DOI: 10.12809/hkmj144327
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Use of cephalosporins in patients with immediate penicillin hypersensitivity: cross-reactivity revisited
QU Lee, MB, ChB, FHKAM (Paediatrics)
Department of Paediatrics and Adolescent Medicine, Princess Margaret
Hospital, Laichikok, Hong Kong
Corresponding author: Dr QU Lee (leequnui@gmail.com)
Abstract
A 10% cross-reactivity rate is commonly cited
between penicillins and cephalosporins. However,
this figure originated from studies in the 1960s and
1970s which included first-generation cephalosporins
with similar side-chains to penicillins. Cephalosporins
were frequently contaminated by trace amount
of penicillins at that time. The side-chain
hypothesis for beta-lactam hypersensitivity is
supported by abundant scientific evidence. Newer
generations of cephalosporins possess side-chains
that are dissimilar to those of penicillins, leading
to low cross-reactivity. In the assessment of cross-reactivity
between penicillins and cephalosporins,
one has to take into account the background beta-lactam
hypersensitivity, which occurs in up to 10%
of patients. Cross-reactivity based on skin testing
or in-vitro test occurs in up to 50% and 69% of
cases, respectively. Clinical reactivity and drug
challenge test suggest an average cross-reactivity
rate of only 4.3%. For third- and fourth-generation
cephalosporins, the rate is probably less than 1%.
Recent international guidelines are in keeping with
a low cross-reactivity rate. Despite that, the medical
community in Hong Kong remains unnecessarily
skeptical. Use of cephalosporins in patients with
penicillin hypersensitivity begins with detailed history
and physical examination. Clinicians can choose
a cephalosporin with a different side-chain. Skin
test for penicillin is not predictive of cephalosporin
hypersensitivity, while cephalosporin skin test is
not sensitive. Drug provocation test by experienced
personnel remains the best way to exclude or
confirm the diagnosis of drug hypersensitivity and to
find a safe alternative for future use. A personalised
approach to cross-reactivity is advocated.
The ten per cent myth about beta-lactam
cross-reactivity
Penicillins and cephalosporins are two groups of
widely prescribed antibiotics. They belong to the
class of beta-lactam (BL) antibiotics because both
possess the same BL nucleus. Allergic reactions are
common side-effects of BL antibiotics. Studies in the
1960s and 1970s frequently estimated 10% cross-reactivity
between penicillins and cephalosporins.1 2
However, at least two recent reviews showed much
lower cross-reactivity.3 4 Notably, cross-reactivity is
higher between penicillins and first- and second-generation
cephalosporins compared with third- and
fourth-generation cephalosporins.5 The latter
two groups are considered safe alternatives for
patients with penicillin hypersensitivity.6 The 10%
cross-reactivity rate has recently been questioned as
a medical myth.4 7 Yet until 2005, an influential drug reference such as the British National Formulary
(BNF) abided by the “10% rule”.8 Faced with such
recommendation, an ordinary physician naturally
avoids all BL antibiotics in patients with a history
suggestive of penicillin hypersensitivity.9 The
implications are far-reaching as physicians often
resort to expensive, broad-spectrum antibiotics,
which may induce antibiotic resistance by selecting
out resistant organisms.10 In order to minimise
unnecessary exposure to expensive broad-spectrum
antibiotics with higher toxicities and to preserve
patients’ right to receive commonly prescribed
antibiotics, a better understanding of BL cross-reactivity
is needed. In the following discussion,
the author will review the use of cephalosporins
in patients with immediate hypersensitivity to
penicillins. Mechanism and epidemiology of cross-reactivity
will be discussed, followed by a suggestion
for a pragmatic approach.
By definition, ‘cross-reaction’ between two
substances is “the interaction of an antigen with an
antibody formed against a different antigen with
which the first antigen shares identical or closely
related antigenic determinants”.11 Hence, antigenic
similarity forms the basis of cross-reactivity. Public
hospitals often suggest avoiding all cephalosporins
indiscriminately for patients with penicillin
hypersensitivity, as exemplified by a recent antibiotic
guideline.12 What remains unsettled is how far
the BL nucleus also acts as a common antigenic
determinant. In other words, does structural
similarity in the drug nucleus translate into clinically
relevant allergic reaction?
Mechanism of beta-lactam hypersensitivity
The BL nucleus is probably the only structure
common to penicillins and cephalosporins. What
differentiates between them is that penicillins
possess a 5-membered thiazolidine ring attached
to the BL nucleus while cephalosporins have a
6-membered dihydrothiazine ring. Secondly, while
penicillins have a single 6-positioned side-chain,
cephalosporins have a 3-positioned as well as a
7-positioned side-chain.3
When a BL antibiotic is absorbed into the body,
the BL nucleus undergoes spontaneous opening.
Covalent bonding between the drug and endogenous
protein results in a hapten-protein conjugate.
In case of penicillins, stable protein conjugates
formed include penicilloyl (major) determinants
and other minor determinants.13 For cephalosporin,
however, haptenic determinants are less clear.14
Once inside the body, cephalosporins undergo rapid
fragmentation of the BL nucleus and dihydrothiazine
rings. The resulting unstable metabolites do not
allow haptenisation of proteins.15 In subjects with
BL hypersensitivity, the hapten-protein conjugate
has the capability to activate T-cells and the ensuing
B-cell response. Specific immunoglobulin (Ig) E
antibodies produced by B-cells become attached to
the surface of effector cells such as mast cells and
basophils. Subsequent exposure to the same drug
induces formation of hapten-protein conjugates.
