Hong Kong Med J 2014 Aug;20(4):313–6 | Epub 6 Jun 2014
DOI: 10.12809/hkmj134190
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
An update on irreversible electroporation of
liver tumours
Enoch SL Yeung, BDS, MB, BS;
Max WY Chung, MB, BS;
Keedon Wong, MB, BS;
Clement YK Wong, MB, BS;
Enoch CT So, BEng (UNSW), MB, BS;
Albert CY Chan, FCSHK, FHKAM (Surgery)
Department of Surgery, Queen
Mary Hospital, The University of Hong
Kong, Pokfulam, Hong Kong
Corresponding author: Dr Albert CY Chan (acchan@hku.hk)
Abstract
Objective: To
investigate the clinical efficacy and
safety of irreversible electroporation for ablation of
liver tumour in humans.
Data sources: The PubMed
and MEDLINE databases
were systematically searched.
Study selection: Clinical
research published in
English in the last 10 years until October 2013
that address clinical issues related to irreversible
electroporation of human liver tumours were
selected. “Liver tumour”, “local ablative therapy”,
and “irreversible electroporation” were used as the
search terms.
Data extraction and synthesis: The
data extracted
for this review was analysed by the authors, with
a focus on the clinical efficacy and the safety of
irreversible electroporation. The complete response
rates look promising, ranging from 72% to 100%,
except in one study in a subgroup of liver tumours
in which the complete response rate was only 50%
that was likely due to the inclusion of larger-size
tumours. In one study, the local recurrence rate at
12 months was approximately 40%. As for the safety
of irreversible electroporation, there were only a few reported
complications (cardiac arrhythmia,
pneumothorax, and electrolyte disturbance) that
were mostly transient and not serious. There was no
reported mortality related to the use of irreversible
electroporation.
Conclusion: Irreversible
electroporation is a
potentially effective liver tumour ablative therapy
that gives rise to only mild and transient side-effects.
Further studies with better patient selection criteria
and longer follow-up are needed to clarify its role as
a first-line liver tumour treatment modality.
Introduction
Local ablative therapies are frequently
employed for
the treatment of primary and secondary malignancies
in the liver. Common choices include radiofrequency
and microwave ablation. These treatment modalities,
however, may cause thermal injury to major bile ducts
within periductal tumours. In addition, the efficacy
of thermal ablative therapy is often undermined by
the heat sink effect, whereby the delivered thermal
energy is dissipated via a continuous high blood flow
in nearby major portal pedicles and hepatic veins.
Recently, the use of irreversible electroporation
(IRE) has been introduced into clinical practice. The
aim of this article was to provide an updated review
of the latest developments of this new technology in
the management of liver tumours.
Methods
A search on the medical literature was
performed to
identify the relevant studies and reviews regarding
the use of IRE as a treatment for primary liver
neoplasm. Both PubMed and MEDLINE databases were searched for
clinical studies published in
English in the last 10 years (until October 2013) that involved the use of IRE
for liver tumours. Key words used for the literature
search were: “liver tumour”, “local ablative therapy”,
and “irreversible electroporation”.
Mechanism and history of
electroporation
Electroporation utilises electrical fields
to induce
changes to plasma membrane permeability. More
specifically, multiple rapid direct current electrical
pulses are applied to the tissue of interest. These
electrical pulses induce nano-scale pores within
the phospholipid bilayer, thus changing cell
permeability.1 There are
two types: reversible
electroporation (RE) and IRE.
As the name suggests, tissues subjected to
RE remain viable after the procedure. The lesser
electrical strength and duration of the applied pulses
during the procedure allow pores in the membrane
to spontaneously seal by themselves. Researchers
exploited this unique effect of a transient increase in cell
permeability to enable foreign materials that were
previously deemed impermeable to pass through the
phospholipid bilayer. A prominent example would
be electrochemotherapy. Since the 1990s, multiple
human clinical trials2 3 4 have shown that RE applied for
this purpose enhances the delivery of chemotherapy
(eg bleomycin) to the desired tissue (eg skin cancer,
breast cancer).
