Emphysematous pyelonephritis (class IIIa) managed with antibiotics alone
DOI: 10.12809/hkmj144301
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Emphysematous pyelonephritis (class IIIa) managed with antibiotics alone
Vivek Chauhan, MD; Rajesh Sharma, MD
Department of Medicine, Dr RPGMC Kangra at Tanda, Kangra, 176001, Himachal Pradesh, India
Corresponding author: Dr Vivek Chauhan (drvivekshimla@yahoo.com)
Abstract
A 54-year-old male with long-standing diabetes
presented with vague left flank pain for 5 days
with uncontrolled blood glucose. The patient was
commenced on insulin and injectable ceftriaxone
empirically, for possibly acute pyelonephritis.
Ultrasound examination revealed extensive
emphysematous pyelonephritis of upper half of
left kidney with involvement of perinephric space.
Computed tomography of abdomen confirmed the
diagnosis of emphysematous pyelonephritis which
was categorised as class IIIa. The recommended
treatment for class IIIa emphysematous
pyelonephritis is nephrectomy but the patient refused
to give consent for surgery or even percutaneous
drainage. Thus, the patient was continued on medical
management alone and surprisingly showed marked
recovery over the next few days. There were no new
complications, and the patient was discharged after
2 weeks of antibiotics with 2 more weeks of oral
antibiotics. After 4 months, the ultrasound showed
normal kidneys. We present this case because it
adds to the little existing evidence that conservative
management can successfully cure patients with
class IIIa emphysematous pyelonephritis, although
supplementation with percutaneous drainage would
have been better in this case.
Introduction
Emphysematous pyelonephritis (EPN) is a
complication seen in patients with long-standing
diabetes and is characterised by formation of gas
in the renal parenchyma.1 Such gas may extend
into the peri-nephric or para-renal spaces and
additionally in advanced case, there may be bilateral
and extensive involvement.1 The primary cause
of EPN is urinary tract infection caused by either
Gram-negative organisms or anaerobes in some
cases. The diagnosis of EPN is confirmed by non-contrast
computed tomography (CT) and treatment
is usually based on the CT classification. In the
case of class IIIa EPN, the current recommended
treatment of choice is nephrectomy or percutaneous
drainage. Herein, however, we managed our patient
with class IIIa EPN conservatively with only medical
management, without percutaneous drainage or
even haemodialysis.
Case report
In August 2010, a 54-year-old male with diabetes
for the past 20 years presented to the Dr Rajendra
Prasad Government Medical College in Himachal
Pradesh, India. His chief complaints were pain in
left flank for 5 days and fever for 1 day. The symptom
of pain was gradually progressive, continuous type
localised in left flank. The patient developed fever on
the fourth day which was recorded as 102°F (38.9°C).
He was given ornidazole and ofloxacin tablets orally
at a primary health centre and was then referred to
our medical department because of uncontrolled
blood glucose. He reported a history suggestive of
osmotic symptoms and burning micturition for 5
days, and there were no other significant present
or past complaints. The vital signs showed blood
pressure of 90/60 mm Hg at presentation, pulse rate
of 106 beats/min, respiratory rate of 16 breaths/min, and
temperature of 103°F (39.4°C) was recorded. On
examination, the patient was conscious, oriented,
and there was tenderness in the left renal angle, but
all the other systems were normal. The biochemistry
results showed a haemoglobin level of 101 g/L
(reference range, 120-150 g/L), total leukocyte count
of 17 700/mm3, neutrophils of 88%, and erythrocyte
sedimentation rate of 68 mm/h (reference range,
0-22 mm/h), with normal platelet counts and liver
functions. Metabolic control was very poor with
glycated haemoglobin level of 12.8%, urea of 19.27
mmol/L (reference range, 4-8.3 mmol/L), and serum
creatinine of 132.6 µmol/L (reference range, 50-110 µmol/L). Urine examination showed no albumin,
traces of sugar, while urine microscopy showed
15 to 20 pus cells per high-power field, and therefore
urine culture and sensitivity was ordered. However,
the urine and blood culture reports later turned out
to be sterile.
The patient was managed for uncontrolled
blood glucose and a possible diagnosis of acute
pyelonephritis of the left kidney was made clinically.