Immediate hypersensitivity is the result of cross-linking
of adjacent surface IgE molecules by the
hapten-protein conjugates that culminates in rapid
degranulation of preformed inflammatory mediators
such as histamine and tryptase.16
Mechanism of cross-reactivity and the side-chain hypothesis
Early cephalosporins before 1980s were
contaminated with trace amounts of penicillin
during the manufacturing process by the
cephalosporium mould. That partly accounted for
the higher cross-reactivity rate between penicillins
and first-generation cephalosporins.14 Cross-reactivity
within penicillins is based on common
antigenic determinants. Antibody binding against
basic structures such as BL ring or penicilloyl
frequently results in higher cross-reactivity rate.
More complex motifs, such as side-chains found
only in certain sub-groups, are associated with
lower cross-reactivity. An in-vitro experiment
has identified two types of T-cells responsible for
penicillin hypersensitivity. The restricted type
is immunologically reactive against a combined
penicilloyl and side-chain structure but exhibits
little cross-reactivity with penicillins with different
side-chains such as amoxicillin or ampicillin. The
broad type does react against different penicillins,
but not against cephalosporins.17
Epitopes (antibody-binding sites) on
penicillin molecules may involve the BL nucleus,
the thiazolidine ring, the side-chain or even the
new antigenic determinant. Side-chain antigenic
determinants account for 42% to 92% of the
penicillin hypersensitivity.18 19 Epitopes on cephalosporin molecules are even more heterogeneous than
penicillin, and involve the whole molecule.20 R1 side-chain
and BL fragment protein conjugates appear to be the major determinants of cephalosporin
hypersensitivity.21 R2 side-chain makes little contribution to cephalosporin hypersensitivity, as it disappears when the BL ring is opened.22
Human studies have provided insight into the
role of similarity in the R1 side-chains in causing
BL cross-reactivity.15 For instance, the 2-amino-2-phenylacetic acid side-chain in ampicillin is also present in first- or second-generation cephalosporins
like cephalexin and cefaclor, respectively, but is
absent in third- or fourth-generation cephalosporins.
Similarly, the same 2-amino-2-(4-hydroxyphenyl)
acetic acid side-chain is present in amoxicillin
and cefadroxil but not in new generations of
cephalosporins.16 In another study on selective
amoxicillin hypersensitivity, Miranda et al23 have
shown that oral challenge with cephadroxil, a first-generation
cephalosporin that shared the same side-chain
mentioned above, resulted in a cross-reactivity
of 38%. On the other hand, use of cefamandole, a
second-generation cephalosporin with a different
side-chain from amoxicillin and cephadroxil, did
not result in any cross-reactivity.23 Notwithstanding,
other authors do not accord with the side-chain
hypothesis.24 Fine structure within the side-chain
such as methylene group has also been suggested as
an antigenic determinant common to penicillins and
cephalosporins.25
Background and co-existing drug hypersensitivity
When dealing with potential cross-reactivity
between penicillins and cephalosporins, one should
take into account the background hypersensitivity
rates in unselected population, which range between
0.7% and 10% for penicillins.26 However, among
patients with a history of penicillin allergy, only 10%
to 20% exhibit positive allergic reaction to skin test or
challenge test.27 28 A non-urticarial, maculopapular skin rash is the most common allergic reaction with a
frequency of 1% to 2%. The frequency of anaphylaxis
per penicillin course is 0.01% to 0.05%.29 Similarly,
background hypersensitivity rates for cephalosporins
range between 1% and 10%, while anaphylaxis
occurs in less than 0.02%.30 In other words, patients
with penicillin hypersensitivity may develop non–cross-reacting allergic response to cephalosporins
by coincidence. They are also at increased risk of non-BL hypersensitivity, with a reported rate of 16%
to 23%.31 32 A caveat is that, as local prevalence data are lacking, epidemiological data can only be applied to the Hong Kong situation by extrapolation.
Cross-reactivity based on cephalosporin skin testing
Skin test is an in-vivo method used to diagnose
IgE-mediated allergic response. Substantial cross-reactivity
in terms of skin testing exists between penicillins and first-generation cephalosporins. In the
1970s, Assem and Vickers33 studied 24 patients with
penicillin hypersensitivity of which 11 (46%) showed
positive intradermal test to cephaloridine; however,
this reaction was not observed in any of the patients
without penicillin hypersensitivity.33 Dash2 studied 100 patients with clinical reaction to penicillin
and demonstrated positive cephalosporin skin test
in 11 (11%) patients. However, seven (9.3%) of 75
control subjects without penicillin hypersensitivity
also tested positive.2 In another study in the 1980s,
Sullivan et al34 recruited 74 patients with penicillin
hypersensitivity confirmed by positive skin prick
test (SPT). Of these, 38 (50%) also exhibited a
positive SPT to cephalothin, another first-generation
cephalosporin.34 Audicana et al35 studied 34 patients allergic to penicillin and found that five (14%) had
positive skin test to cephalexin, a first-generation cephalosporin, but none to ceftazidime, a third-generation
cephalosporin. Romano et al36 studied 128 adult subjects with a history of immediate
penicillin hypersensitivity; positive cephalosporin skin test was observed in 11% of them. Of the 128 subjects, 101 (94 skin test negative and 7 skin test positive for cephalosporins) who accepted the challenge could tolerate oral cefuroxime axetil and intramuscular ceftriaxone.36 Although controlled trial is not possible, the implication is that cephalosporins can be safely given to patients with a history of penicillin hypersensitivity but who have
negative cephalosporin skin test.
Cross-reactivity based on in-vitro tests
Substantial in-vitro cross-reactivity also
exists between penicillins and first-generation
cephalosporins. In an early study in 1960s, Abraham
et al37 were able to demonstrate haemagglutination
antibody against cephalothin (titre of 1:8 or greater)
in 22 (69%) of 32 patients who had been given
penicillin but denied a history of cephalothin
therapy. A subsequent adsorption study using
penicilloic acid-solid phase by Zhao et al25 further
identified cross-reacting specific IgE antibodies
against both benzylpenicillin and cephalothin.