By contrast, IRE relies on delivering
electrical
pulses whose strength and/or duration exceeds the
threshold of spontaneous cell membrane repair. The
permanent permeability of the cell membrane that
they induce disrupts the homeostasis of the cells,
leading to cell death. Interestingly, this technique
was largely ignored by the medical community until
2005, when Davalos et al5
proved its theoretical basis
via a mathematical analysis. This predicted that
“irreversible electroporation can ablate substantial
volumes of tissue, comparable to those achieved
with other ablation techniques, without causing any
detrimental thermal effects and without the need of
adjuvant drugs.”5
Rubinsky et al6
performed the first
experimental IRE in 2007 by performing 35 ablations
on 14 swine livers. Interestingly, all animals survived
till the electrical pulse applications ceased, but
reversible chemical paralysis was necessary to
prevent unwanted muscular contractions during the
procedure. Histologically there was haemorrhagic
necrosis of the liver, but preservation of the vessels
and bile ducts within the zones of ablation.7
Since then, many other animal studies have
been performed in various organs and tissues,
including liver, prostate, pancreas, small bowel,
kidney, carotid artery, atrial appendages, and lung.
The results were encouraging, in that IRE conferred
three key advantages in all of these studies:
(1) It was effective in ablating tissues of interest, including tumours.
(2) It lacked the heat-sink effect. Traditional thermal ablation relied on tissue temperature reaching a certain threshold (60°C) in order to induce cell death. Cells near the large vessels were therefore prone to the continuously cooling effect from the flowing fluids within the vessels. This leads to incomplete necrosis, making local recurrence of tumours more likely.1
(3) It was capable of preserving critical structures (blood vessels, bile ducts, urethra, and nerves) within the zone of ablation. Maor et al8 performed an experiment by applying IRE directly onto the carotid arteries of six rats. All rats survived with no apparent side-effects. Carotid arteries remained intact with no evidence of aneurysm, thrombus, or necrosis 28 days later. Histologically, there was a statistically significant decrease in mean vascular smooth muscle cell density (24 ± 11 vs 139 ± 14; P<0.001) but with no apparent damage to extracellular matrix components and structure. This may explain why critical structures appear to be preserved with this new technology.
(1) It was effective in ablating tissues of interest, including tumours.
(2) It lacked the heat-sink effect. Traditional thermal ablation relied on tissue temperature reaching a certain threshold (60°C) in order to induce cell death. Cells near the large vessels were therefore prone to the continuously cooling effect from the flowing fluids within the vessels. This leads to incomplete necrosis, making local recurrence of tumours more likely.1
(3) It was capable of preserving critical structures (blood vessels, bile ducts, urethra, and nerves) within the zone of ablation. Maor et al8 performed an experiment by applying IRE directly onto the carotid arteries of six rats. All rats survived with no apparent side-effects. Carotid arteries remained intact with no evidence of aneurysm, thrombus, or necrosis 28 days later. Histologically, there was a statistically significant decrease in mean vascular smooth muscle cell density (24 ± 11 vs 139 ± 14; P<0.001) but with no apparent damage to extracellular matrix components and structure. This may explain why critical structures appear to be preserved with this new technology.
Clinical efficacy of irreversible
electroporation on human liver
tumours
To date, four published case series9 10 11 12 have
evaluated
the safety and efficacy of IRE on human liver
tumours. All of them adopted the NanoKnife system
(AngioDynamics, New York, US), which consists
of a footswitch, a control panel with a screen
and a cardiac synchroniser, and a direct current
generator connected with unipolar or bipolar needle
electrodes. The number and placement of electrodes
are determined by the size of the target tumour. The
current, the applied voltage, and duration of ablation
can be varied according to tumour characteristics.