He was started on human mixed insulin along with
ceftriaxone (1 g administered intravenously every
12 hours). Chest X-ray showed raised left dome of
diaphragm. Ultrasound examination of abdomen
revealed EPN of left kidney with destruction of
upper portion and extending into the perinephric
space near the upper pole. The treatment given was
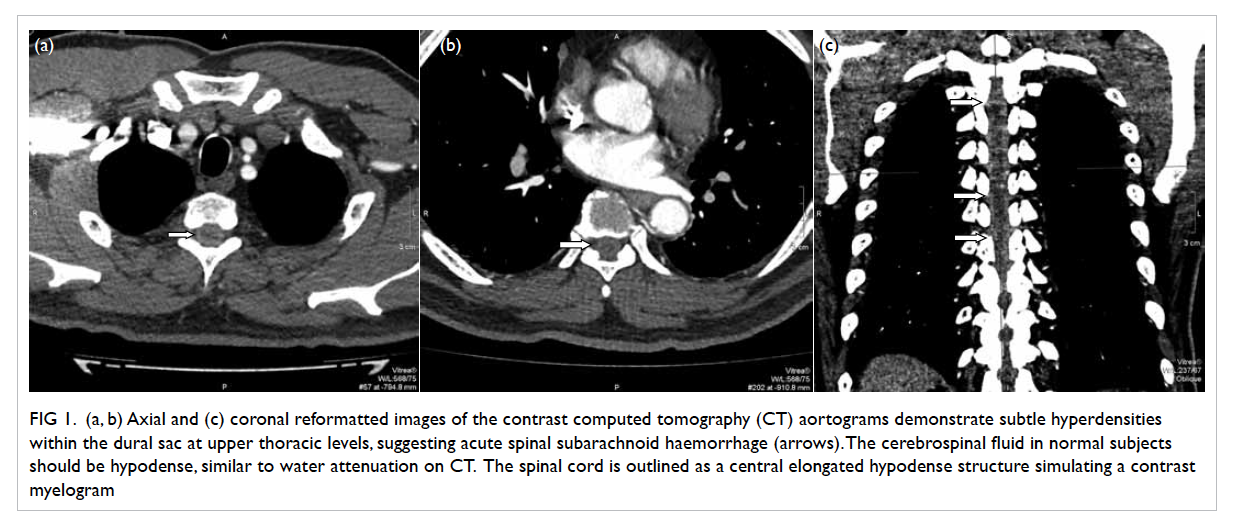
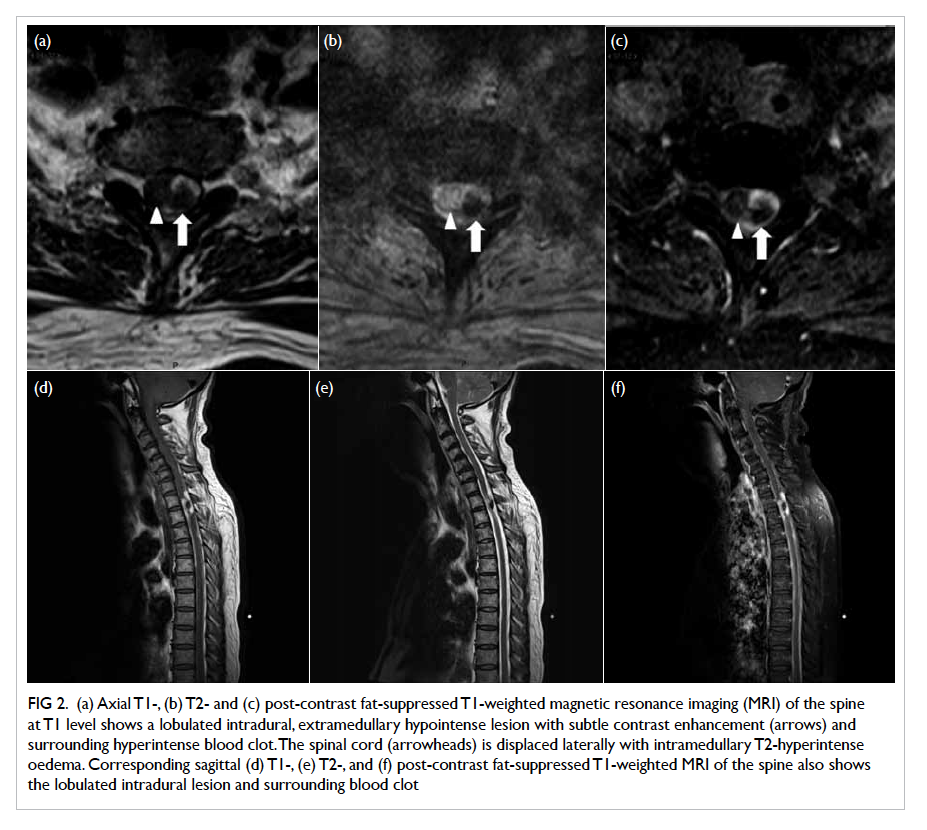
continued and the abdominal CT scan revealed evidence of
a small renal calculus on the left side with extensive
EPN of the left kidney and the presence of small
amount of fluid in the left perinephric space (Fig). In
this patient, the CT classification was class IIIa EPN.
