DOI: 10.12809/hkmj134082
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Pallidal deep brain stimulation: an effective treatment in Chinese patients with tardive dystonia
Peter YM Woo, MB, BS, FRCS (Edin)1; Danny TM Chan, MB, ChB, FRCS (Edin)1; XL Zhu, BMed (Jinan), FRCS (Edin)1; Jonas HM Yeung, MB, ChB, FRCP (London)1; Anne YY Chan, MB, ChB, MRCP1; Angie CW Au, MB, ChB2; KM Cheng, MRCPsych, FHKAM (Psychiatry)2; KY Lau, MSc1; YK Wing, FRCPsych, FHKAM (Psychiatry)3; Vincent CT Mok, MB, BS, FRCP (Edin)1; WS Poon, MB, ChB, FRCS (Edin)1
1 Movement Disorder Group, Division of Neurosurgery, Department of
Surgery and Department of Medicine and Therapeutics, Prince of Wales
Hospital, The Chinese University of Hong Kong, Hong Kong
2 Department of General Adult Psychiatry, Castle Peak Hospital, Hong
Kong
3 Department of Psychiatry, Prince of Wales Hospital, The Chinese
University of Hong Kong, Hong Kong
Corresponding author: Prof WS Poon (wpoon@cuhk.edu.hk)
Abstract
Tardive dystonia is an iatrogenic complication of
dopamine receptor antagonist medication such
as first-generation antipsychotics. It occurs in
up to 2% of patients and only 10% recover after
stopping medication. Deep brain stimulation for
primary dystonia has proven to be effective and
its application for secondary dystonias is gaining
acceptance. We report our experience in treating
three ethnic Chinese schizophrenia patients with
severe medically refractory tardive dystonia by
globus pallidus internus deep brain stimulation.
Preoperatively, all required assistance with essential
activities of daily living and two were bed-bound.
The mean Burke-Fahn-Marsden Dystonia Rating
Scale score was 61 (range, 44-80) and mean Global
Dystonia Rating Scale score was 47 (range, 40-52). No
procedure-related complications were encountered.
By 3 months all could return to unassisted living
and walk with support with a mean of 77% and 66%
improvement in the Burke-Fahn-Marsden Dystonia
Rating Scale and Global Dystonia Rating Scale scores,
respectively. Quality-of-life assessment performed
for two patients using the EuroQol-5 dimensions
visual analogue scale showed a mean improvement
of 86% at 3 months. On clinical follow-up, the effect
was well maintained for a period of 3 to 10 years.
Pallidal deep brain stimulation is a safe and highly
effective form of symptomatic treatment for patients
with medically refractory tardive dystonia.
Introduction
Tardive dystonia (TD) is an iatrogenic extrapyramidal
movement disorder caused by the
use of dopamine receptor antagonists (DRAs).
Antipsychotic medications, especially belonging to
the first-generation class, are typically responsible
for the condition.1 The reported prevalence of this
adverse drug reaction among adults varies from
1% to 2% and only 10% of patients recover after
medication termination.2 The latency of onset could
range from several days to 20 years.3
Initial management strategies include
withdrawal of the offending antipsychotic,
switching to a second-generation antipsychotic
such as clozapine, and suppressive therapies such
as benzodiazepines or tetrabenazine, but none has
demonstrable efficacy.3 In recent years deep brain
stimulation (DBS) of the globus pallidus interna (GPi)
has proven to be effective for secondary dystonias
such as TD.4 5 6 7 We report our experience with using
DBS for treating three young ethnic Chinese patients
with severe TD refractory to pharmacological
management.
Case reports
Clinical assessment
Three paranoid schizophrenia patients were referred
by psychiatrists for TD satisfying the proposed
diagnostic criteria by Adityanjee et al.2 All three
patients were managed by the Movement Disorder
Group, Division of Neurosurgery, Department
of Surgery and Department of Medicine and
Therapeutics, Prince of Wales Hospital, Hong Kong.
