Hong Kong Med J 2014;20(6):519–28 | Epub 29 Aug 2014
DOI: 10.12809/hkmj134116
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Management of secondary lymphoedema
TW Chiu, FHKAM (Surgery)
Division of Plastic Aesthetic and Reconstructive Surgery, Prince of Wales
Hospital, Shatin, New Territories, Hong Kong
Corresponding author: Dr TW Chiu (torchiu@surgery.cuhk.edu.hk)
Abstract
Lymphoedema is a chronic, progressive condition.
There is no cure but it is most easily managed with
early recognition and therapy; those who do not
have treatment tend to worsen rapidly and advanced
disease is more difficult to treat than early disease.
Surgery for lymphoedema is often regarded as a
last resort but traditional excisional techniques that
have been slightly modified for modern practice
have shown good results, whilst newer microsurgical
reconstruction techniques show promise although long-term results are lacking. This report provides
an update on the therapy of lymphoedema.
Introduction
Lymphoedema is characterised by an imbalance of
lymphatic flow leading to accumulation of protein-rich
fluid in the interstitium of subcutaneous tissues.
The consequent swelling may cause cosmetic and
functional impairment, with significant physical
and psychological morbidity. There is progressive
damage to the lymphatics with inflammation,
fibrosis and more swelling, eventually leading to
elephantiasis. Recurrent infection is a common
complication whilst lymphangiosarcomas are rare,
occurring in 0.03% of patients surviving more than
10 years after mastectomy.1
It is traditional to classify lymphoedema into
primary or secondary forms—in the former there
is congenital lymphatic dysfunction related to
dysplasia/malformation whilst in the latter there is
disruption of lymphatic outflow related to another
disease process or due to iatrogenic mechanisms.
Primary lymphoedema
The traditional subdivision of primary lymphoedema
according to the time of onset has little clinical
significance. There is confusion in the literature
regarding the terms used, particularly, the various
eponymous syndromes that have been described;
most cases of primary lymphoedema are not
associated with specific syndromes.
Congenita (10% of cases): the swelling is often
present at birth (any swelling that begins before
the age of 2 years is included in this group).
Praecox (80% of cases): patients present with
swelling before the age of 35 years.
Tarda (10% of cases): patients present after the age
of 35 years with swelling that is usually bilateral.
Lymphatic vessels tend to be hyperplastic.
It is important to appreciate that primary
lymphoedema is a heterogeneous group with many
subtypes occurring due to many different causes
which are generally poorly understood. The term
Milroy’s disease is often used interchangeably with
lymphoedema congenita—it should only be applied
to the group of inherited congenital lymphoedemas
that demonstrates autosomal dominant inheritance;
it is linked to vascular endothelial growth factor
(VEGF) receptor–3 mutations (FLT4 gene on locus
5q35.3)2 3 and some evidence reveals that there is a
functional defect of absorption rather than a gross
structural defect.
Lymphoedema praecox is the commonest type
(80% of cases) and the swelling may be unilateral and
limited to the foot/calf region. Patients lack distal
lymphatics (hypoplasia) and often have a strong
family history, some demonstrating autosomal
dominant inheritance with different mutations in
FOXC2 gene on chromosome 16.4 Some patients
with praecox have distichiasis (double row of
eyelashes) and form a distinct syndromic entity—lymphoedema distichiasis (mutation on 16q24.3).5
Patients with Meige’s disease, which is a subset of
lymphoedema, usually present at puberty and the
term should be reserved for those with the familial
form of the disease (with as-yet unknown mutation).
Secondary lymphoedema
Worldwide, the commonest cause of secondary
lymphoedema is filariasis, caused by infection
with Wuchereria bancrofti. However, this condition
is rare in developed countries such as Hong Kong
where lymphoedema is most commonly related to
malignant disease and, particularly, its treatment
with surgery and/or irradiation.6 Upper limb lymphoedema most often follows the treatment
of breast cancer, typically after a latent period of
variable duration—77% of cases present within 3
years of surgery7 but the condition can arise at any
time.
Although the exact mechanism of the response
of lymphatic channels to trauma is unknown, in
general, the more extensive the surgery the greater is
the damage. Thus, a formal axillary node dissection
carries a risk of up to 20% compared to 4% to 10%
following sentinel node biopsy.8 9 10 11 Irradiation
causes fibrosis and inhibits lymphangiogenesis
and, approximately, doubles the risk of developing
lymphoedema after nodal surgery. Lymph vessels do
have some regenerative capacity12 and not all patients
undergoing surgery and/or radiotherapy develop
lymphoedema; it may be related to some underlying
susceptibility that is as yet undefined, and may be,
possibly, genetic.13 Patients with lymphoedema often
have subtle lymphatic anomalies in the contralateral
limb.14
Staging
The International Society of Lymphology staging is
the most commonly used system (Table 115), but it is
somewhat flawed in that it is based only on physical
findings. Some experts suggest incorporating
measures of quality of life (QOL) to improve its
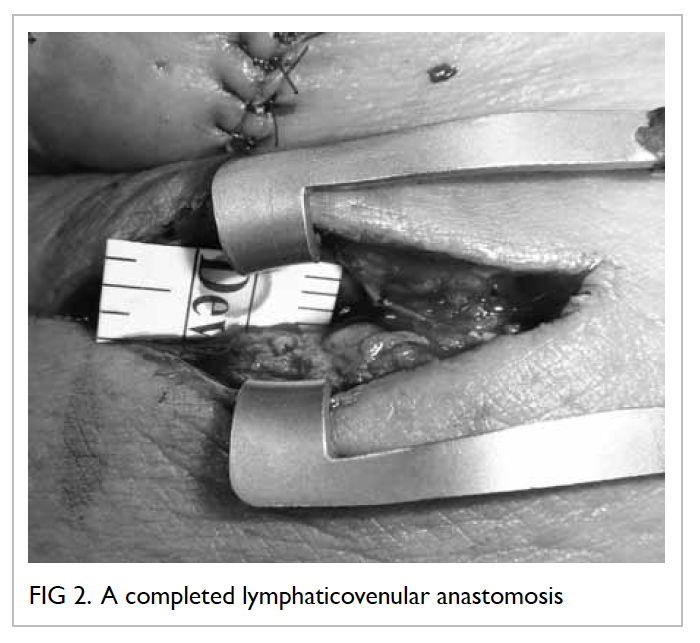
usefulness.16 The classification offered by Campisi
et al17 shows congruence with indocyanine green
(ICG) dermal backflow patterns, which provides
an indication of lymphatic function (Table 2).17 18 19
Bioimpedance spectroscopy uses electric current
to measure the degree of tissue fluid retention and
is useful in detecting early-stage lymphoedema
including stage 0 disease.
