
might not have been reached or might have refused to participate in the
study; hence, the results may be underestimates. Thirdly, we could not
attain our target number of 150 students from each university, except from
the Hong Kong Polytechnic University. Importantly, this study used
screening questionnaires to assess the depressive and anxiety symptoms
experienced by the students, but no clinical diagnoses were made by health
care professionals. However, with limited resources, the use of validated
screening questionnaires was considered a cost-effective approach to
explore the situation in general and thus was used in this study. Possible
directions for further studies include the implementation of stratified
random sampling, expansion of the sample size of the study, and
performance of a reliability test to reduce information bias. Including
detailed backgrounds of students’ disciplines and faculties, as well as
ensuring a diverse population to include both local and non-local
students, as well as Chinese-speaking and non-Chinese-speaking students,
could allow further subgroup analysis; students from different faculties
may experience depressive symptoms or anxiety symptoms at different
severities due to differences in curricula. In this research, participants
who were screened to have depressive and/or anxiety symptoms were not
notified because of the lack of identifiable personal information.
Provision of the results to participants and recommendations regarding
help-seeking information are suggested for future studies. This study
result may not fully reflect the severity of depressive and anxiety
symptoms among students, as the study method could not reach severely
depressed individuals who had socially isolated themselves.
Conclusion
This study showed that over 50% of university
students in the eight UGC-funded universities expressed some degree of
depressive symptoms (68.5%) or anxiety symptoms (54.4%). Notably, 9% of
these university students exhibited moderate to severe depressive symptoms
and 5.8% of the studied students showed severe anxiety symptoms. Students
with regular exercise, higher self-confidence, better satisfaction with
academic performance, and more optimism towards the future experienced
fewer depressive symptoms. Students with better satisfaction with
friendship, better sleep quality, higher self-confidence, and lower levels
of academic difficulty experienced fewer anxiety symptoms.
Author contributions
Concept or design of study: KWC Lun, CK Chan.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the article: All authors.
Critical revision for important intellectual content: KWC Lun, CK Chan.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the article: All authors.
Critical revision for important intellectual content: KWC Lun, CK Chan.
Acknowledgement
We thank Dr LM Ho, assistant IT director of the
Division of Epidemiology and Biostatistics of School of Public Health of
the University of Hong Kong, for his statistical support and advice in
conducting this study. We also thank the School of Public Health of the
University of Hong Kong, for its support in conducting this study.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
Funding/support
The LKS Faculty of Medicine, University of Hong
Kong reimbursed project expenses under the title of Health Research
Project for HKD500.
Ethical approval
This study was reviewed and approved by the
Institutional Review Board of the University of Hong Kong/Hospital
Authority Hong Kong West Cluster.
References
1. Hong Kong Jockey Club Centre for Suicide
Research and Prevention, The University of Hong Kong. Statistics.
Available from: https://csrp.hku.hk/statistics/. Accessed 18 Sep 2018.
2. Centre for Health Protection, Department
of Health, Hong Kong SAR Government. Depression: beyond feeling blue.
Non-Communicable Diseases Watch 2012. Available from:
https://www.chp.gov.hk/files/pdf/ncd_watch_sep2012.pdf. Accessed 20 Apr
2016.
3. Centre for Health Protection, Department
of Health, Hong Kong SAR Government. Population Health Survey 2003/2004.
Collaborative project of Department of Health and Department of Community
Medicine, University of Hong Kong, Available from:
https://www.chp.gov.hk/files/pdf/report_on_population_health_survey_2003_2004_en.pdf.
Accessed 20 Apr 2016.
