Hong Kong Med J 2023 Dec;29(6):545–7 | Epub 18 Dec 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Novel contrast echocardiographic features of cardiac myxoma with cystic degeneration: a case report
Derek PH Lee, MB, ChB, FHKCP1; TW Ho, MB, BS2; Ivan MH Wong, MB, BS, FHKCP1; Eric CY Wong, MB, BS, FRCP1; Michael KY Lee, MB, BS, FRCP1
1 Department of Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Pathology, Queen Elizabeth Hospital, Hong Kong SAR, China
Corresponding author: Dr Derek PH Lee (lph748@ha.org.hk)
Case presentation
A 63-year-old lady presented to our emergency
department with a 6-month history of chronic cough
and progressive shortness of breath on exertion with
New York Heart Association class III heart failure.
She also reported episodic non-exertional chest pain
and bilateral ankle swelling. On examination, she was
afebrile, with blood pressure 121/77 mm Hg, heart
rate 97 beats/min and respiratory rate 16 breaths/min.
Cardiac examination revealed an elevated jugular
venous pressure at 4 cm above the sternal notch with
a right parasternal heave. A loud pulmonary second
heart sound and a diastolic murmur were detected.
The murmur was variable with posture and was
best heard at the apex in the right lateral position
on end expiration. Respiratory examination showed
clear lung fields, and mild pitting ankle oedema
was noted. Chest X-ray revealed enlarged bilateral
pulmonary trunks. Electrocardiogram showed sinus
rhythm of 94 beats/min, right axis deviation and
tall right precordial R waves. Inflammatory markers
were within normal range. However, levels of highly
sensitive troponin I and N-terminal prohormone
of brain natriuretic peptide were elevated (85 ng/L
and 1786 ng/L, respectively). Bedside transthoracic
echocardiography screening in the emergency
department revealed a large left atrial mass. The
clinical impression was pulmonary hypertension
secondary to a left-sided cardiac tumour.
The patient had a history of left parotid
pleomorphic adenoma for which she had undergone
partial excision 15 years ago. She had no history of
cardiopulmonary disease and no family history of
cardiac tumours.
Differential diagnosis
The top differential diagnosis of primary cardiac
tumour was cardiac myxoma but other primary
malignant tumours such as sarcoma and secondary
cardiac tumours were possible. Given the clinical
history, absence of fever and normal inflammatory
markers, an infective process was deemed unlikely.
Investigations
A detailed transthoracic echocardiography
examination demonstrated a double-barrel–shaped
left atrial mass with central echolucent space of
5.8 cm × 2.7 cm in size (Fig 1a). The mass was seen
attached to a thick echogenic base at the interatrial
septum and was prolapsing through the mitral valve
into the left ventricle during each diastolic phase,
causing a mild degree of mitral regurgitation and
significant mitral inflow obstruction. The average
mean gradient across the mitral valve was 15 mm Hg
(Fig 1b). Bi-atrial enlargement was seen. The
right ventricle was dilated with preserved systolic
function. There was a significant degree of pulmonary
hypertension and right ventricular systolic pressure
was 87 mm Hg. Left ventricular size and systolic
function were normal. A contrast echocardiographic
study was performed by intravenous administration
of SonoVue (Bracco Diagnostics Inc, Milan, Italy)
and revealed an initial contrast enhancement at the
base of the stalk (Fig 1c), followed by delayed contrast
enhancement of the central echolucent space (Fig 1d and 1e) and subsequent early contrast washout prior
to the cardiac chambers (Fig 1f).