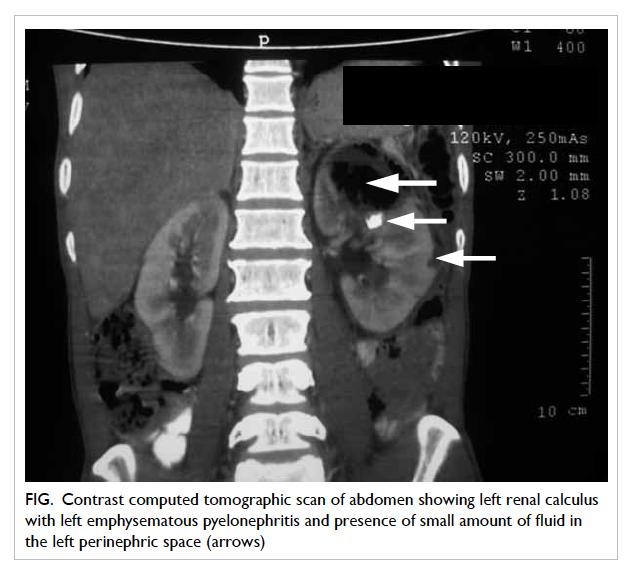
Figure. Contrast computed tomographic scan of abdomen showing left renal calculus with left emphysematous pyelonephritis and presence of small amount of fluid in the left perinephric space (arrows)
With regard to further management, we gave an
option of nephrectomy to the patient, but he refused
to give consent for any surgical intervention. The
patient refused even for percutaneous nephrostomy,
so we continued with medical management alone. To
our surprise, throughout the hospital stay, the patient
remained afebrile and fully conscious. He showed a
steady recovery in clinical and laboratory parameters
without any new complaints or complications.
After 1 week of insulin and antibiotic (ceftriaxone)
treatment, his renal functions remained normal
with a serum creatinine of 106.1 µmol/L and blood
glucose was adequately controlled with human
mixed insulin injections over the next few days.
The patient was started on intravenous ceftriaxone
empirically on admission and this was continued for
a total of 2 weeks. During this period, the patient
became fully active and mobile. Due to absence of
any complications or features of sepsis after 2 weeks
of treatment, the patient was discharged on oral
antibiotics for a further 2 weeks. After 4 months,
ultrasound examination showed normal kidneys and
even the small calculus had disappeared.
Discussion
Emphysematous pyelonephritis is a well-known
condition which mainly affects the diabetic
population (90%) and seen in patients with chronic
diabetes.1 Some interesting facts about EPN are that
it has a strong female preponderance with female-to-male
ratio of 5:1, mean age of occurrence is around
the fifth decade, and most often it involves left kidney
in almost 60% of cases.1 The gas contains carbon
dioxide mainly because of bacterial fermentation
of glucose along with some hydrogen and nitrogen.
Presentation is often vague with non-specific
abdominal pain, fever, and tenderness in the renal
angle.1
Since most cases are sporadic and spread over
time, it is not easy to formulate definitive guidelines
for all cases of EPN. The best evidence available for
EPN is dependent on a few large prominent studies
by Huang and Tseng2 in 2000 with 48 patients and
a review by Pontin and Barnes1 in 2009 with 52
patients. Such studies serve as a good guide for the
rest of the world, but may suffer from bias from the
treating physicians and surgeons. So it is prudent to
report all cases of EPN with details of presentation,
extent of involvement, line of management, and
outcomes.
The medical management of EPN was
proposed a long time ago when it was first described
by Schultz and Klorfein in 1962.3 In fact, in their
report it was stated that “Vigorous treatment of
uncontrolled diabetes and the use of appropriate
antibiotics, avoiding anaesthesia and surgery seems
a more rational approach.”3
The current management recommendations
are based on the CT-based classification used by
Huang and Tseng2 in which they divide EPN into
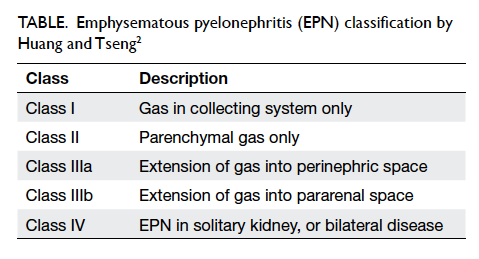
classes I, II, IIIa, IIIb, and IV (Table).