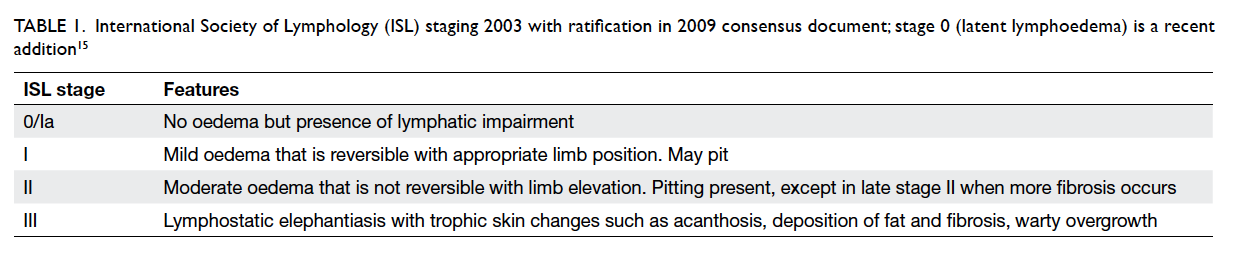
Table 1. International Society of Lymphology (ISL) staging 2003 with ratification in 2009 consensus document; stage 0 (latent lymphoedema) is a recent addition15
The most commonly used method of objectively
assessing the swelling is some form of conal
measurement, such as measuring the circumference
at 4 cm intervals, which is more practical than water
displacement methods20 21 but is, supposedly, similar
in accuracy.22 A perometer uses infrared rays to
measure limb cross-sections at multiple intervals
and, thus, determine the volume of the limb.23 The
author has been exploring the idea of using Kinect
(part of the Microsoft Xbox game system) as a three-dimensional
scanner to gauge volume in collaboration with the
Department of Computer Science and Engineering
at The Chinese University of Hong Kong.
Imaging
In most cases, diagnosis can be made from the
clinical history and examination, although some
co-morbidities may confound the clinical picture.
Lymphoedema can be assessed by common imaging
techniques including computed tomography,
magnetic resonance imaging, and duplex
ultrasonography that can reliably show volume
differences between the affected and normal limbs,
the presence of subcutaneous fatty fibrosis, and help
exclude proximal obstruction in late-onset unilateral
lymphoedema which may be due to an occult visceral
tumour.
Lymphangiography involves direct cannulation
of a lymphatic vessel on the dorsum of the foot or
hand (under magnification). An oil-based contrast
material is then injected through this vessel and serial
plain radiographs of the limb are taken, allowing the
lymphatics to be precisely delineated. Due to the
potential of damage to the lymphatic vessels, it can
theoretically worsen lymphoedema and, thus, is not
commonly used.
Radionuclide lymphoscintigraphy is the
current standard investigation for evaluation of
lymphatic function. Technetium-labelled colloid is
injected into the web spaces of the toes or fingers,
and drainage of the colloid from the injection site
and the time taken to move proximally are recorded
using a gamma camera. It is minimally invasive and
enables making both qualitative and quantitative
analyses. It does not require dye injection, a method
that has been occasionally complicated by allergic
skin reactions or anaphylaxis.
Magnetic resonance lymphangiography may
replace lymphoscintigraphy as it does not require
direct injection of contrast and avoids the use of
ionising radiation using a magnetic contrast medium
to provide a sharp image of lymphatic vessels.
In our early experience with this investigation,
reproducibility has been a potential issue and needs
further evaluation.
Near-infrared fluorescence imaging with ICG
is a promising emerging imaging modality that allows
dynamic study of even small lymphatics in the skin.
Treatment
There is no cure for lymphoedema but it is easily
managed with early recognition and therapy. Those
who do not have treatment tend to worsen rapidly
and advanced disease tends to be more difficult to
treat than early disease. Patients are best treated
in a specialised clinic24; inexperienced staff may
delay treatment, or worse, advocate inappropriate
treatments.
The standard of care for lymphoedema is, what
is commonly referred as, complex (or combined or
complete) physical therapy (CPT, sometimes called
complex decongestive therapy [CDT]), which is
a staged combination of various components in
two phases. The actual treatment regimen varies
significantly by locality but, in general, 60% to 70%
of compliant patients will respond to CPT when
administered by specially trained therapists, with an
average volume reduction of 50%.25 26 However, the
time and effort involved, as well as the associated
moderate discomfort, lead to decreased patient
compliance, particularly in the long term.
(1) Phase 1, also known as the ‘decongestive phase’,
often requires the patient to be admitted as an
in-patient as the regimen is intensive. Treated 5
days a week, it may take 4 to 6 weeks or more to
have an effect on the limb volume, depending on
the severity of the disease. A lack of effect may
be due to improper technique, non-compliance,
or incorrect diagnosis.
Manual lymph drainage (MLD) is not the
same as massage; it is a much lighter, slow
and specific action that aims to promote
lymph movement in the superficial tissues
away from the swelling.
Compression bandage is applied to reduce
limb size but the bandages used are not
of the standard variety—they are short
and non-stretch, and applied with more
pressure distally than proximally. This aims
to move fluid out of the limb (hence the
proximal portions need to be emptied with
MLD first). It is only really effective within
a CPT programme; the efficacy is reduced
considerably in advanced disease.
Nail/skin care and exercises with bandaging/pressure garments as tight as tolerated to
oppose the filtration pressure and provide
counterforce to muscle contractions.
Concerns that exercise may exacerbate or
trigger lymphoedema have not been proven
in studies.
(2) Phase 2, or the maintenance phase, can be out-patient–based or as self-care in selected trained
patients. Patients should wear pressure garments
during the day and compression bandages at
night (alternatives include specifically designed
garments such as Reid sleeves). Intermittent
pneumatic compression machines are
sometimes used but care needs to be taken to
ensure that they have appropriate design, action,
and pressures (usually 30-60 mm Hg though
pressures of >45 mm Hg can cause lymphatics
to rupture). Pneumatic compression is to be
avoided in those with chronic non-pitting
disease or those with active infection.