Recently, Liu et al38 employed radioallergosorbent
test to identify specific IgE antibodies against
penicillins and cephalosporins in 420 subjects with
penicillin hypersensitivity; cross-reacting specific
IgE antibodies occurred in 22.6% of the subjects.
Specific cephalosporin IgE antibodies were present in
27.1% of those with specific penicillin IgE antibodies,
compared with 14.6% in those without specific
penicillin IgE antibodies.38 However, in the absence
of cross-linking, demonstration of antibodies cannot
be equated with clinical reactivity.2
Clinical reaction to cephalosporins in patients with a history of penicillin hypersensitivity
As skin test and in-vitro test are often inadequate
for confirmation of cephalosporin hypersensitivity,
one has to rely on a provocation test or the result
of drug exposure. In an early review of 701 patients
with a history of penicillin hypersensitivity, Petz39
reported an 8.1% reactivity rate to first- or second-generation
cephalosporins, compared with 1.9% among those without penicillin hypersensitivity. In
another cohort study in the 1980s by Solley et al,40
178 patients with a history of penicillin allergy were
given cephalosporins. Positive reaction resulted in
two patients, equivalent to a clinical cross-reactivity
rate of 1.1%.40 Goodman et al41 reviewed the medical records of 413 patients with a self-reported history of penicillin allergy who underwent anaesthetic
procedures that included antibiotic therapy. Only one patient (0.24%) probably developed cross-reactivity
to cephalexin, a first-generation cephalosporin.41
Despite the retrospective nature and the lack of confirmatory tests, the low clinical cross-reactivity
is reassuring.
Fonacier et al42 reviewed 83 patients with
penicillin hypersensitivity who were subsequently
given cephalosporins. Seven (8.4%) of them
developed an adverse drug reaction. A definite
history of penicillin hypersensitivity was found in
six (85.7%) of the seven patients. Eleven (13.3%)
patients with penicillin hypersensitivity also
reported hypersensitivity reaction to other drugs
such as non-BL antibiotics and codeine. Regarding
the types of cephalosporin, clinical cross-reactivity
rates between penicillin and first-, second-, third-,
and fourth-generation cephalosporins are 4.6%,
50%, 10.5% and 0%, respectively. Small sample size
and potential recall bias undermine the reliability of
the study. The role of side-chain is highlighted by a
4-fold increase in the cross-reactivity rate between
penicillins and cephalosporins with similar amino-benzyl ring side-chain.
In a large prospective study by Atanasković-Marković et al43 that included 644 children with a
history of hypersensitivity reaction to penicillins,
rate of cross-reactivity to cephalosporins was
31.5%. If the generations of cephalosporins were
taken into account, the cross-reactivity rate with
aminopenicillins differed by 100-fold, ranging from
0.3% to 0.7% in third-generation cephalosporins to
around 32.4% to 38.5% in first- or second-generation
cephalosporins, respectively. This, again, illustrates
the relevance of side-chain in cross-reactivity. An
interesting corollary is that, in patients with negative
skin test to penicillins or cephalosporins, 0% to
1.8% of patients showed positive drug challenge to
the test drug. Hence the false-negative rate of skin
test is quite low. On the other hand, as patients with
positive skin test were not further challenged with
drugs to confirm clinical hypersensitivity, the true-positive
rate cannot be ascertained.
A 5-year retrospective study by Apter et al32
reviewed 534 810 patients in the United Kingdom
who received a penicillin followed by cephalosporin
of which 64% were tested with first-generation
cephalosporins. The authors compared 3920 patients
with allergy-like events (ALE) within 30 days of
receiving penicillin with 530 890 patients without
ALE. Among 3920 patients with ALE after receiving
penicillin, 1% cross-reacted with cephalosporins. The
unadjusted risk ratios for ALE after the subsequent
cephalosporin and sulphonamide challenges were
10.0 (95% confidence interval [CI], 7.4-13.6) and
7.2 (95% CI, 3.8-13.5), respectively, suggesting that
patients allergic to penicillin may have an increased
tendency for drug hypersensitivity via a mechanism
other than cross-reactivity.
In another retrospective study, Daulat et al44
reviewed medical records of 606 patients with a
history of penicillin allergy who were subsequently
given a cephalosporin. Confirmatory penicillin
skin testing was not reported. Clinical allergy
occurred in only one patient given cefazolin, a first-generation
cephalosporin. This is tantamount to
a cross-reactivity rate of 0.165%. As drug allergy
was suspected from diagnostic coding only, true
penicillin allergy, and hence cephalosporin cross-reactivity,
might have been higher.
In a landmark meta-analysis in 2007, Pichichero
and Casey15 reviewed nine studies that compared
allergic reaction rate to cephalosporins in patients
with or without penicillin allergy. Among 47 284
patients with a history of penicillin allergy alone, the
odds ratio (OR) for cephalosporin cross-reactivity
in general was 2.63 (95% CI, 2.11-3.28; P<0.00001).
However, the increased cross-reactivity rate was
mainly due to first-generation cephalosporins, as
the corresponding ORs for first-, second-, and third-generation
cephalosporins were 4.79 (95% CI, 3.71-6.17; P<0.00001), 1.13 (95% CI, 0.61-2.12; P=0.70),
and 0.45 (95% CI, 0.18-1.13; P=0.09), respectively.
There was actually a trend towards decreased risk of
cross-reactivity to third-generation cephalosporins,
although the result did not reach statistical
significance.