Class I and II EPN can be managed by medical
therapy alone or combined with percutaneous
drainage. Huang and Tseng2 found that class IIIa EPN
showed a 71% failure rate following percutaneous
drainage with a 29% mortality rate, and those
belonging to class IIIb had a 30% failure rate with
a 19% mortality rate. Thus surgery became the
norm for extensive disease except where there was
a single kidney or bilateral involvement. Unilateral
nephrectomy can be done with a low mortality risk
in most centres.1
The most recent updates, however, recommend
a more conservative approach. Recently, reports of
successful management using percutaneous drainage
and medical management alone have started coming
from across the world even with bilateral and
extensive disease.4 5 6 A recent case series describes
eight cases of EPN with medical management alone.6
Of these eight cases, four were class IV EPN, five
cases required haemodialysis, whereas four needed
percutaneous drainage. The authors successfully
used injections of imipenem for 10 days in five
patients, cefoperazone + sulbactam for 14 days
in two patients, and piperacillin + tazobactam for 14
days in one patient.6 Antibiotics that are effective in
treating EPN include quinolones, beta-lactamase
inhibitors, cephalosporins, and aminoglycosides,
alone or in combinations mainly to cover Gram-negative
bacteria like Escherichia coli, Klebsiella
pneumoniae, Proteus spp, Streptococcus spp,
Pseudomonas spp, and anaerobes like Bacteroides
fragilis, and Clostridium septicum.7 8
The reason for successful shift in management
is probably because nowadays EPN gets picked up
early on CT showing very small air pockets. Such
patients are therefore more likely to respond to
conservative management. Even for larger EPN,
percutaneous drainage rather than nephrectomy can
be the standard of care in a majority of patients.1
Since India is one of the epicentres of diabetes
epidemic, we wish to emphasise that systematic
reporting of all EPN cases from this region can aid in
formulating guidelines, especially for poor resource
settings like ours. Most of our cases go unreported
due to lack of proper record maintenance. Our
patient had received ciprofloxacin tablets for 5 days
before presenting to our hospital. Early initiation of
antibiotics may have limited the progress of EPN
in this patient and may have also been responsible
for the sterile cultures that were reported. The
patient had long-standing diabetes and also had
a renal calculus, both of which can predispose to
infection and subsequently lead to EPN. The patient
was categorised as class IIIa EPN, who responded
exceptionally well to antibiotics alone and had
complete resolution of EPN on follow-up.
In conclusion, this is a rare case report because
our patient recovered without drainage, surgery, or
even haemodialysis. There have been reports that
in class IIIa EPN that medical management alone
with percutaneous drainage has a 92% failure rate.2
We wish to emphasise sensitisation of treating
physicians regarding early investigations using
contrast CT scan in diabetes patients with fever,
renal angle tenderness, and vague abdominal pain.
We do not recommend medical treatment for all
such cases, but follow-up should be based upon
patient’s response to the initial treatment.
References
1. Pontin AR, Barnes RD. Current management of
emphysematous pyelonephritis. Nat Rev Urol 2009;6:272-9. Crossref
2. Huang JJ, Tseng CC. Emphysematous pyelonephritis:
clinicoradiological classification, management, prognosis,
and pathogenesis. Arch Intern Med 2000;160:797-805. Crossref
3. Schultz EH Jr, Klorfein EH. Emphysematous pyelonephritis.
J Urol 1962;87:762-6.
4. Flores G, Nellen H, Magaña F, Calleja J. Acute bilateral
emphysematous pyelonephritis successfully managed by
medical therapy alone: a case report and review of the
literature. BMC Nephrol 2002;3:4. Crossref
5. Tahir H, Thomas G, Sheerin N, Bettington H, Pattison
JM, Goldsmith DJ. Successful medical treatment of acute
bilateral emphysematous pyelonephritis. Am J Kidney Dis
2000;36:1267-70. Crossref
6. Kolla PK, Madhav D, Reddy S, Pentyala S, Kumar
P, Pathapati RM. Clinical profile and outcome of
conservatively managed emphysematous pyelonephritis.
ISRN Urol 2012;2012:931982. Crossref
7. Chen MT, Huang CN, Chou YH, Huang CH, Chiang
CP, Liu GC. Percutaneous drainage in the treatment of
emphysematous pyelonephritis: 10-year experience. J Urol
1997;157:1569-73. Crossref
8. Shokeir AA, El-Azab M, Mohsen T, El-Diasty T.
Emphysematous pyelonephritis: a 15-year experience with
20 cases. Urology 1997;49:343-6. Crossref