Systematic reviews support the use of CDT and
MLD. Conservative therapy can give good results but
the effects are temporary without the maintenance
and continued compression. In Hong Kong, the
climate may reduce patient compliance with pressure
garments. General care is also important; patients
are advised to avoid even minor degrees of trauma
such as venepuncture, insect bites, and acupuncture.
The evidence for some of the following preventive
practices is low but they are simple to follow.
Air travel: maintain hydration and mobilisation,
whilst some experts suggest wearing pressure
garments.
Avoid overuse of the affected limb.
Avoid trauma; take care when cutting nails, avoid
needlestick/venepuncture, and blood pressure
measurement. Patients can wear medical alert
bracelets to inform others of the condition.
Avoid extremes of heat/cold and overtight
clothing.
Medications such as benzopyrenes and
diuretics are not useful and will not be discussed
any further. Oral penicillins such as amoxicillin and
dicloxacillin should be started early when there is
evidence of infection in a lymphoedematous limb
and continued until the signs of inflammation
resolve. There is borderline support for the use of
prophylactic antibiotics in those who have more
than two to three episodes of infection a year. Many
modalities have been promoted for the non-surgical
treatment of lymphoedema but at present only low-level
laser therapy has approval from the US Food
and Drug Administration (FDA), although evidence
of its long-term effects is lacking—it generates low-intensity
light (650-1000 nm) that is believed to
promote lymph vessel regeneration and increase
lymph pumping. The typical response is moderate
and slow, and requires repeated treatments. Near-infrared
light therapy aims to increase nitric oxide
in the tissues to improve tissue repair and lymph
regeneration but has not received FDA approval,
whilst electrical stimulation is not recommended
based on current evidence.27
Surgery
For reasons such as concerns of scarring or perceived
lack of effect, surgery has often been regarded as
a ‘last resort’, meaning that there is often a delay
before patients are referred28 by which time they
may only be suited for ‘salvage’ procedures. Whilst
initial attempts at ‘physiological’ reconstruction
were met with disappointing late results, improved
understanding of the pathophysiology accompanied
by improved microsurgical techniques have seen
the development of newer techniques that seem to
potentially offer better outcomes.
Physiological/reconstructive
techniques
Physiological techniques aim to repair the damage
and increase the return of lymph to the circulation,
by reconnecting the lymphatic pathways above and
beyond the obstruction, either directly (lymphatic to
lymphatic) or indirectly via another segment such as
veins/venules. Campisi et al17 29 30 have been a pioneer
of these microsurgical techniques and classified
them into ‘derivative’ techniques (essentially
a lymphovenous bypass) and ‘reconstructive’
techniques (lymphatic-to-lymphatic connections).
Lymphovenous anastomosis or
bypass
When interpreting the literature describing
lymphovenous bypass, it is important to note
the fundamental difference between early
(lymphovenous) and newer (lymphaticovenular)
techniques. The bypass concept was first described
in 1963 in a rat model31 whilst Yamada32 and O’Brien
et al33 were the first to use it in patients. O’Brien
et al33 anastomosed lymphatics to veins measuring
approximately 1 to 3 mm diameter and reported
an average volume reduction of 44% in this series
with 14 years’ follow-up. Campisi et al30 reported an
average of 67% reduction in volume and 87% reduction
in cellulitis in this large long-term series. Overall,
85% of patients were able to stop conservative
management. However, one criticism of these
retrospective studies was patient heterogeneity.
However, Damstra et al34 found no
improvement in patients with postmastectomy
lymphoedema who were treated with lymphovenous
anastomosis/bypass; a prospective study of 10
patients demonstrated only a 4.8% volume reduction
at 3 months that was further reduced to 2% after
1 year, with minimal improvement in reported
QOL. Boccardo et al35 36 performed lymphovenous
bypass at the time of breast surgery with the aim
of preventing lymphoedema—they anastomosed
tributaries of the axillary vein to lymphatics with a
patency rate of 95.6% but found no difference in limb
volume compared with controls at up to 18 months’ follow-up.
Lymphaticovenular anastomoses
In Koshima et al’s opinion,37 38 39 there were inherent
problems in lymphovenous anastomoses: (1) it is
difficult to find larger lymphatic trunks, most are
0.8 mm or under in diameter; (2) reduced lymphatic
pumping function; and (3) increased venous
pressure and high rate of thrombosis due to blood
at anastomotic site. According to these authors,
lymphaticovenular anastomosis (LVA) offered better
vessel size match compared with lymphovenous
anastomoses. In 52 patients with an average of 2.1
LVAs per patient, there was a mean reduction of
41.8% in leg circumference at a mean follow-up of
14.5 months. Benefit could be demonstrated even
in patients with stage III/IV disease, with recurrent
lymphangitis and fibrosis. Koshima et al37 38 39 have
also applied the technique in early disease for prophylaxis against development of fibrotic disease.
The Tokyo group uses ICG fluorescent
lymphography to stage the disease severity based
on the amount of dermal backflow, and thus,
select patients for surgery.40 41 Subdermal vessels
are explored through small (<3 cm) skin incisions,
aiming to use lymphatic vessels of approximately
0.2 mm in diameter with venules sized 0.5 mm or
less on the basis that smaller veins will have lower
pressures. Some call this ‘supermicrosurgery’
although the use of the term is rather arbitrary. The
procedure can be performed under local anaesthesia
with short hospital stay, which, in the author’s
opinion, is one of the biggest advantages of this
technique.
The studies from Koshima et al’s unit37 38 39 have
demonstrated an average volume reduction of 82.5%
in those not responding to CPT. The Chang’s series42 demonstrated a 35% reduction of lymphoedema in
breast cancer patients 1 year after LVA. Cormier et
al43 reviewed eight studies and calculated a mean
volume reduction of 54.0%. It is generally accepted
that microsurgery will offer better results in early
disease when patients have some healthy lymphatics
before progressing to the fibrotic stage when damage
is irreversible.
Lymph node transfer
Wongtrungkapun44 performed lymph node transfer
in patients with filariasis; one to two groin nodes were
partially decapsulated and anastomosed to the
saphenous vein. Becker et al45 46 described the
transfer of lymph nodes from the groin to the axilla
in 24 patients with postmastectomy lymphoedema;
62.5% of the patients were said to be ‘cured’ and able
to discontinue physiotherapy. Overall, in Becker
et al’s experience,45 46 98% of the patients had some
improvement; only 2% had repeat infections. Whilst
40% of patients with stage 1 or 2 lymphoedema
had complete normalisation and did not require
additional conservative therapy, those with stage 3
lymphoedema needed conservative therapy. Lin et
al47 anastomosed superficial circumflex iliac nodes
at the wrist to treat upper limb lymphoedema and
reported a 55% reduction in volume at 56 months,
with fewer episodes of cellulitis.