Clinical reaction to cephalosporins in patients with penicillin hypersensitivity confirmed by investigations
In a cohort study by Solley et al,40 none of the 27
patients with a history of penicillin allergy and
a positive penicillin skin test developed clinical
reactivity to cephalosporins. On the contrary, two
of the 151 patients with allergic history but negative
penicillin skin test reacted to cephalosporins, putting
to doubt the value of penicillin skin test in predicting
cross-reactivity to cephalosporins.40 In another
small cohort study by Blanca et al,45 19 patients with
confirmed penicillin hypersensitivity were given
parenteral cephamandole, a second-generation
cephalosporin, followed by oral cephaloridine, a
first-generation cephalosporin, if the former was
tolerated. Two (10.5%) of the 19 patients cross-reacted
with cephamandole while all the remaining
17 patients tolerated cephaloridine.
Sastre et al24 subjected 16 patients with
selective amoxicillin hypersensitivity confirmed
by skin test or drug challenge with cephadroxil, a
first-generation cephalosporin. Two (12%) were
found to be cross-reactors.24 Novalbos et al46
recruited 41 patients with a history of penicillin
hypersensitivity confirmed by either skin test or
drug challenge. Patients were then challenged with
three cephalosporins (cephazoline, cefuroxime and
ceftriaxone) with side-chains which were different
from that of penicillin. None of them cross-reacted
with the cephalosporins.46 Hameed and Robinson47
recruited 158 patients with positive penicillin
test. Seven (4.4%) of them developed immediate
hypersensitivity when given cephalosporins. None of
the cephalosporins was from the third generation.47
There is a lack of published reports on anaphylactic
reaction to cephalosporins in children with a history
of anaphylaxis to penicillins, and only a few such
reports in adults have been published.47
Macy and Burchette48 studied 83 patients with a
history of adverse reaction to penicillin confirmed by
skin test. Post–skin test exposure to cephalosporins in
42 resulted in adverse reaction in one, amounting to
2.4% cross-reactivity rate. The corresponding figure
for non-BL was eight (10.8%) out of 74, suggesting
that in patients allergic to penicillin, cross-reactivity
for non-BLs may be even higher than that for BLs.48 This
study and the one by Apter et al32 have significant
implications for practitioners who routinely employ
non-BL antibiotics for patients with penicillin
hypersensitivity.
In a preoperative assessment clinic, Park et
al49 recruited 1072 patients with a history of BL
allergy for penicillin skin testing. Among the 999
patients who underwent the skin test, 43 had a
positive skin test for penicillin and three of those 43
eventually received cefazolin. None developed cross-reactivity.49 Ahmed et al50 reviewed 173 children
with a history of penicillin hypersensitivity, with or
without a skin test, who underwent cephalosporin
challenge. None among those with positive skin test
showed reactivity. However, one (0.7%) of the 152
patients with negative skin test had an immediate
allergic reaction after cephalexin, underscoring the
lack of predictive power of the penicillin skin test.50
In a meta-analysis by Pichichero and Casey,15
1831 patients with a history of penicillin allergy
also received penicillin skin test. Compared with
patients with negative skin test, the OR for cross-reactivity
to any cephalosporin for patients with
positive results was 1.48 (95% CI, 0.64-3.41; P=0.36).
Corresponding ORs for first-, second-, and third-generation
cephalosporins were 4.13 (95% CI, 0.70-24.51; P=0.11), 1.33 (95% CI, 0.32-5.52; P=0.69),
and 0.75 (95% CI, 0.15-3.66; P=0.72), respectively.15
There was a trend towards increased risk for first-generation
cephalosporins, although the result did not reach statistical significance.
Studies on cephalosporin drug challenge in
patients with a history of penicillin hypersensitivity
have several inherent limitations. Firstly, retrospective
studies are subjected to recall bias. Secondly,
the so-called ‘positive reaction’ may include ‘nocebo
effects’, ie untoward effects after administration of an
inert substance, which may occur in around 27% of
subjects.51 Thirdly, as most studies excluded patients
with positive penicillin skin test, investigators had
no way to tell whether these patients could actually
tolerate cephalosporins. Lastly, most studies of
cephalosporin challenge were performed in an open,
uncontrolled manner. Patients with penicillin allergy
who may have underlying multiple drug allergy
syndrome will be missed in the absence of a control
arm, such as non-BL group.52
Ten per cent cross-reactivity: an over-estimation
Review of published studies, as described above,
shows that cross-reactivity between penicillins and
cephalosporins, if restricted to clinical reaction
or positive drug challenge, varies between 0% and
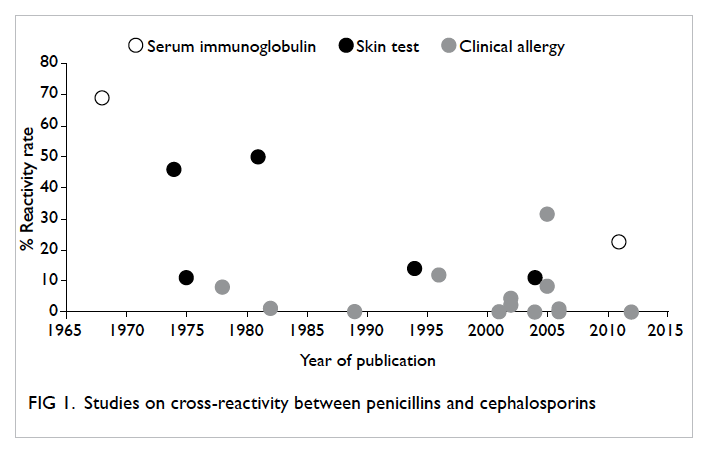
31.5% (Fig 1). Among the 14 studies that included a total of 6464 patients with penicillin hypersensitivity,
279 showed clinical reactivity or positive challenge
to cephalosporins, resulting in an average cross-reactivity
rate of 4.32%. Corresponding figures for patients with a history of penicillin allergy alone
and those confirmed by investigations are 4.34% and
3.76%, respectively. Studies reporting rates higher
than 10% are mainly those involving first- or second-generation
cephalosporins, especially when in-vitro or skin tests were employed. It must be emphasised
that although cross-reactivity is substantial with first-generation
cephalosporins (up to 32%), it is less than 1% for third- and fourth-generation cephalosporins.