4. Wong JG, Cheung EP, Chan KK, Ma KK, Tang
SW. Web-based survey of depression, anxiety and stress in first-year
tertiary education students in Hong Kong. Aust N Z J Psychiatry
2006;40:777-82. Crossref
5. Lam LC, Wong CS, Wang MJ, et al.
Prevalence, psychosocial correlates and service utilization of depressive
and anxiety disorders in Hong Kong: the Hong Kong Mental Morbidity Survey
(HKMMS). Soc Psychiatry Psychiatr Epidemiol 2015;50:1379-88. Crossref
6. Cairns KE, Yap MB, Pilkington PD, Jorm
AF. Risk and protective factors for depression that adolescents can
modify: a systematic review and meta-analysis of longitudinal studies. J
Affect Disord 2014;169:61-75. Crossref
7. Yu X, Tam WW, Wong PT, Lam TH, Stewart
SM. The Patient Health Questionnaire-9 for measuring depressive symptoms
among the general population in Hong Kong. Compr Psychiatry
2012;53:95-102. Crossref
8. Wang W, Bian Q, Zhao Y, et al.
Reliability and validity of the Chinese version of the Patient Health
Questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry
2014;36:539-44. Crossref
9. Martin A, Rief W, Klaiberg A, Braehler
E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9)
in the general population. Gen Hosp Psychiatry 2006;28:71-7. Crossref
10. Spitzer RL, Kroenke K, Williams JB,
Löwe B. A brief measure for assessing generalized anxiety disorder: the
GAD-7. Arch Intern Med 2006;166:1092-7. Crossref
11. Tong X, An D, McGonigal A, Park SP,
Zhou D. Validation of the Generalized Anxiety Disorder-7 (GAD-7) among
Chinese people with epilepsy. Epilepsy Res 2016;120:31-6. Crossref
12. Yeh YC, Yen CF, Lai CS, Huang CH, Liu
KM, Huang IT. Correlations between academic achievement and anxiety and
depression in medical students experiencing integrated curriculum reform.
Kaohsiung J Med Sci 2007;23:379-86. Crossref
13. O’Keeffe GS, Clarke-Pearson K, Council
on Communications and Media. The impact of social media on children,
adolescents, and families. Pediatrics 2011;127:800-4. Crossref
14. Hall RC, Platt DE, Hall RC. Suicide
risk assessment: a review of risk factors for suicide in 100 patients who
made severe suicide attempts. Evaluation of suicide risk in a time of
managed care. Psychosomatics 1999;40:18-27. Crossref
15. Blanco C, Rubio J, Wall M, Wang S, Jiu
CJ, Kendler KS. Risk factors for anxiety disorders: common and specific
effects in a national sample. Depress Anxiety 2014;31:756-64. Crossref
16. van Ameringen M. Comorbid anxiety and
depression in adults: Epidemiology, clinical manifestations, and
diagnosis. 15 Jun 2017. Available from:
https://www.uptodate.com/contents/comorbid-anxiety-and-depression-in-adults-epidemiology-clinical-manifestations-and-diagnosis.
Accessed 24 Jul 2017.
17. Muris P. Normal and Abnormal Fear and
Anxiety in Children and Adolescents. London: Elsevier; 2007.
18. Richards CS, O’Hara MW, editors. The
Oxford Handbook of Depression and Comorbidity. New York: Oxford University
Press; 2014.Crossref
19. Buckworth J, Dishman RK. Exercise
Psychology. Champaign: Human Kinetics; 2002.
20. Faulkner GE, Taylor AH, editors.
Exercise, Health and Mental Health: Emerging Relationships. New York:
Routledge; 2005. Crossref
21. Wang KT. Perfectionism, depression,
and self-esteem: a comparison of Asian and Caucasian Americans from a
collectivistic perspective [dissertation]. Pennsylvania: The Pennsylvania
State University; 2007.
22. Stirling K, Toumbourou JW, Rowland B.
Community factors influencing child and adolescent depression: A
systematic review and meta-analysis. Aust N Z J Psychiatry 2015;49:869-86.
Crossref
23. Anxiety and Depression Association of
America. Test Anxiety. Available from:
https://www.adaa.org/living-with-anxiety/children/test-anxiety. Accessed
23 Mar 2017.
24. Anxiety and Depression Association of
America. Stress and Anxiety Interferes with sleep. Available from:
https://www.adaa.org/understanding-anxiety/related-illnesses/other-related-conditions/stress/stress-and-anxietyinterfere.
Accessed 23 Mar 2017.