Proponents of node surgery say that LVA
or lymphatic-lymphatic anastomosis eventually
become occluded (possibly due to the effect of
interstitial pressure on low-pressure thin vessels)
whilst lymph nodes are supposedly less susceptible.
The lymphatic vessels are not actually anastomosed;
it seems that the transplanted nodes develop new
drainage pathways—proposed theories for this
drainage include nodes acting as suction pumps
whilst others suggest they are a source of VEGF-C
that promotes lymphangiogenesis.
Some experts have developed a procedure
to transfer lymph nodes as part of a breast reconstruction procedure48; there may be several other
benefits of such a procedure including release of scar
tissue in the axilla and the provision of vascularised
tissue as a lymphatic bridge.49 Isotope scans at 3
and 6 months demonstrated improved function in
all but one patient.48 With this type of surgery, it is
important to only harvest nodes of lower abdomen
and not the leg; axillary reverse mapping may help to
spare limb lymphatics.50
Overall, good long-term functional data
are lacking and some authors have had difficulty
reproducing published results,25 possibly, due to the
significant learning curve. Some have found 38%
risk of complications, although mostly transient
(eg lymphocele and hydrocele); however, some
complications such as iatrogenic lymphoedema and
chronic pain may be more persistent.51 They also
found no volume difference after a median follow-up
of 40 months with interval CDT and pressure
garments. Viitanen et al52 found reduced lymphatic
flow in the donor site/limb with lymphoscintigraphy
without overt clinical lymphoedema in a small group
of 10 patients.
Preliminary results suggest that node transfer
is more successful if performed sooner after nodal
dissection surgery.45 53 Saaristo et al48 regard it as
largely experimental but can be justified if performed
at the time of breast reconstruction. Some surgeons
have looked at animal models, combining node
transfer with additional VEGF-C and -D,54 55 and
this may offer a new type of treatment; however,
the effect on lymphatic metastasis is unknown and
deserves attention.
Lymphatic grafting/transplantation
Other less commonly used procedures include
lymphatic grafting. Baumeister et al56 57 58 59 have
significant experience in using this technique to
treat secondary lymphoedema with an average
volume reduction of 65%.60 61 In the lower limb, a
long segment of lymphatic vessel is dissected out
from the upper inner thigh and tunnelled over to
affected contralateral leg for a lymphatic-lymphatic
anastomosis. The efficacy in the upper limb where
the lymphatic tissue is used as a free graft has also
been demonstrated with significant improvement in
over 90% of patients, with a mean volume reduction
of 22% to 31% whilst scintigraphy shows continued
graft function.62 63 64 The dissection of lymphatics is
technically challenging; Campisi65 used veins instead
of lymphatic vessels to bridge lymphatics.
Some experts have used flaps for lymphatic
bridging; for instance, the inclusion of random
lymphatics in tissues such as the omentum66 or an
axial flap such as the deltopectoral.67 The use of
omentum has largely been abandoned as there is no
evidence that it actually promotes lymph drainage,
and the surgery is associated with high rates of
morbidity. Similarly, enteromesenteric bridge
operations and tube/thread implants should be
avoided, based on current evidence.
Debulking or excision
Early attempts at treating lymphoedema involved
techniques that were mainly based on debulking,
that is, removing the oedematous tissue to restore
form. The underlying lymphatic dysfunction is
not addressed and may actually lead to further
deterioration of the condition.
Charles procedure
The Charles procedure used in 1912 has been wrongly
attributed to Sir Richard Charles for the treatment
of leg lymphoedema by McIndoe.68 The surgeon had
primarily treated scrotal swelling69 and had only
described one unsuccessful case involving the lower
limb. In simple terms, the lymphoedematous tissue
is excised down to the fascial level and the defect
is covered with skin grafts. The grafts can be taken
from the excised tissue if it is not grossly abnormal70;
otherwise, it has to be harvested from another site.
Complications include graft breakdown/ulceration,
scarring, and recurrence. The aesthetic results are
rather poor and, thus, usually reserved for cases with
severe skin changes.
Homans-Miller procedure
The Homans-Miller procedure, first used in 1936, is
based on a multistage procedure initially described
by Sistrunk71—skin flaps are elevated along one
border of the limb, and after the deeper swollen
tissue has been excised along with the fascia, the
skin flaps are replaced.72 73 It is a traditional practice
to avoid surgery at or below ankle, taking care to
avoid damage to the common peroneal and sural
nerves. A study with 14 years’ follow-up showed
that this type of surgery was capable of long-lasting
reduction in size in 80% of the treated patients, and
associated with improved function and reduced
cellulitis.73 It is the most common excisional surgery
for lymphoedema. Others have refined this further
by preserving the perforator vessels during flap
elevation, allowing the flaps to be thinner (5 mm)
and, potentially, for both sides to be treated in one
stage.74
The Thompson technique used in 1962 involves
similar tissue resection whilst also harvesting a
dermal flap75 that is buried into the muscle next to
the neurovascular bundle, with the aim of creating
a bridge for lymphatic return. However, long-term
results were similar to excision alone and did not
support the theoretical aim of a physiological effect
and, thus, has largely been abandoned.
The proven benefits of excisional surgery are
often ignored, in part due to misconceptions of
morbidity and complications; these were mostly
related to early aggressive use of the technique
leading to almost total abandonment of procedures
in the mid-20th century. Recently Karri et al76
demonstrated that good results are possible with
modern application of the Charles procedure that
may also be combined with negative-pressure
wound therapy.77 Similarly, surgeons have modified
the Homans-Miller procedure25 and combined this
with postoperative pressure garments to achieve
good results.
Liposuction
Liposuction is a relatively recently described
debulking technique based on the observation that
there is adipose hypertrophy in lymphoedema. The
fat accumulation may be related to altered lipid
transport78 79 and corresponds to a non-pitting type
of swelling that is not responsive to compression.
O’Brien et al80 were one of the first surgeons
to use liposuction to treat lymphoedema, and
reported a volume reduction of 23%. Brorson et
al81 used liposuction to treat patients with post–breast cancer lymphoedema that had been resistant
to conservative therapy with reasonable effects
that were confirmed with volume measurements,
computed tomographic scans, and plethysmography.
