DOI: 10.12809/hkmj134049
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Tumefactive acute disseminated encephalomyelitis complicating human swine influenza (H1N1)
Amanda CY Chan, FHKCP, FHKAM (Medicine)1; SH Ng, FRCP (Lond & Edin), FHKAM (Medicine)2
1 Department of Medicine and Geriatrics, Tuen Mun Hospital, Tuen Mun,
Hong Kong
2 Division of Neurology, Department of Medicine and Therapeutics, The
Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Dr Amanda CY Chan (acychan@gmail.com)
Abstract
This report illustrates an adult patient
presenting with tumefactive acute disseminated
encephalomyelitis complicating human swine
influenza. Its presentation, diagnosis, investigation
findings, course, and response to treatment are
discussed herein.
Introduction
A new outbreak of influenza caused by a new strain
of H1N1, also known as ‘human swine influenza’
was first described in April 2009 in Mexico. This
strain was different from the rest, in that it had a
propensity to infect very healthy and young subjects,
and also caused severe manifestations, such as
acute respiratory distress, pneumonia, and even
death. Approximately 80% of affected patients were
younger than the age of 25 years.1
Since April 2009, there have been few reports
of the neurological complications of human swine
influenza.2 3 4 We report a case of severe human
swine influenza causing acute demyelinating
encephalomyelitis of the tumefactive form.
Case report
A 21-year-old woman with a history of diplegic
cerebral palsy and epilepsy was hospitalised for
breathlessness, cough, and fever for 3 days in January
2011. The initial chest X-ray was unremarkable, the
white cell count (WCC) was elevated (13 x 109 /L;
reference range [RR], 3.4-9.6 x 109 /L) and showed
neutrophil predominance (8.9 x 109 /L; RR, 1.27-6.2 x 109 /L). An initial nasopharyngeal swab for influenza A and influenza B was negative. She was treated
empirically with amoxicillin and clavulanic acid. She
developed generalised tonic-clonic convulsion and
desaturation 2 days later, for which she was intubated
and received intensive care. Computed tomography
of the brain showed multiple patchy hypodensities at
the grey/white junction and white matter of frontal,
parietal and temporal lobes on both sides, suspicious
of underlying white matter disease.
Bedside bronchoscopy showed an inflamed
mucosa, a small-sized airway with a distorted right
bronchus, and purulent sputum. Bronchoalveolar
lavage was positive for human swine influenza.
The patient was given a course of oseltamivir, and
later she received treatment with piperacillin and
tazobactam.
One week later, the patient remained
comatosed despite discontinuing sedation. Physical
examination showed an absent deep pain response,
wandering eyes with bilateral tonic pupils, and
sluggish response to light. The doll’s eye reflex was
absent, and the limbs were hypotonic and areflexic.
Autoimmune blood testing revealed nil
abnormal. Her erythrocyte sedimentation rate and
C-reactive protein level were elevated at 71 mm/h
(RR, 5-15 mm/h) and 92 mg/L (RR, 0-10 mg/L),
respectively. The serum antibody titre for influenza
type A showed a significant increase from 10 to 640
over 10 days. Other virus and atypical pneumonia
titres were also negative.
Electroencephalogram showed alpha coma
pattern, and intermittent generalised slow waves at
1-2 Hz, 50-100 µV. Periodic lateralised epileptiform
discharges at 1 Hz, 40-60 µV were evident over the
right frontocentral region lasting for 4 to 5 seconds.
Overall, the features were supportive of severe
encephalopathy and cerebral dysfunction.
Lumbar puncture yielded a high opening
pressure, and cerebrospinal fluid (CSF) protein was
elevated at 4.26 g/L (RR, 0.1-0.4 g/L), glucose 2.2
mmol/L (RR, 2.2-3.9 mmol/L), WCC 0.3 x 106 /L, red
cell count 0.6 x 106 /L, oligoclonal bands and Gram
stain were negative. Three serial CSF specimens
were sent for influenza A viral titres and showed
an upward trend. Herpes simplex virus polymerase
chain reaction (PCR), tuberculosis PCR, and
Cryptococcus were negative.