A probable reason for the low cross-reactivity
stems from the fact that, despite having the same
BL nucleus, penicillins and cephalosporins are
immunologically different. If the BL nucleus is
the common antigenic determinant, one should
expect a very high cross-reactivity. However, this
is not the case because the BL ring opens in the
process of metabolism to form major or minor
determinants. Secondly, newer generations of
cephalosporin do not share similar side-chains with
penicillins, hence cross-reactivity will, generally,
not occur. Thirdly, among patients with alleged
penicillin hypersensitivity, less than 10% show
genuine hypersensitivity. The majority of cases may
suffer from transient adverse reaction followed by
subsequent tolerance to cephalosporins.7
Despite current recognition of the low cross-reactivity
rate, international guidelines are not
unanimous in their recommendations regarding
the use of cephalosporins in patients with penicillin
hypersensitivity. A recent practice parameter from
a Joint Task Force in the United States stated that
“most patients with a history of penicillin allergy
tolerate cephalosporins”.10 If patients with positive
penicillin skin test are given cephalosporins,
around 2% may cross-react, including some with
anaphylactic reactions. If a clinician chooses not to
skin test a patient with a history of penicillin allergy
but directly prescribe a cephalosporin, the chance
of developing a reaction is probably less than 1%.
In treating otitis media in children with penicillin
allergy, the American Academy of Pediatrics simply
suggested prescribing, rather than avoiding, either
second- or third-generation cephalosporins.53 Basing
on dissimilarity in chemical structures, the Academy
considered cross-reactivity between penicillin and
second- or third-generation cephalosporins to be
‘highly unlikely’.
A relatively conservative approach is adopted
by the Infectious Diseases Society of America
(IDSA). In the 2012 clinical practice guideline for
bacterial rhinosinusitis, the IDSA recommended
third-generation cephalosporins only for patients
with non–type I penicillin allergy. Non-BL antibiotics
were recommended for those with type I penicillin
allergy.54 Even more conservative is the British
Medical Association; in the 2014 edition, the BNF
advised against using cephalosporins in patients
with penicillin hypersensitivity. Nevertheless, if no
other alternatives are available, third- and fourth-generation
cephalosporins can be used, albeit with
caution.55
Skepticism still lingers within the medical community
in Hong Kong. For instance, a recent public
hospital antibiotic guideline does not differentiate
between different generations of cephalosporins,
but treats all cephalosporins as having the potential
to cross-react with penicillins.56 The IMPACT
guideline in Hong Kong has aptly pointed out a deep-rooted
preoccupation with cross-reactivity among
the medical profession. The guideline suggests that
“second, third and fourth generation cephalosporins
have negligible cross-reactivity with penicillin”.
However, it also raises a common concern that
contra-indications indicated in product inserts have
resulted in “medico-legal implications when using
cephalosporins in patients with penicillin allergy”.57
This concern is understandable but unfounded, for
two reasons. Firstly, a legal case appealed to the New
Jersey Supreme Court in 1998 has come to the final
decision that product inserts alone do not establish
a standard of care.3 Secondly, a review of the inserts
shows that, rather than contra-indicating the use
of cephalosporins, pharmaceutical companies only
issue words of caution in patients with penicillin
allergy.58 59
Pragmatic approach to cross-reactivity
For patients with suspected penicillin
hypersensitivity, one should begin with careful
history and physical examination to establish the
likelihood of adverse drug reaction. Clinicians will
not do justice by simply avoiding all cephalosporins
in patients with so-called penicillin hypersensitivity.
Injudicious use of non-BL antibiotics without
precaution is falsely reassuring and will expose
patients with allergic tendency to further drug
hypersensitivity.
Allergological investigations should preferably
be done 4 to 6 weeks after resolution of adverse
drug reaction.60 One should start from skin testing
to confirm penicillin hypersensitivity. Ideally, skin
test reagents should include penicilloyl polylysine
(PPL) and minor determinant mixture (MDM).
Unfortunately, the two major manufacturers,
Allergopharma (Hamburg, Germany) and Hollister-Stier (Spokane, WA, US) ceased production of PPL
and MDM in 2004. Although Diater (Madrid, Spain)
has launched the production of PPL and MDM
since 2003, the reagents have not gained widespread
popularity in Hong Kong.61 Besides, diagnosis of
selective reaction to a single BL requires a long
algorithm, which begins testing with PPL and MDM,
followed by the culprit drug.5 This may be tedious
and time-consuming in daily clinical practice. For
pragmatic purposes, it is often the culprit drug and/or a potentially safe alternative that will be tested
and prescribed. Non-irritating concentration of the
culprit drug should be employed for skin testing.62
Patients with negative penicillin skin test may
undergo supervised drug provocation test (DPT) of
the culprit drug.3 The aim of DPT is to exclude or
confirm the diagnosis of drug hypersensitivity and to
find a safe alternative for future use. Drug provocation
test generally has a high negative predictive value of
94% to 98%. A caveat is that anaphylactic reactions
can still occur among a few cross-reacting patients.
Hence, DPT must be performed by experienced
personnel in a setting with resuscitation facilities.60
Patients with remote or severe hypersensitivity may
be re-tested 2 to 4 weeks later to exclude a small but
possible risk of re-sensitisation after initial negative
testing. Contra-indications to DPT include a history
of severe cutaneous drug reactions (eg Stevens-Johnson syndrome or toxic epidermal necrolysis),
severe anaphylaxis or certain medical conditions
(eg uncontrolled asthma or pregnancy).63 In the
absence of severe or recent immediate penicillin
hypersensitivity, patients may choose to receive
penicillin directly without skin testing.10 To further
ensure drug safety, the first dose may be divided into
incremental steps similar to DPT.