Liposuction for lymphoedema is similar but not
the same as cosmetic liposuction. The technique
has evolved in several ways, particularly, with the
adoption of a tumescent technique with injection
of adrenaline combined with use of a tourniquet
that causes less bleeding—13% versus 25% without
tourniquet.82 More recently, Schaverien et al83 used
the Brorson technique (1997) and demonstrated a
101% reduction compared to normal limb at 1 year,
and that was maintained at 5 years.
The technique seems to be straightforward and
safe, and produces consistent results84 85 86 with treated
patients reporting improved QOL and suffering
from decreased episodes of cellulitis. Preliminary
results with laser Doppler scanning seem to support
the theory that liposuction can reduce the lymphatic
load87 without damaging lymph function.88 The National Institute for Health and Care Excellence89
suggests that liposuction may be considered in those
patients with severe disease (massive incapacitating
disease, unresponsive to conservative therapy). The
morbidity may be less than traditional debulking
surgeries and, thus, it can be regarded as the first
choice of a debulking operation, if the skin is normal.
Its main disadvantage is that patients are required
to wear lifelong compression garments90; otherwise,
the treated limb enlarges again; this may make the
modality less suited in regions such as Hong Kong.
Lymphoedema surgery in Hong
Kong
Since 1993, breast cancer has become the most
common cancer in women in Hong Kong with an
incidence of 79.4/100 000 in 2009.91 A local study
presented at the 2010 Hospital Authority Convention
found that 11.3% of patients had lymphoedema at
3 months after breast cancer surgery with axillary
dissection.92 Mak et al93 found that previous
infection-inflammation (odds ratio [OR]=4.49),
surgery on the side of the dominant hand (OR=2.97),
increased body mass index, and older age at the
time of axillary dissection were significant risk
factors for the development of moderate-to-severe
lymphoedema in our local population.
Despite the significant number of patients
liable to suffer from breast cancer–related
lymphoedema (BCRL), the general awareness of
lymphoedema in Hong Kong is low among both
health care professionals and patients. It is a common
misconception that nothing can be done for the
condition; thus, patients tend to be diagnosed late
with symptomatic moderate-to-severe disease, and
salvage-type surgical procedures are often the only
therapeutic option.
Medical costs for women with BCRL are
substantially higher than for those without,94 with
the difference mostly accounted for by the costs
of treating infections. A local study95 found that
instituting effective and standardised primary
intervention for BCRL in the form of CDT/MLD would be beneficial to both patients and the
health care institute, with savings of as much as
HK$444 200 per year in a local hospital.
Improvements in care for lymphoedema
patients in Hong Kong require establishment of
integrated treatment protocols which may include
the following:
(1) Education for caregivers and patients is
important. Public/teaching hospitals should
take the lead; in addition, support groups such
as the Hong Kong Breast Cancer Foundation
have a particularly important role. Medical staff
with an interest in treating lymphoedema should
keep themselves updated.
(2) Establishment of multidisciplinary care
units focusing around nurse-led clinics with
formalised protocols following proven MLD/CDT programmes such as Foldi or Vodder
that should be offered as a first option for
lymphoedema patients. These clinics should
be supported by medical staff who would offer
medical advice, discuss surgical options, as well
as treat complications when they occur.
(3) Although CDT/MLD remains the mainstay
of treatment for patients with lymphoedema,
surgery may be considered in those patients who
do not respond to conservative therapy. Newer
microsurgical techniques (LVA and lymph node
transfer) may be useful in early-stage disease
though they have a significant learning curve
and, therefore, should be undertaken only by
experienced microsurgeons.
(a) The author prefers offering LVA under local
anaesthesia as a first option in stage I/II
lymphoedema, with lymph node transfer for
stage II/III disease.
(b) Debulking surgery (Homans-Miller
procedure with perforator preservation or
Charles procedure with negative pressure
dressings) may be considered in those
with severe disabling swelling; the role of
liposuction is likely to be minor in Hong
Kong but can be offered to those willing to
wear pressure garments continuously.
A number of local surgeons have travelled to
regional centres of excellence for training and our
unit has organised courses in supermicrosurgery
and lymph node transfer to encourage the uptake of
these techniques among other surgeons. Lymphatic
venous anastomosis has been performed in Hong
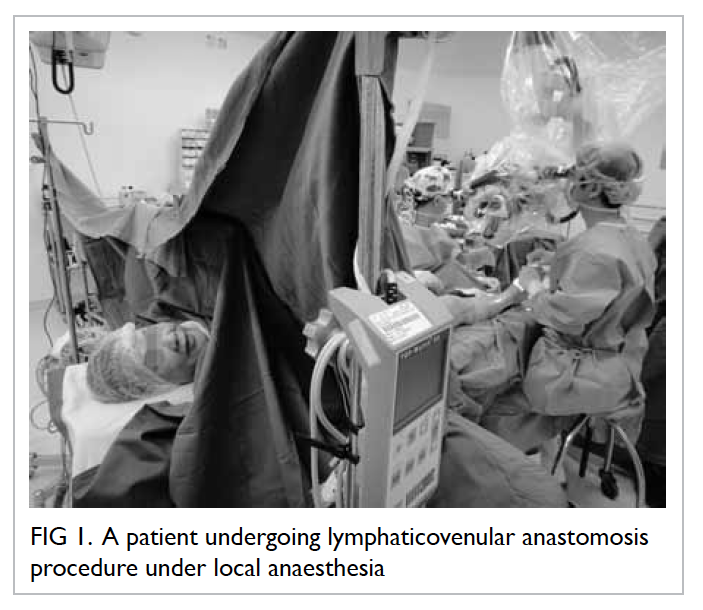
Kong since 2012 (Figs 1 and 2) and the preliminary
results are encouraging, with patients often
describing early relief, particularly from symptoms of
‘tightness’. Given the short period of experience and
small number of patients, it is too early to comment
on local results; however, there is no reason to
suggest that the results would not be comparable
with international findings in the long term.
References
1. de Jong MA, Oldenborg S, Bing Oei S, et al. Reirradiation
and hyperthermia for radiation-associated sarcoma.