Magnetic resonance imaging (MRI) of the
brain, cervical and thoracic spines showed multiple
T1-weighted hypointense, and T2-weighted
hyperintense lesions up to 3 cm in diameter in
the cerebral white matter bilaterally, the genu
and splenium of the corpus callosum, external
and internal capsules, midbrain, dorsal pons, and
medulla oblongata. These lesions were contrast
non-enhancing. The lesions involving the cerebral
white matter and corpus callosum showed ring-like
peripheral restricted diffusion.
Long segments of T1-weighted hypointense
and T2-weighted hyperintense lesions were detected
along the whole spinal cord, with the cervical cord
being the most severely involved. The lesions at
the cervical cord were continuous with that at the
medulla oblongata (Fig). Overall, the features were in favour of acute disseminated encephalomyelitis
(ADEM) with tumefactive demyelination.
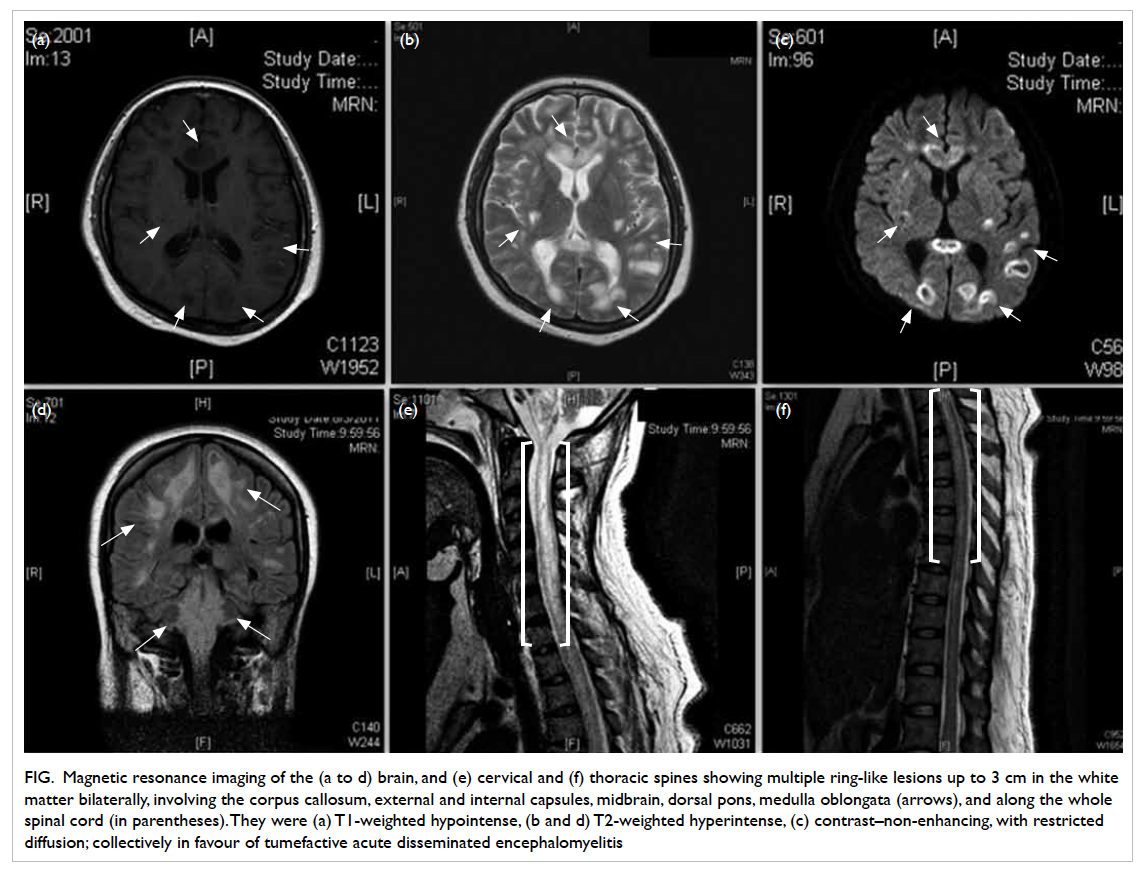
Figure. Magnetic resonance imaging of the (a to d) brain, and (e) cervical and (f) thoracic spines showing multiple ring-like lesions up to 3 cm in the white matter bilaterally, involving the corpus callosum, external and internal capsules, midbrain, dorsal pons, medulla oblongata (arrows), and along the whole spinal cord (in parentheses). They were (a) T1-weighted hypointense, (b and d) T2-weighted hyperintense, (c) contrast–non-enhancing, with restricted diffusion; collectively in favour of tumefactive acute disseminated encephalomyelitis
This patient was treated with pulsed
methylprednisolone, and later given two courses of
intravenous immunoglobulin. However, there was
no neurological improvement and the patient finally
succumbed.