Despite withdrawal of the antipsychotic responsible
for the condition, and switching to second-generation
medication, they all experienced progressive TD with
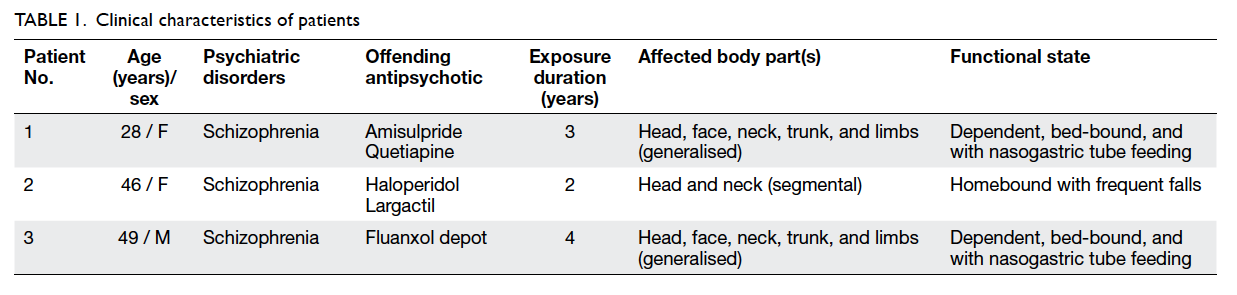
severe disability. Their clinical details are outlined in
Table 1. Investigations excluding other movement
disorders included serum levels of ceruloplasmin,
copper, thyroid-stimulating hormone, thyroxine
as well as syphilis, and antinuclear antibody titres
were either undetected or within normal limits.
Magnetic resonance imaging of the brain was also
unremarkable. For objective clinical evaluation video
filming, the Burke-Fahn-Marsden Dystonia Rating
Scale (BFMDRS) and the Global Dystonia Rating
Scale (GDS) scores were documented before the
procedure, at 1 week and 3 months postoperatively.8 9 Quality of life (QoL) assessments were performed
for two of the patients, preoperatively and 3 months
postoperatively, using the Chinese-version validated
EuroQoL-5 dimensions (EQ-5D) instrument.10
This instrument serves as a basis for comparing
health outcomes using a basic ‘common core’ of
health-related QoL characteristics. The dimensions
covered were mobility, self-care, usual activities,
pain/discomfort, and anxiety or depression.11 After
targeted questioning of these five domains, the
patient was required to report on a visual analogue
scale (VAS) from a score of zero (worst imaginable
health state) to 100 (best imaginable health state).
Operative procedure
We performed frame-based stereotaxy using
the Leksell coordinate frame and multipurpose
stereotactic arc system (Elekta AB, Stockholm,
Sweden). The target was set at the ventroposterior
part of GPi. The target coordinates were 19 mm lateral
to the inter-commissural line (from the anterior
commissure to the posterior commissure [AC-PC
line]), 2 mm anterior to the mid-commissural point,
and 4 mm inferior to the AC-PC line. The trajectory
on the coronal view was 0 degree from the mid-sagittal
plane. On sagittal view, it was 60 degrees
from the AC-PC line.
The operation was performed under local
anaesthesia for patients 1 and 2 in December 2004
and March 2009, respectively. Patient 3 was operated
on under total intravenous general anaesthesia in
April 2011 because of uncontrolled and vigorous
trunk and limb movement. For patient 3, propofol
was stopped 10 minutes before commencing
microelectrode recording (MER). In all patients,
MER was successfully performed and bilateral
pallidal discharges were recorded.
For patients 1 and 2, visual-evoked MERs
were registered at the target point by shining a
light source in their eyes. If no capsular responses
were evoked during macrostimulation below a
threshold of 4 mA (0.1 ms PW, 130 Hz), a permanent
quadripolar electrode (Medtronic 3387; Medtronic,
Minneapolis [MN], US) was implanted bilaterally.
During the same operative sitting, a pulse generator
(Kinetra; Medtronic, Minneapolis [MN], US) was
connected and implanted subcutaneously in the left
infraclavicular region. A postoperative computed
tomographic scan verified the final DBS electrode
positions.
Results
Patients’ mean age was 41 years, ranging from 28
to 49 years with a mean duration of antipsychotic
exposure of 3.7 years. Patients 2 and 3 received
first-generation antipsychotics and patient 1 was
administered second-generation medication.