Cancer 2012;118:180-7. CrossRef
2. Karkkainen MJ, Ferrell RE, Lawrence EC, et al. Missense
mutations interfere with VEGFR-3 signalling in primary
lymphoedema. Nat Genet 2000;25:153-9. CrossRef
3. Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula
M. Congenital hereditary lymphoedema caused by a
mutation that inactivates VEGFR3 thyrosine kinase. Am J
Hum Genet 2000;67:295-301. CrossRef
4. Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S. FOXC2 is a winged helix gene that counteracts
obesity, hypertriglyceridemia, and diet-induced insulin
resistance. Cell 2001;106:563-73. CrossRef
5. Yildirim-Toruner C, Subramanian K, El Manjra L, Chen
E, Goldstein S, Vitale E. A novel frameshift mutation of
FOXC2 gene in a family with hereditary lymphoedema-distichiasis
syndrome associated with renal disease and
diabetes mellitus. Am J Med Genet A 2004;131:281-6. CrossRef
6. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema:
a comprehensive review. Ann Plast Surg 2007;59:464-72. CrossRef
7. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema
in a cohort of breast carcinoma survivors 20 years after
diagnosis. Cancer 2001;92:1368-77. CrossRef
8. Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel
lymph node biopsy (SLN) alone versus SLN and completion
axillary lymph node dissection after breast cancer surgery:
a prospective Swiss multicenter study on 659 patients. Ann
Surg 2007;245:452-61. CrossRef
9. Lucci A, McCall LM, Beitsch PD, et al. Surgical
complications associated with sentinel lymph node
dissection (SLND) plus axillary lymph node dissection
compared with SLND alone in the American College
of Surgeons Oncology Group Trial Z0011. J Clin Oncol
2007;25:3657-63. CrossRef
10. Veronesi U, Paganelli G, Viale G, et al. A randomized
comparison of sentinel-node biopsy with routine axillary
dissection in breast cancer. N Engl J Med 2003;349:546-53. CrossRef
11. Wilke LG, McCall LM, Posther KE, et al. Surgical
complications associated with sentinel lymph node biopsy:
results from a prospective international cooperative group
trial. Ann Surg Oncol 2006;13:491-500. CrossRef
12. Tammela T, Alitalo K. Lymphangiogenesis: molecular
mechanisms and future promise. Cell 2010;140:460-76. CrossRef
13. Newman B, Lose F, Kedda MA, et al. Possible genetic
predisposition to lymphedema after breast cancer.
Lymphat Res Biol 2012;10:2-13. CrossRef
14. Aldrich MB, Guilliod R, Fife CE, et al. Lymphatic
abnormalities in the normal contralateral arms of subjects
with breast cancer–related lymphedema as assessed by
near-infrared fluorescent imaging. Biomed Opt Express
2012;3:1256-65. CrossRef
15. International Society of Lymphology. The diagnosis and
treatment of peripheral lymphedema. 2009 Concensus
Document of the International Society of Lymphology.
Lymphology 2009;42:51-60.
16. Lee BB, Laredo J, Neville RF. Combined clinical and
laboratory (lymphoscintigraphic) staging. In: Lee B,
Bergan J, Rockson SG, editors. Lymphedema: a concise
compendium of theory and practice. London: Springer;
2011: 97-104. CrossRef
17. Campisi C, Bellini C, Campisi C, Accogli S, Bonioli E,
Boccardo F. Microsurgery for lymphoedema: clinical
research and long-term results. Microsurgery 2010;30:256-60.
18. Yamamoto T, Narushima M, Doi K, et al. Characteristic
indocyanine green lymphography findings in lower
extremity lymphedema: the generation of a novel
lymphedema severity staging system using dermal
backflow patterns. Plast Reconstr Surg 2011;127:1979-86. CrossRef
19. Yamamoto T, Matsuda N, Doi K, et al. The earliest finding
of indocyanine green lymphography in asymptomatic
limbs of lower extremity lymphedema patients secondary
to cancer treatment: the modified dermal backflow stage
and concept of subclinical lymphedema. Plast Reconstr
Surg 2011;128:314e-321e. CrossRef
20. Stanton A, Modi S, Mellor R, Levick R, Mortimer P.
Diagnosing breast cancer–related lymphoedema in the arm.
J Lymphoedema 2006;1:12-5.
21. Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity
volume measurements in women with
lymphedema: a comparison of measurements obtained
via water displacement with geometrically determined
volume. Phys Ther 2002;82:1201-12.
22. Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J.
Reliability and validity of arm volume measurements for
assessment of lymphedema. Phys Ther 2006;86:205-14.
23. Stanton AW, Northfield JW, Holroyd B, Mortimer PS,
Levick JR. Validation of an optoelectronic limb volumeter
(Perometer). Lymphology 1997;30:77-97.
24. Garfein ES, Borud LJ, Warren AG, Slavin SA. Learning from
a lymphedema clinic: an algorithm for the management of
localized swelling. Plast Reconstr Surg 2008;121:521-8. CrossRef
25. Lee BB, Kim YW, Kim DI, Hwang JH, Laredo J, Neville R.
Supplemental surgical treatment to end stage (stage IV-V)
of chronic lymphedema. Int Angiol 2008;27:389-95.
26. Ko DS, Lerner R, Klose G, Cosimi AB. Effective treatment
of lymphedema of the extremities. Arch Surg 1998;133:452-8. CrossRef
27. Moseley AL, Carati CJ, Piller NB. A systematic review of
common conservative therapies for arm lymphoedema
secondary to breast cancer treatment. Ann Oncol
2007;18:639-46. CrossRef
28. Lee BB. Contemporary issues in management of chronic
lymphedema: personal reflection on an experience with
1065 patients. Lymphology 2005;38:28-31.
29. Campisi C, Davini D, Bellini C, et al. Lymphatic
microsurgery for the treatment of lymphoedema.
Microsurgery 2006;26:65-9. CrossRef
30. Campisi C, Boccardo F, Zilli A, Macciò A, Napoli F. Long-term
results after lymphatic-venous anastomoses for the
treatment of obstructive lymphoedema. Microsurgery
2001;21:135-9. CrossRef
31. Laine JB, Howard JM. Experimental lymphatico-venous
anastomosis. Surg Forum 1963;14:111-2.
32. Yamada Y. The studies on lymphatic venous anastomosis in
lymphoedema. Nagoya J Med Sci 1969;32:1-21.