Discussion
Since the first appearance of human swine influenza
in April 2009 until now, there have been reports of
neurological complications that mostly occurred in
the paediatric population. A few were also reported
in adults,2 3 4 but in them the presentations were not
as florid and radiologically severe. We report this
case of a 21-year-old, ambulatory and independent
woman, with a history of cerebral palsy and epilepsy.
She is one of the few adults to have ADEM as a
complication of human swine influenza, and the first
reported to have the tumefactive form.
Acute disseminated encephalomyelitis is an
inflammatory demyelinating disorder of the central
nervous system (CNS), which is thought to be due
to a T-cell hypersensitivity reaction.5 6 It is one of the many syndromes that can develop after vaccination
or a microbial infection, and has a 2- to 30-day
latency period.3 7
The typical MRI appearance is of demyelinating
lesions preferentially affecting white matter tracts in
a periventricular distribution. Diagnostic difficulty
occurs whenever these demyelinating lesions appear
to be solitary, large, or tumefactive. Tumefactive
lesions are usually defined as solitary lesions,
typically greater than 2 cm in diameter and imaging
characteristics resembling a tumour. They tend to
be circumscribed and have little in the way of mass
effect or vasogenic oedema, typically involving
the supratentorium, and are centred within the
white matter, although they may extend to involve
the cortical grey matter. The exact pathogenesis
is unknown. Approximately half of tumefactive
demyelinating lesions show pathological contrast
enhancement, usually in the form of rings. Commonly
they occur in the form of an open ring, with the
incomplete portions on the grey matter side of the
lesion. The enhancing portion of the ring is believed
to represent the leading edge of demyelination and
thus favours the white matter side of the lesion.
The central non-enhancing core represents a more
chronic phase of the inflammatory process.8
One must distinguish between infectious and
post-infectious encephalitis, as all causes of the
former should be excluded before concluding to the
latter diagnosis. This involves systemic screening for
herpes CNS infections, viruses endemic to specific
regions, and other common causes of infective
encephalitis. Other mimickers of ADEM include
CNS lymphomas, systemic diseases like systemic
lupus erythematosus, CNS vasculitis, and vascular,
toxic or infectious leukoencephalopathies.5 The time
course of ADEM, however, is usually hyperacute
or acute, whereas the others are usually more
chronic. Multiple sclerosis (MS) is also a differential
diagnosis, but less likely as in the CSF oligoclonal
bands were not present and protein was elevated,
and radiologically there were no plaques or lesions
disseminated in time. Although in most cases, ADEM
is seemingly diagnosed clinically by exclusion, the
definitive diagnosis of ADEM is histopathological.