Preoperatively, all patients required either assisted
living or was homebound. The craniospinal regions
were the most seriously affected regions and all
demonstrated opisthotonus and retrocollis. Chronic
rhythmic neck hyperextension movements resulted
in premature cervical spondylosis, and patients 1 and
3 required nasogastric tube feeding. At the time of
surgery, all were unable to walk independently. The
mean preoperative BFMDRS score was 61, ranging
from 44 to 80, and mean GDS score was 47, ranging
from 40 to 52. There were no treatment-related
complications and the procedure was well tolerated.
As expected, there was no minimal response before
stimulation but after pulse generator activation,
marked amelioration of dystonic symptoms was
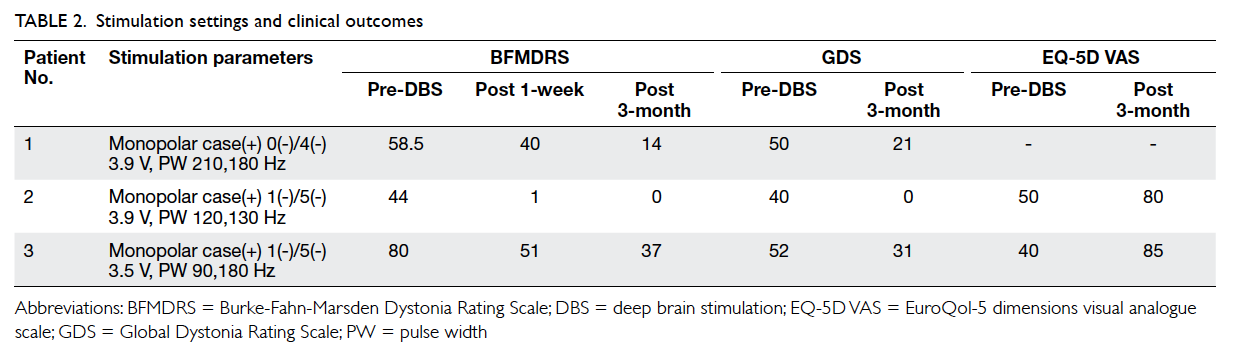
observed. The stimulation settings were set at
monopolar mode (anodal case and cathodal target
contact) and are presented in Table 2. Notable
improvement was found in patients 2 and 3 within
the first week. In that week, the mean BFMDRS score
decreased by over 30%. By 3 months, all patients
reached a stable state with a mean of 77% and 66%
improvement in the BFMDRS and the GDS scores,
respectively. Patients 1 and 3 overcame dysphagia to
resume oral dietary intake and all patients were able
to perform basic activities of daily living without
assistance. No psychiatric side-effects were detected.
Patients 2 and 3 experienced an improvement in
QoL as reflected by a mean increase in the EQ-5D
VAS score by 86% (Table 2).
Discussion
Tardive dystonia is an iatrogenic complication
belonging to a group of DRA-induced movement
disorders known as the tardive syndromes. Although
it may co-exist with tardive dyskinesia, TD is a
distinct condition with different epidemiology,
clinical features, prognosis, and treatment outcome.3
Literature shows a higher prevalence of TD in men
than in women, with a male-to-female ratio of 2.4:1,
and a mean age of onset of 36 years.2 The first-generation
antipsychotics such as chlorpromazine,
flupenthixol, and haloperidol are the strongest
aetiological factors although second-generation
medications and antiemetics such as metoclopramide
have also been implicated.1 2 Although the mean
duration of medication exposure varies from 3.3
to 6.6 years, there does not appear to be a minimal
‘safe’ period and symptom onset can occur as early
as within days or weeks.1
In this report, all patients fulfilled Adityanjee et
al’s diagnostic criteria2 for TD, namely, (1) chronic
dystonia characterised by sustained, stereotypical
involuntary muscle contractions or posture; (2)
dystonia developing during or within 2 months’
discontinuation of treatment with DRAs; (3) other
secondary dystonias adequately ruled out, and (4)
a negative family history for dystonia. The onset of
symptoms is insidious initially, involving one body
region and, typically, progressing to segmental, ie
craniocervical, or generalised dystonia. Torticollis
or retrocollis is characteristic of TD and chronic
repetitive movements could result in spondylotic
myelopathy or even fractures.12 Truncal involvement
manifests as opisthotonus and, in the severest of
cases, patients could become bed-bound, reduced
to a state of dependency.2 In contrast to classic
orobuccolingual tardive dyskinesia, TD is largely
irreversible with 90% of patients failing to achieve
remission at a mean follow-up of 6.6 years.13
The limitations of medical treatment
reflect incomplete understanding of the complex
pathophysiology of TD. Multiple theories have been
proposed of which the most prominent describe
postsynaptic dopamine receptor hypersensitivity,
degeneration of striatal cholinergic neurons, and
gamma-amino-butyric acid–containing neurons.13
In contrast, the success of pallidal DBS in the
treatment of primary dystonias led to its adoption
for secondary dystonias such as TD.5 6 7 14 15 In this
series we demonstrate a significant beneficial effect
for medically refractory TD where rapid remarkable