Patients who have a history of severe penicillin
hypersensitivity, a positive penicillin skin test or DPT
may resort to cephalosporins. However, a positive
penicillin skin test does not predict cross-reactivity
with cephalosporin.30 50 Clinicians may perform
skin tests using a cephalosporin with a different side-chain to guide clinical use.10 Unfortunately, the diagnostic accuracy of cephalosporin skin test is difficult to establish.5 Studies generally have shown low sensitivity and positive predictive value.64 65 Nevertheless, skin test for BL hypersensitivity is still
considered ‘good’ by the International Consensus in 2014.60 Patients with negative cephalosporin skin test should pass a DPT before finally receiving a cephalosporin. Patients who fail the DPT may be given a non-BL antibiotic or undergo desensitisation, if the cephalosporin is essential.60 A suggested algorithm for investigation and management of suspected immediate penicillin hypersensitivity is
summarised in Figure 2.
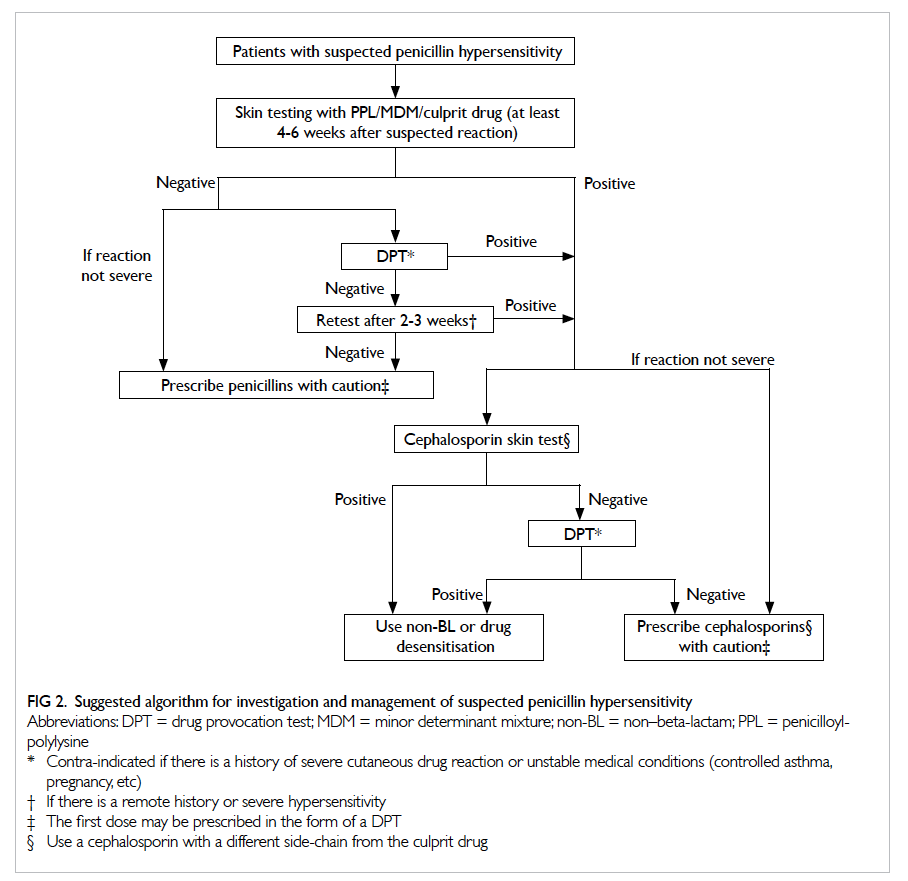
Figure 2. Suggested algorithm for investigation and management of suspected penicillin hypersensitivity
Conclusion
The available evidence to date does not support
the notion of a 10% cross-reactivity rate between
penicillins and cephalosporins. Above all, 10% is
an oversimplified and indiscriminate generalisation
of cross-reactivity. Scientific evidence supports the
side-chain hypothesis and a low cross-reactivity rate.
Clinicians should adopt a personalised approach
towards BL cross-reactivity. Finally, future research
on the local prevalence of BL hypersensitivity and
cross-reactivity is needed.
Declaration
No conflicts of interests were declared by the author.
References
1. Petz LD, Fudenberg HH. Coombs-positive hemolytic
anemia caused by penicillin administration. N Engl J Med
1966;274:171-8. CrossRef
2. Dash CH. Penicillin allergy and the cephalosporins. J
Antimicrob Chemother 1975;1(3 Suppl):107-18. CrossRef
3. DePestel DD, Benninger MS, Danziger L, et al.
Cephalosporin use in treatment of patients with penicillin
allergies. J Am Pharm Assoc (2003) 2008;48:530-40. CrossRef
4. Campagna JD, Bond MC, Schabelman E, Hayes BD. The
use of cephalosporins in penicillin-allergic patients: a
literature review. J Emerg Med 2012;42:612-20. CrossRef
5. Torres MJ, Blanca M, Fernandez J, et al. Diagnosis of
immediate allergic reactions to beta-lactam antibiotics.
Allergy 2003;58:961-72. CrossRef
6. Prematta T, Shah S, Ishmael FT. Physician approaches to
beta-lactam use in patients with penicillin hypersensitivity.
Allergy Asthma Proc 2012;33:145-51. CrossRef
7. Herbert ME, Brewster GS, Lanctot-Herbert M. Medical
myth: ten percent of patients who are allergic to penicillin
will have serious reactions if exposed to cephalosporins.