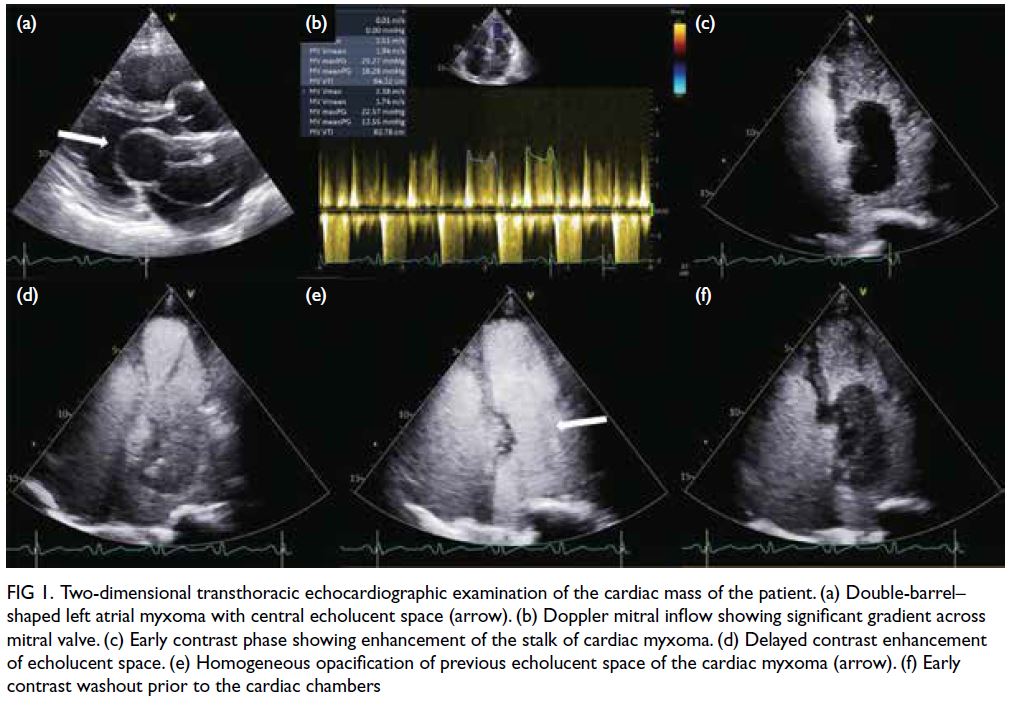
Figure 1. Two-dimensional transthoracic echocardiographic examination of the cardiac mass of the patient. (a) Double-barrel–shaped left atrial myxoma with central echolucent space (arrow). (b) Doppler mitral inflow showing significant gradient across mitral valve. (c) Early contrast phase showing enhancement of the stalk of cardiac myxoma. (d) Delayed contrast enhancement of echolucent space. (e) Homogeneous opacification of previous echolucent space of the cardiac myxoma (arrow). (f) Early contrast washout prior to the cardiac chambers
Urgent in-house cardiothoracic surgical
consultation was obtained and immediate open-heart
excision of the cardiac tumour was performed.
Intraoperative findings showed a large left atrial
cystic mass with an internal solid component
and wide-based stalk attaching to the interatrial
septum. The mitral valve appeared normal. The
left atrial mass, along with the stalk and part of the
involved interatrial septum, was resected en bloc.
No significant mitral regurgitation was detected
with saline testing and there was no interatrial
flow detected on intraoperative transoesophageal
echocardiography following resection. The patient
was successfully decannulated and the sternum was
closed uneventfully.
Gross examination of the specimen showed
a solid cystic tumour measuring 5.5 cm × 4.5 cm
× 2.5 cm with a base measuring 2.5 cm × 1.5 cm
(Fig 2a). The tumour was carefully cut open to reveal multilocular surfaces with small gelatinous
semitranslucent areas. Serially sectioned specimens
showed prominent cystic change with solid myxoid
areas adjacent to the base (Fig 2b). Microscopic
examination showed thick-walled blood vessels at the
stalk of the mass (Fig 2c). The tumour cells exhibited
a classic concentric arrangement around capillary
and a halo of matrix around cellular clusters (Fig 2d).
Sampling from the cystic area revealed morphology
similar to the rest of the tumour. Immunostaining
for calretinin showed positive nuclear staining in
tumour cells (Fig 2e). Overall histopathological
features were compatible with cardiac myxoma.
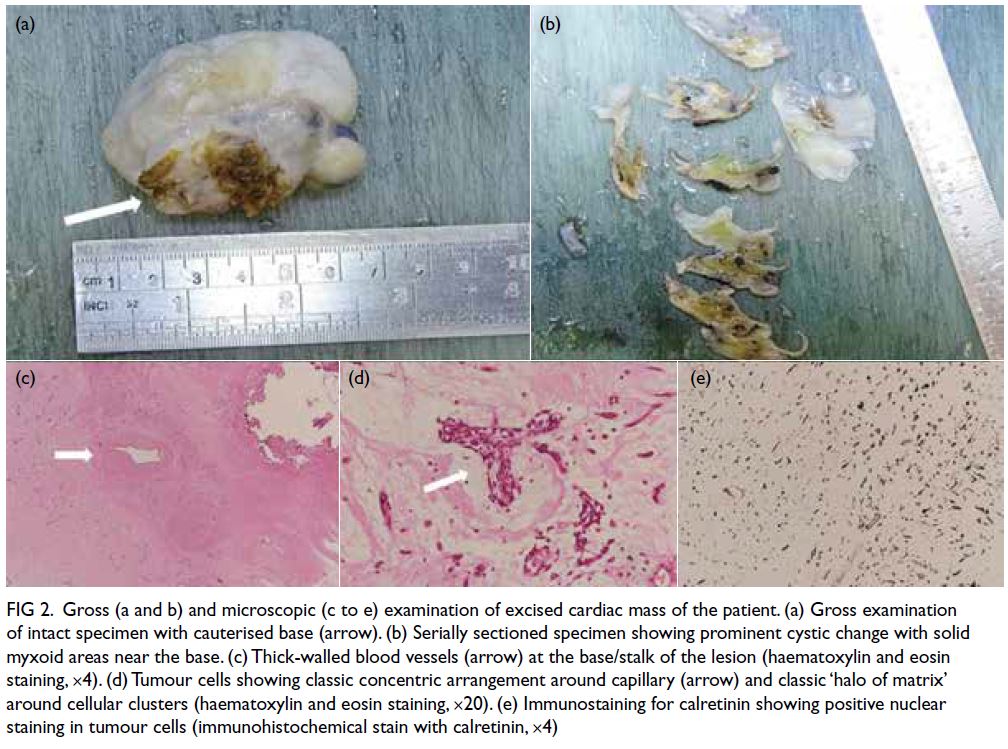
Figure 2. Gross (a and b) and microscopic (c to e) examination of excised cardiac mass of the patient. (a) Gross examination of intact specimen with cauterised base (arrow). (b) Serially sectioned specimen showing prominent cystic change with solid myxoid areas near the base. (c) Thick-walled blood vessels (arrow) at the base/stalk of the lesion (haematoxylin and eosin staining, ×4). (d) Tumour cells showing classic concentric arrangement around capillary (arrow) and classic ‘halo of matrix’ around cellular clusters (haematoxylin and eosin staining, ×20). (e) Immunostaining for calretinin showing positive nuclear staining in tumour cells (immunohistochemical stain with calretinin, ×4)
Management
The patient made an uneventful postoperative
recovery and pre-discharge echocardiogram showed
no residual mass, significant mitral regurgitation or
pericardial effusion. She did not report any chest
pain or shortness of breath. She was discharged
uneventfully on postoperative day 7.
Follow-up
The patient was followed up in the outpatient clinic 2 months after discharge. Her exercise tolerance had improved and ankle swelling resolved. She did not complain of any chest pain or shortness of breath.