33. O’Brien BM, Sykes P, Threlfall GN, Browning FS.
Microlymphaticovenous anastomoses for obstructive
lymphoedema. Plast Reconstr Surg 1977;60:197-211. CrossRef
34. Damstra RJ, Voesten HG, van Schelven WD, van der Lei
B. Lymphatic venous anastomosis (LVA) for treatment of
secondary arm lymphedema. A prospective study of 11
LVA procedures in 10 patients with breast cancer related
lymphedema and a critical review of the literature. Breast
Cancer Res Treat 2009;113:199-206. CrossRef
35. Boccardo F, Casabona F, De Cian F, et al. Lymphedema
microsurgical preventive healing approach: a new
technique for primary prevention of arm lymphedema
after mastectomy. Ann Surg Oncol 2009;16:703-8. CrossRef
36. Boccardo FM, Casabona F, Friedman D, et al. Surgical
prevention of arm lymphedema after breast cancer
treatment. Ann Surg Oncol 2011;18:2500-5. CrossRef
37. Koshima I, Nanba Y, Tsutsui T, Takahashi Y, Itoh S. Long-term
follow-up after lymphaticovenular anastomosis
for lymphoedema in the leg. J Reconstr Microsurg
2003;19:209-15. CrossRef
38. Koshima I, Nanba Y, Tsutsui T, Tahahashi Y, Itoh S, Fujitsu
M. Minimal invasive lymphaticovenular anastomosis
under local anesthesia for leg lymphedema: is it effective
for stage III and IV? Ann Plast Surg 2004;53:261-6. CrossRef
39. Koshima I, Kawada S, Moriguchi T, Kajiwara Y.
Ultrastructural observations of lymphatic vessels in
lymphedema in human extremities. Plast Reconstr Surg
1996;97:397-405; discussion 406-7. CrossRef
40. Mihara M, Uchida G, Hara H, et al. Lymphaticovenous
anastomosis for facial lymphoedema after multiple courses
of therapy for head-and-neck cancer. J Plast Reconstr
Aesthet Surg 2011;64:1221-5. CrossRef
41. Furukawa H, Osawa M, Saito A, et al. Microsurgical
lymphaticovenous implantation targeting dermal
lymphatic backflow using indocyanine green fluorescence
lymphography in the treatment of postmastectomy
lymphedema. Plast Reconstr Surg 2011;127:1804-11. CrossRef
42. Chang DW. Lymphaticovenular bypass for lymphedema
management in breast cancer patients: a prospective study.
Plast Reconstr Surg 2010;126:752-8. CrossRef
43. Cormier JN, Rourke L, Crosby M, Chang D, Armer J. The
surgical treatment of lymphedema: a systematic review of
the contemporary literature (2004-2010). Ann Surg Oncol 2012;19:642-51. CrossRef
44. Wongtrungkapun R. Microsurgical lymphonodovenous
implantation for chronic lymphedema. J Med Assoc Thai
2004;87:877-82.
45. Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy
lymphedema: long-term results following microsurgical
lymph node transplantation. Ann Sur 2006;243:313-5. CrossRef
46. Becker C, Vasile JV, Levine JL, et al. Microlymphatic
surgery for the treatment of iatrogenic lymphedema. Clin
Plast Surg 2012;39:385-98. CrossRef
47. Lin CH, Ali R, Chen SC, et al. Vascularized groin
lymph node transfer using the wrist as a recipient site
for management of postmastectomy upper extremity
lymphedema. Plast Reconstr Surg 2009;123:1265-75. CrossRef
48. Saaristo AM, Niemi TS, Viitanen TP, Tervala TV, Hartiala
P, Suominen EA. Microvascular breast reconstruction and
lymph node transfer for postmastectomy lymphedema
patients. Ann Surg 2012;255:468-73. CrossRef
49. Card A, Crosby MA, Liu J, Lindstrom WA, Lucci
A, Chang DW. Reduced incidence of breast cancer–related
lymphedema following mastectomy and breast
reconstruction versus mastectomy alone. Plast Reconstr
Surg 2012;130:1169-78. CrossRef
50. Klimberg VS. A new concept toward the prevention of
lymphedema: axillary reverse mapping. J Surg Oncol
2008;97:563-4. CrossRef
51. Vignes S, Blanchard M, Yannoutsos A, Arrault M.
Complications of autologous lymph-node transplantation
for limb lymphoedema. Eur J Vasc Endovasc Surg
2013;45:516-20. CrossRef
52. Viitanen TP, Mäki MT, Seppänen MP, Suominen EA,
Saaristo AM. Donor-site lymphatic function after
microvascular lymph node transfer. Plast Reconstr Surg
2012;130:1246-53. CrossRef
53. Slavin SA, Van den Abbeele AD, Losken A, Swartz MA, Jain
RK. Return of lymphatic function after flap transfer for
acute lymphedema. Ann Surg 1999;229:421-7. CrossRef
54. Tammela T, Saaristo A, Holopainen T, et al. Therapeutic
differentiation and maturation of lymphatic vessels after
lymph node dissection and transplantation. Nat Med
2007;13:1458-66. CrossRef
55. Lähteenvuo M, Honkonen K, Tervala T, et al. Growth
factor therapy and autologous lymph node transfer in
lymphedema. Circulation 2011;123:613-20. CrossRef
56. Baumeister RG, Seifert J, Hahn D. Autotransplantation of
lymphatic vessels. Lancet 1981;1:147. CrossRef
57. Baumeister RG, Seifert J, Wiebecke B, Hahn D.
Experimental basis and first application of clinical lymph
vessel transplantation of secondary lymphedema. World J
Surg 1981;5:401-7. CrossRef
58. Baumeister RG. Microsurgery of the lymphatic system [in
German]. Chirurg 1983;54:374-8.
59. Baumeister RG, Siuda S. Treatment of lymphedemas by
microsurgical lymphatic grafting: what is proved? Plast
Reconstr Surg 1990;85:64-74; discussion 75-6. CrossRef
60. Baumeister RG, Siuda H, Bohmert H, Moser E. A
microsurgical method for reconstruction of interrupted
lymphatic pathways: autologous lymph-vessel
transplantation for treatment of lymphedemas. Scand J
Plast Reconstr Surg 1986;20:141-6. CrossRef
61. Kleinhans E, Baumeister RG, Hahn D, Siuda S, Büll U, Moser
E. Evaluation of transport kinetics in lymphoscintigraphy:
follow-up study in patients with transplanted lymphatic
vessels. Eur J Nucl Med 1985;10:349-52. CrossRef
62. Weiss M, Baumeister RG, Hahn K. Post-therapeutic
lymphedema: scintigraphy before and after autologous
lymph vessel transplantation: 8 years of long-term follow-up.