Lesions are usually bilateral, although not
symmetrical, and mainly they involve the cerebral
white matter and brainstem. Occasionally the
cerebellum and spinal cord are involved. Small veins
and venules in the white matter are surrounded by
lymphocytes, macrophages and occasional plasma
cells, whereas arteries and arterioles are relatively
free of inflammation. Perivascular haemorrhages,
axonal fragmentation, inflammatory cells within the
leptomeninges, and subpial demyelination in the
brainstem and spinal cord may be present.7
For our patient, the diagnosis of tumefactive
ADEM was mainly made on clinical grounds and
typical radiological features. However, postmortem
brain biopsy was not performed. We undertook
reverse-transcription (RT) PCR analysis for swine
flu on CSF samples, but all results came back
negative. However, paired CSF and serum samples
for influenza A viral titres showed an increasing
trend. For patients with suspected neurological
complications of swine flu, the sensitivity and
specificity of RT PCR and viral titres specifically
on CSF samples have not been studied in detail
and warrant further investigation. Previous reports
of children with influenza A encephalitis in the US
showed that CSF PCR were all negative in the three
cases.9
The treatment of ADEM is borrowed from
that of MS. First-line treatment mainly involves
corticosteroids, which have been found to shorten the
duration of symptoms and halt disease progression.
Patients are given 6-methylprednisolone 6 to 8 g
over 6 to 8 days, followed by oral prednisolone at
tapering doses, but the prognosis remains variable.
Approximately 80% of patients have a full recovery
and ADEM is classically a monophasic disease.
However, relapses have been reported in 5% to
10% of cases. If relapses occur on more than one
occasion, a diagnosis of MS rather than multiphasic
disseminated encephalomyelitis is probably more
likely. Around 30% of patients are non-responders to
steroids. Half of these non-responders benefit from
treatment with intravenous immunoglobulin. Some
authors recommend the use of cyclophosphamide
in patients at high risk for relapse, either during the
first attack or when relapse occurs. However, overall
results have been disappointing.10
Conclusion
Herein we report one of the few adult cases of severe
tumefactive demyelinating ADEM complicating
human swine influenza infection. As different strains
of influenza continue to spread throughout the
world and in different populations, it is expected that
more neurological complications will be reported.
As it seems that neurological complications are
more common in young age-groups with existing
neurological diseases, prophylactic vaccination
should be considered for such patients. In addition,
resorting to early antiviral and immunomodulating
therapy should also be emphasised for this patient
group.
References
1. Novel Swine-Origin Influenza A (H1N1) Virus Investigation
Team, Dawood FS, Jain S, Finelli L, et al. Emergence of a
novel swine-origin influenza A (H1N1) virus in humans. N
Engl J Med 2009;360:2605-15. CrossRef
2. Kimura E, Okamoto S, Uchida Y, et al. A reversible lesion
of the corpus callosum splenium with adult influenza-associated
encephalitis/encephalopathy: a case report. J
Med Case Rep 2008;2:220. CrossRef
3. Athauda D, Andrews TC, Holmes PA, Howard RS.
Multiphasic acute disseminated encephalomyelitis
(ADEM) following influenza type A (swine specific H1N1).
J Neurol 2012;259:775-8. CrossRef
4. Wang J, Duan S, Zhao J, Zhang L. Acute disseminated
encephalomyelitis associated with Influenza A H1N1
infection. Neurol Sci 2011;32:907-9. CrossRef
5. Gupte G, Stonehouse M, Wassmer E, Coad NA,
Whitehouse WP. Acute disseminated encephalomyelitis: a
review of 18 cases in childhood. J Paediatr Child Health
2003;39:336-42. CrossRef
6. Ozkale Y, Erol I, Ozkale M, Demir S, Alehan F. Acute
disseminated encephalomyelitis associated with influenza
A H1N1 infection. Pediatr Neurol 2012;47:62-4. CrossRef
7. Love S. Demyelinating diseases. J Clin Pathol 2006;59:1151-9. CrossRef
8. Given CA 2nd, Stevens BS, Lee C. The MRI appearance of
tumefactive demyelinating lesions. AJR Am J Roentgenol
2004;182:195-9. CrossRef
9. Centers for Disease Control and Prevention (CDC).
Neurologic complications associated with novel influenza
A (H1N1) virus infection in children—Dallas, Texas, May
2009. MMWR Morb Mortal Wkly Rep 2009;58:773-8.
10. Marchioni E, Tavazzi E, Minoli L, et al. Acute disseminated
encephalomyelitis. Neurol Sci 2008;29 Suppl 2:S286-8. CrossRef