West J Med 2000;172:341. CrossRef
8. British Medical Association and the Royal Pharmaceutical
Society of Great Britain. British National Formulary BNF
49 March 2005. London: BMJ Books; 2005.
9. Solensky R, Earl HS, Gruchalla RS. Clinical approach to
penicillin-allergic patients: a survey. Ann Allergy Asthma
Immunol 2000;84:329-33. CrossRef
10. Joint Task Force on Practice Parameters; American
Academy of Allergy, Asthma and Immunology; American
College of Allergy, Asthma and Immunology; Joint Council
of Allergy, Asthma and Immunology. Drug allergy: an
updated practice parameter. Ann Allergy Asthma Immunol
2010;105:259-73. CrossRef
11. Cross reaction. In: Anderson DM, editor. Dorland’s
illustrated medical dictionary. 31st ed. Philadelphia: W.B.
Saunders; 2007.
12. Kowloon West Cluster Antibiotic Subcommittee. KWC
Antimicrobial Therapy Guide 2012, Second Edition [cited
2014 June 5]. Available from: https://gateway.ha.org.hk/f5-w-687474703a2f2f706d682e686f6d65$$/sites/mg/General/KWC%20Antimicrobial%20Thearpy%20
Guide%202012.pdf. Accessed Jun 2014.
13. Park B, Sanderson J, Naisbitt D. Drugs as haptens,
antigens, and immunogens. In: Pichler WJ, editor. Drug
hypersensitivity. Basel: Karger; 2007: 55-65. CrossRef
14. Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med
2001;345:804-9. CrossRef
15. Pichichero ME, Casey JR. Safe use of selected
cephalosporins in penicillin-allergic patients: a meta-analysis.
Otolaryngol Head Neck Surg 2007;136:340-7. CrossRef
16. Torres M, Mayorga C, Blanca M. Urticaria and anaphylaxis
due to betalactams (penicillins and cephalosporins). In:
Pichler WJ, editor. Drug hypersensitivity. Basel: Karger;
2007: 190-203. CrossRef
17. Mauri-Hellweg D, Zanni M, Frei E, et al. Cross-reactivity
of T cell lines and clones to beta-lactam antibiotics. J
Immunol 1996;157:1071-9.
18. Mayorga C, Obispo T, Jimeno L, et al. Epitope mapping
of beta-lactam antibiotics with the use of monoclonal
antibodies. Toxicology 1995;97:225-34. CrossRef
19. Torres MJ, Romano A, Mayorga C, et al. Diagnostic
evaluation of a large group of patients with immediate
allergy to penicillins: the role of skin testing. Allergy
2001;56:850-6. CrossRef
20. Pham NH, Baldo BA. beta-Lactam drug allergens: fine
structural recognition patterns of cephalosporin-reactive
IgE antibodies. J Mol Recognit 1996;9:287-96. CrossRef
21. Sánchez-Sancho F, Perez-Inestrosa E, Suau R, et al.
Synthesis, characterization and immunochemical
evaluation of cephalosporin antigenic determinants. J Mol
Recognit 2003;16:148-56. CrossRef
22. Blanca M, Romano A, Torres MJ, et al. Update on the
evaluation of hypersensitivity reactions to betalactams.
Allergy 2009;64:183-93. CrossRef
23. Miranda A, Blanca M, Vega JM, et al. Cross-reactivity
between a penicillin and a cephalosporin with the same
side chain. J Allergy Clin Immunol 1996;98:671-7. CrossRef
24. Sastre J, Quijano LD, Novalbos A, et al. Clinical cross-reactivity
between amoxicillin and cephadroxil in patients
allergic to amoxicillin and with good tolerance of penicillin.
Allergy 1996;51:383-6. CrossRef
25. Zhao Z, Baldo BA, Rimmer J. beta-Lactam allergenic
determinants: fine structural recognition of a cross-reacting
determinant on benzylpenicillin and cephalothin.
Clin Exp Allergy 2002;32:1644-50. CrossRef
26. Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and
extent of penicillin side-reactions, with particular reference
to fatalities from anaphylactic shock. Bull World Health
Organ 1968;38:159-88.
27. Graff-Lonnevig V, Hedlin G, Lindfors A. Penicillin
allergy—a rare paediatric condition? Arch Dis Child
1988;63:1342-6. CrossRef
28. Park MA, Li JT. Diagnosis and management of penicillin
allergy. Mayo Clin Proc 2005;80:405-10. CrossRef
29. Shepherd GM. Allergy to β-lactam antibiotics. Immunol
Allergy Clin N Am 1991;11:611-33.
30. Annè S, Reisman RE. Risk of administering cephalosporin
antibiotics to patients with histories of penicillin allergy.
Ann Allergy Asthma Immunol 1995;74:167-70.
31. Khoury L, Warrington R. The multiple drug allergy
syndrome: a matched-control retrospective study in
patients allergic to penicillin. J Allergy Clin Immunol
1996;98:462-4. CrossRef
32. Apter AJ, Kinman JL, Bilker WB, et al. Is there cross-reactivity
between penicillins and cephalosporins? Am J
Med 2006;119:354.e11-9.
33. Assem ES, Vickers MR. Tests for penicillin allergy in man.
II. The immunological cross-reaction between penicillins
and cephalosporins. Immunology 1974;27:255-69.
34. Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW.
Skin testing to detect penicillin allergy. J Allergy Clin
Immunol 1981;68:171-80. CrossRef
35. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions
to betalactams: studies in a group of patients allergic
to penicillin and evaluation of cross-reactivity with
cephalosporin. Allergy 1994;49:108-13. CrossRef
36. Romano A, Guéant-Rodriguez RM, Viola M, Pettinato
R, Guéant JL. Cross-reactivity and tolerability of
cephalosporins in patients with immediate hypersensitivity
to penicillins. Ann Intern Med 2004;141:16-22. CrossRef
37. Abraham GN, Petz LD, Fudenberg HH.
Immunohaematological cross-allergenicity between
penicillin and cephalothin in humans. Clin Exp Immunol
1968;3:343-57.