Clin Nucl Med 2002;27:788-92. CrossRef
63. Weiss M, Baumeister RG, Hahn K. Dynamic lymph flow
imaging in patients with oedema of the lower limb for
evaluation of the functional outcome after autologous
lymph vessel transplantation: an 8-year follow-up study.
Eur J Nucl Med Mol Imaging 2003;30:202-6. CrossRef
64. Baumeister RG, Frick A. The microsurgical lymph vessel
transplantation [in German]. Handchir Mikrochir Plast
Chir 2003;35:202-9.
65. Campisi C. Use of autologous interposition vein graft in
management of lymphedema: preliminary experimental
and clinical observations. Lymphology 1991;24:71-6.
66. Goldsmith HS, De los Santos R. Omental transposition in
primary lymphoedema. Surg Gynecol Obstet 1967;125:607-10.
67. Withey S, Pracy P, Wood S, Rhys-Evans P. The use of a
lymphatic bridge in the management of head and neck
lymphoedema. Br J Plast Surg 2001;54:716-9. CrossRef
68. Dumanian GA, Futrell JW. The Charles procedure:
misquoted and misunderstood since 1950. Plast Reconstr
Surg 1996;98:1258-63. CrossRef
69. Charles R. Elephantiasis scroti. In: Latham A, English TC,
editors. A system of treatment. London: Churchill; 1912.
70. Gibson T, Tough JS. A simplified one-stage operation for
the correction of lymphedema of the leg. AMA Arch Surg
1955;71:809-17. CrossRef
71. Sistrunk WE. Further experience with the Kondoleon
operation for elephantiasis. JAMA 1918;71:800-6. CrossRef
72. Homans J. The treatment of elephantiasis of the legs. A
preliminary report. N Engl J Med 1936;215:1099-104. CrossRef
73. Miller TA, Wyatt LE, Rudkin GH. Staged skin and
subcutaneous excision for lymphedema: a favorable report
of long-term results. Plast Reconstr Surg 1998;102:1486-98; discussion 1499-501. CrossRef
74. Salgado CJ, Mardini S, Spanio S, Tang WR, Sassu P, Chen
HC. Radical reduction of lymphedema with preservation
of perforators. Ann Plast Surg 2007;59:173-9. CrossRef
75. Thompson N. Surgical treatment of chronic lymphoedema
of the lower limb. With preliminary report of new
operation. Br Med J 1962;2:1566-73. CrossRef
76. Karri V, Yang MC, Lee IJ, et al. Optimizing outcome
of Charles procedure for chronic lower extremity
lymphoedema. Ann Plast Surg 2011;66:393-402. CrossRef
77. van der Walt JC, Perks TJ, Zeeman BJ, Bruce-Chwatt AJ,
Graewe FR. Modified Charles procedure using negative
pressure dressings for primary lymphedema: a functional
assessment. Ann Plast Surg 2009;62:669-75. CrossRef
78. Brorson H. Surgical treatment of postmastectomy
lymphedema—liposuction. In: Lee BB, Bergan J, Rockson
SG, editors. Lymphedema: a concise compendium of
theory and practice. London: Springer; 2011: 409-18. CrossRef
79. Dixon JB. Lymphatic lipid transport: sewer or subway?
Trends Endocrinol Metab 2010;21:480-7. CrossRef
80. O’Brien BM, Khazanchi RK, Kumar PA, Dvir E, Pederson
WC. Liposuction in the treatment of lymphoedema; a
preliminary report. Br J Plast Surg 1989;42:530-3. CrossRef
81. Brorson H, Ohlin K, Svensso B. The facts about liposuction
as a treatment for lymphoedema. J Lymphoedema
2008;3:38-47.
82. Wojnikow S, Malm J, Brorson H. Use of tourniquet with and
without adrenaline reduces blood loss during liposuction
for lymphoedema of the arm. Scand J Plast Reconstr Surg
Hand Surg 2007;41:243-9. CrossRef
83. Schaverien MV, Munro KJ, Baker PA, Munnoch DA.
Liposuction for chronic lymphoedema of the upper
limb: 5 years of experience. J Plast Reconstr Aesthet Surg
2012;65:935-42. CrossRef
84. Brorson H. Liposuction in arm lymphoedema treatment.
Scand J Surg 2003;92:287-95.
85. Brorson H, Svensson H. Complete reduction of
lymphoedema of the arm by liposuction after breast cancer.
Scand J Plast Reconstr Surg Hand Surg 1997;31:137-43. CrossRef
86. Brorson H, Svensson H. Liposuction combined with
controlled compression therapy reduces arm lymphoedema
more effectively than controlled compression therapy
alone. Plast Reconstr Surg 1998;102:1058-67; discussion
1068. CrossRef
87. Brorson H. Liposuction gives complete reduction of
chronic large arm lymphedema after breast cancer. Acta
Oncol 2000;39:407-20. CrossRef
88. Brorson H, Svensson H, Norrgren K, Thorsson O.
Liposuction reduces arm lymphoedema without
significantly altering the already impaired lymph transport.
Lymphology 1998;31:156-72.
89. National Institute for Health and Clinical Excellence. Liposuction for chronic lymphoedema, February 2008. Available from: http://www.nice.org.uk/guidance/ipg251/resources/guidance-liposuction-for-chronic-lymphoedema-pdf. Accessed Jan 2014.
90. Brorson H, Ohlin K, Olsson G, Långström G, Wiklund
I, Svensson H. Quality of life following liposuction and
conservative treatment of arm lymphoedema. Lymphology
2006;39:8-25.
91. Hong Kong Cancer Registry. Available from: http://www3.ha.org.hk/cancereg/statistics.html. Accessed Jan 2014.
92. Leung SY. A prospective cohort study on physiotherapy
service to identify service gaps for post-operative breast
cancer patients. Proceedings of the Hospital Authority
Convention 2010; 2010 May 10-11; Hong Kong.
93. Mak SS, Yeo W, Lee YM, et al. Predictors of lymphedema
in patients with breast cancer undergoing axillary lymph
node dissection in Hong Kong. Nurs Res 2008;57:416-25. CrossRef
94. Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment
costs, and complications of lymphedema after breast
cancer among women of working age: a 2-year follow-up
study. J Clin Oncol 2009;27:2007-14. CrossRef
95. Tang KS. A clinical guideline for management of
lymphoedema using nurse-led manual lymphatic drainage
therapy. Hong Kong: The University of Hong Kong; 2013.