improvements in motor symptoms were observed
within days without exacerbation of psychiatric
symptoms. Our results are in agreement with other
DBS outcome trials for TD. A systematic review of
17 studies involving 50 TD patients concluded that
pallidal stimulation led to a mean improvement in
BFMDRS scores by 77.5% (95% confidence interval,
71.4%-83.3%).4 The motor benefits are sustainable
with a documented duration of 41 months (range,
18-80 months).5 7 16 Long-term responses for 8 to
10 years have also been reported.6 17 Although most
data were from case studies or small trials, our
experience supports DBS as an effective and safe
surgical treatment modality that can considerably
improve QoL.
Involuntary dystonic movement imposes
unique technical difficulties not only for obtaining
preoperative brain scans of acceptable quality, but also
for performing the actual surgery. Sedation or general
anaesthesia may be needed for such procedures as
was needed in our patient 3. Furthermore, correct
choice of anaesthetic drug is critical for successful
MER. For example, propofol was found to affect
the recording quality of the subthalamic nucleus
of Parkinson’s disease patients.18 To overcome this
interference, lower doses of propofol were used;
these proved to be equally feasible to perform MER
under general anaesthesia.19 20 Due to the relatively rapid offset action of intravenous propofol, after 10
minutes of cessation, we were able to observe the
characteristic mean discharge frequencies of 20 to 40
Hz at the GPi target. The discharge pattern qualities
were similar to those of the other two patients for
whom the procedure was performed under local
anaesthesia. Our experience demonstrates that in
severely dystonic patients, total intravenous general
anaesthesia with propofol can produce comparable
clinical outcomes.
In 2014, patient 1 has received DBS for 10
years. In that period, she was capable of performing
daily activities at home without assistance and was
considered to have dystonia remission. However,
when the pulse generator battery approached
its end-of-life state, 2 years after implantation,
segmental dystonia reappeared over the neck and
hand regions. Her BFMDRS score rapidly rose
from 0 to 23 within a week. The symptoms were
relieved after battery replacement. The high voltage
and pulse width requirements in GPi stimulation
for TD can considerably shorten battery longevity
(Kinetra; Medtronic) to an average of 2 years. The
introduction of a non-invasive transcutaneous
rechargeable battery system (Activa; Medtronic,
Minneapolis [MN], US) with a 9-year life span is
an improved solution to frequent replacements.
Not only is it more cost-effective in terms of overall
battery costs, but also reduces the number of
surgical procedures. All three patients are currently
implanted with this new device. The daily recharging
process was convenient and non-disruptive, and
the pulse generator performance was as effective
as the non-rechargeable counterparts. With these
encouraging results, we have continued to provide
DBS as treatment for patients with refractory TD.
Two more cases were treated in 2013 and 2014,
respectively, with results as good as those in the
three cases reported here.
Conclusion
Medically refractory TD is a disabling and essentially
irreversible condition that can be successfully
managed by pallidal DBS. There is a need to
conduct multicentre trials to reliably assess and
define appropriate selection criteria for DBS as a
new therapeutic option. In our series, response to
stimulation can be observed as soon as within 1 week.
Our patients experienced remarkable alleviation
of dystonia symptoms at 3 months enabling them
to return to performing daily activities without
assistance, with no additional psychiatric side-effects.
On clinical follow-up, the effect was well
maintained for a period of 3 to 10 years.
Acknowledgements
The Oriental Daily News Charitable Fund, Oriental
Press Group Limited subsidised the purchase of the
implantable hardware devices.