38. Liu XD, Gao N, Qiao HL. Cephalosporin and penicillin
cross-reactivity in patients allergic to penicillins. Int J Clin
Pharmacol Ther 2011;49:206-16. CrossRef
39. Petz LD. Immunologic cross-reactivity between penicillins
and cephalosporins: a review. J Infect Dis 1978;137
Suppl:S74-S79. CrossRef
40. Solley GO, Gleich GJ, Van Dellen RG. Penicillin allergy:
clinical experience with a battery of skin-test reagents. J
Allergy Clin Immunol 1982;69:238-44. CrossRef
41. Goodman EJ, Morgan MJ, Johnson PA, Nichols BA, Denk
N, Gold BB. Cephalosporins can be given to penicillin-allergic
patients who do not exhibit an anaphylactic
response. J Clin Anesth 2001;13:561-4. CrossRef
42. Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions
to a cephalosporins in hospitalized patients with a history
of penicillin allergy. Allergy Asthma Proc 2005;26:135-41.
43. Atanasković-Marković M, Velicković TC, Gavrović-Jankulović M, Vucković O, Nestorović B. Immediate allergic reactions to cephalosporins and penicillins and
their cross-reactivity in children. Pediatr Allergy Immunol
2005;16:341-7. CrossRef
44. Daulat S, Solensky R, Earl HS, Casey W, Gruchalla RS.
Safety of cephalosporin administration to patients with
histories of penicillin allergy. J Allergy Clin Immunol
2004;113:1220-2. CrossRef
45. Blanca M, Fernandez J, Miranda A, et al. Cross-reactivity
between penicillins and cephalosporins: clinical and
immunologic studies. J Allergy Clin Immunol 1989;83:381-5. CrossRef
46. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross-reactivity
to cephalosporins among patients allergic to
penicillins. Clin Exp Allergy 2001;31:438-43. CrossRef
47. Hameed TK, Robinson JL. Review of the use of
cephalosporins in children with anaphylactic reactions
from penicillins. Can J Infect Dis 2002;13:253-8.
48. Macy E, Burchette RJ. Oral antibiotic adverse reactions
after penicillin skin testing: multi-year follow-up. Allergy
2002;57:1151-8. CrossRef
49. Park M, Markus P, Matesic D, Li JT. Safety and effectiveness
of a preoperative allergy clinic in decreasing vancomycin
use in patients with a history of penicillin allergy. Ann
Allergy Asthma Immunol 2006;97:681-7. CrossRef
50. Ahmed KA, Fox SJ, Frigas E, Park MA. Clinical outcome
in the use of cephalosporins in pediatric patients with a
history of penicillin allergy. Int Arch Allergy Immunol
2012;158:405-10. CrossRef
51. Liccardi G, Senna G, Russo M, et al. Evaluation of the
nocebo effect during oral challenge in patients with
adverse drug reactions. J Investig Allergol Clin Immunol
2004;14:104-7.
52. American Academy of Allergy Asthma & Immunology.
Work group report: cephalosporin administration to
patients with a history of penicillin allergy May, 2009 [cited
2014 April 6]. Available from: https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.
pdf. Accessed Apr 2014.
53. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The
diagnosis and management of acute otitis media. Pediatrics
2013;131:e964-99. CrossRef
54. Chow AW, Benninger MS, Brook I, et al. IDSA clinical
practice guideline for acute bacterial rhinosinusitis in
children and adults. Clin Infect Dis 2012;54:e72-e112. CrossRef
55. British Medical Association and the Royal Pharmaceutical
Society of Great Britain. British National Formulary BNF
66 September 2013. London: BMJ Books; 2014.
56. Ching B. Hospital Authority guideline on known drug
allergy checking. Hong Kong: Hospital Authority; 2013.
57. Reducing bacterial resistance with IMPACT—Interhospital
Multi-disciplinary Programme on Antimicrobial
ChemoTherapy. 4th ed. Hong Kong: Centre for Health
Protection; 2012.
58. Ceftriaxone for Injection, USP [package insert] Lake
Forest, IL: Hospira; [updated Dec 2010; cited 2014 June
22]. Available from: http://www.hospira.com/Images/EN-2726_32-91495_1.pdf. Accessed Jun 2014.
59. Cefotaxime for Injection, USP [package insert]
Bridgewater, NJ: Sanofi; [updated Feb 2014; cited 2014 June
22]. Available from: http://products.sanofi.us/claforan/claforan.pdf. Accessed Jun 2014.
60. Demoly P, Adkinson NF, Brockow K, et al. International
Consensus on drug allergy. Allergy 2014;69:420-37. CrossRef
61. Romano A, Viola M, Bousquet PJ, et al. A comparison of the
performance of two penicillin reagent kits in the diagnosis
of beta-lactam hypersensitivity. Allergy 2007;62:53-8. CrossRef
62. Brockow K, Garvey LH, Aberer W, et al. Skin test
concentrations for systemically administered drugs—an
ENDA/EAACI Drug Allergy Interest Group position
paper. Allergy 2013;68:702-12. CrossRef
63. Aberer W, Bircher A, Romano A, et al. Drug provocation
testing in the diagnosis of drug hypersensitivity reactions:
general considerations. Allergy 2003;58:854-63. CrossRef
64. Romano A, Gaeta F, Valluzzi RL, Alonzi C, Viola M,
Bousquet PJ. Diagnosing hypersensitivity reactions to
cephalosporins in children. Pediatrics 2008;122:521-7. CrossRef
65. Yoon SY, Park SY, Kim S, et al. Validation of the
cephalosporin intradermal skin test for predicting
immediate hypersensitivity: a prospective study with drug
challenge. Allergy 2013;68:938-44. CrossRef