Her chronic cough had subsided.
Discussion
Cystic degeneration of cardiac myxoma is rare. It
is caused by foci of myxoma stromal liquefaction
resulting in a cyst-like structure with clear fluid
content. The stalk of cardiac myxoma is typically
dominated by the presence of large, thick-walled
and occasionally dysplastic arteries giving rise to the
described ‘tumour blush’ occasionally observed at
coronary angiography.1 Previous histopathological
studies of cardiac myxomas have shown that these
tumours produce vascular endothelial growth factor
that likely induces angiogenesis for tumour growth.2 3
On microscopic tissue examination of our patient,
we also found these thick-walled blood vessels
at the stalk of cardiac myxoma (Fig 2c). Vascular
communication between the stalk and the fluid
content of cardiac myxoma with cystic degeneration
has not been described before.
The use of contrast agent in echocardiography
is particularly helpful in assessing vascular
communications, vascularity of cardiac masses and
to differentiate masses from intracardiac thrombi.4
Compared with contrast computed tomography, contrast echocardiography offers the advantage
of capturing the extended real-time contrast
enhancement sequence. To the best of our knowledge,
only one case report has discussed the feature of
cystic degeneration of cardiac myxoma on contrast
echocardiography.5 We have described a novel feature
on contrast echocardiography of cystic degeneration
of myxoma: initial enhancement of the base of stalk
(Fig 1c), followed by delayed contrast enhancement
of the central echolucent space (Fig 1d and 1e) and
early contrast washout prior to the cardiac chambers
(Fig 1f). This suggested the presence of some degree
of vascularity at the contrast-enhanced base of stalk
with vascular communication between the stalk and
the echolucent cystic space of the mass.
Conclusion
Left atrial tumour is a rare cause of pulmonary
hypertension. Variable diastolic murmur may
be detected on physical examination. Cystic
degeneration of cardiac myxoma is rare with
specific features on contrast echocardiography.
Histopathological examination of the cardiac mass
provided a histological basis for the unique contrast
enhancement pattern on echocardiography.
Author contributions
Concept or design: DPH Lee.
Acquisition of data: All authors.
Analysis or interpretation of data: DPH Lee.
Drafting of the manuscript: DPH Lee.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: DPH Lee.
Drafting of the manuscript: DPH Lee.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and has provided consent for all procedures and publication.
References
1. Tazelaar HD, Maleszewski JJ. Tumors of the heart and pericardium. In: Fletcher CD, author. Diagnostic histopathology of tumors. 5th ed. Philadelphia (PA): Elsevier; 2021: 13.
2. Kono T, Koide N, Hama Y, et al. Expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: a study of fifteen patients. J Thorac Cardiovasc Surg 2000;119:101-7. Crossref
3. Sakamoto H, Sakamaki T, Kanda T, et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ J 2004;68:488-93. Crossref
4. Lanzoni L, Bonapace S, Dugo C, et al. Cardiac masses
and contrast echocardiography. Eur Heart J Cardiovasc
Imaging 2022;23 (Suppl 1):jeab289.296. Crossref
5. Yousaf H, Patel M, Khandheria BK, et al. Myxoma blush with contrast echocardiography. Int J Cardiol 2013;166:e1-2. Crossref