Declaration
No conflicts of interests were declared by authors.
References
1. Burke RE, Fahn S, Jankovic J, et al. Tardive dystonia and
inappropriate use of neuroleptic drugs. Lancet 1982;1:1299. CrossRef
2. Adityanjee, Aderibigbe YA, Jampala VC, Mathews T.
The current status of tardive dystonia. Biol Psychiatry
1999;45:715-30. CrossRef
3. Kiriakakis V, Bhatia KP, Quinn NP, Marsden CD. The
natural history of tardive dystonia. A long-term follow-up
study of 107 cases. Brain 1998;121:2053-66. CrossRef
4. Mentzel CL, Tenback DE, Tijssen MA, Visser-Vandewalle
VE, van Harten PN. Efficacy and safety of deep brain
stimulation in patients with medication-induced tardive
dyskinesia and/or dystonia: a systematic review. J Clin
Psychiatry 2012;73:1434-8. CrossRef
5. Trottenberg T, Volkmann J, Deuschl G, et al. Treatment of
severe tardive dystonia with pallidal deep brain stimulation.
Neurology 2005;64:344-6. CrossRef
6. Chang EF, Schrock LE, Starr PA, Ostrem JL. Long-term
benefit sustained after bilateral pallidal deep brain
stimulation in patients with refractory tardive dystonia.
Stereotact Funct Neurosurg 2010;88:304-10. CrossRef
7. Gruber D, Trottenberg T, Kivi A, et al. Long-term effects
of pallidal deep brain stimulation in tardive dystonia.
Neurology 2009;73:53-8. CrossRef
8. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz
C, Friedman J. Validity and reliability of a rating scale for
the primary torsion dystonias. Neurology 1985;35:73-7. CrossRef
9. Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura
T; Dystonia Study Group. Rating scales for dystonia: a
multicenter assessment. Mov Disord 2003;18:303-12. CrossRef
10. Luo N, Chew LH, Fong KY, et al. Do English and Chinese
EQ-5D versions demonstrate measurement equivalence? An exploratory study. Health Qual Life Outcomes 2003;1:7. CrossRef
11. Pinto Prades JL. A European measure of health: the
EuroQol [in Spanish]. Rev Enferm 1993;16:13-6.
12. Konrad C, Vollmer-Haase J, Gaubitz M, Nabavi DG,
Reilmann R, Knecht S. Fracture of the odontoid process
complicating tardive dystonia. Mov Disord 2004;19:983-5. CrossRef
13. Margolese HC, Chouinard G, Kolivakis TT, Beauclair
L, Miller R. Tardive dyskinesia in the era of typical and
atypical antipsychotics. Part 1: pathophysiology and
mechanisms of induction. Can J Psychiatry 2005;50:541-7.
14. Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain
stimulation in primary generalized or segmental dystonia.
N Engl J Med 2006;355:1978-90. CrossRef
15. Vidailhet M, Jutras MF, Roze E, Grabli D. Deep brain
stimulation for dystonia. Handb Clin Neurol 2013;116:167-87. CrossRef
16. Sako W, Goto S, Shimazu H, et al. Bilateral deep brain
stimulation of the globus pallidus internus in tardive
dystonia. Mov Disord 2008;23:1929-31. CrossRef
17. Boulogne S, Danaila T, Polo G, Broussolle E, Thobois S.
Relapse of tardive dystonia after globus pallidus deep-brain
stimulation discontinuation. J Neurol 2014;261:1636-7. CrossRef
18. Raz A, Eimerl D, Zaidel A, Bergman H, Israel Z. Propofol
decreases neuronal population spiking activity in the
subthalamic nucleus of Parkinsonian patients. Anesth
Analg 2010;111:1285-9. CrossRef
19. Pinsker MO, Volkmann J, Falk D, et al. Deep brain
stimulation of the internal globus pallidus in dystonia:
target localisation under general anaesthesia. Acta
Neurochir (Wien) 2009;151:751-8. CrossRef
20. Hertel F, Züchner M, Weimar I, et al. Implantation of
electrodes for deep brain stimulation of the subthalamic
nucleus in advanced Parkinson’s disease with the aid of
intraoperative microrecording under general anesthesia.
Neurosurgery 2006;59:E1138; discussion E1138.