The procedure can be performed via percutaneous,
laparoscopic, or open surgical approaches.9 10 11
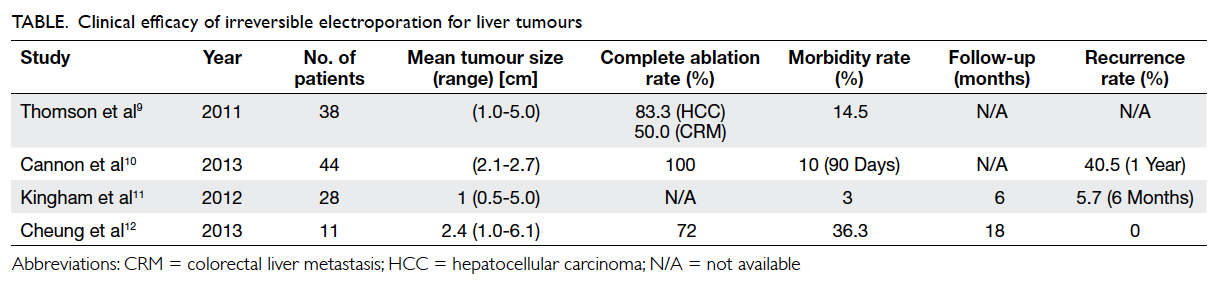
The Table9 10 11 12 illustrates the efficacy and
postoperative outcomes after IRE in various case
series. The complete response rate ranged from
72% to 100%,9 10 11 12 except in Thomson et al’s study9
in which the complete response rate was only 50%
for colorectal liver metastasis. That study also
demonstrated lack of significant tumour response
when the size of the liver metastatic lesion was larger than 5 cm.
In Cannon et al’s study,10 however, the complete
response rates were the same for hepatocellular
carcinoma (HCC) and colorectal liver metastasis.
The lower complete
response rate in Thomson et al’s study9
could be
partially attributed to the inclusion of larger-size
tumours and greater use of the percutaneous
approach. Consistent with Thomson et al’s study,9
they also showed a trend towards higher recurrence
rates for tumours exceeding 4 cm in diameter.
The longest follow-up among these studies
was 12 months, which was in Cannon et al’s study,10
by which time the local recurrence rate was about
40% for both HCC and colorectal liver metastases.
To date, no randomised controlled trial comparing
the efficacy of IRE and other ablation modalities
has been published. The latest quoted figures for
recurrence rates are approximately 2% to 15% 2
years after radiofrequency ablation, and 11% to 35%
2 years after percutaneous ethanol injection.13
The complete response rate of liver tumour
to
IRE looked promising, but a 40% local recurrence
rate after 1 year was too high to justify its use as first-line
treatment. Moreover, tumour size seems to be
an important consideration affecting the outcome.
The inclusion of large tumours may have contributed
to the high local recurrence rate in Cannon et al’s
study.10 More prospective
studies are warranted to
define standard selection criteria in order to achieve
satisfactory outcomes.
Adverse effects of irreversible
electroporation
Like any other local ablative therapy, IRE
is also
associated with a few other adverse effects, be they
general or procedure-related.
General intra-operative complications
As with all operations, IRE carries the
risk of general
anaesthesia9 and positional
neuropraxia.14 In two
retrospective studies, such effects occurred in an
isolated number of patients but were transient and
self-limiting, and resolved without any long-term
disability.9 14
Specific intra-operative complications
One of the specific complications related
to IRE is
unintended injury to other organs and structures
during manipulation of the electrodes. One instance
of pneumothorax due to direct injury caused by
an electrode was reported by Thomson et al.9 That
pneumothorax resolved spontaneously and did not
result in a delayed discharge. The same authors9
reported another instance of direct damage by an
instrument due to an unplanned tip insertion during
an attempt to treat a renal tumour. That particular
patient had transient acute hypotension, and
subsequent mild hypotension for a further 2 months.
Cardiac arrhythmia is a potential
life-threatening
complication associated with IRE, which
is presumed due to application of a large current close
to the heart, especially for liver tumours situated
below the right hemidiaphragm. Thomson et al9 and
Ball et al14 reported
cases of ventricular bigeminy,
ventricular tachycardia, and atrial fibrillation during
the procedure, in which a drop in blood pressure was
associated with the arrhythmia. Furthermore, IRE
has been incorporated with electrocardiographic
synchronisation, rendering the possibility of intra-operative
arrhythmia less common. Although
none of the studies reported mortality due to cardiac
arrhythmia, this potentially devastating effect should
not be ignored.
In addition to cardiac arrhythmia,
discharges
from the electrode could cause muscle stimulation.
In one case, insufficient muscle relaxant was used,
resulting in an upper body contraction similar to what ensues
during a grand mal seizure.14
Hence,
IRE treatment should be performed under general
anaesthesia with deep neuromuscular blockade, in
order to prevent excessive body movement during
treatment.
Since IRE involves the disruption of the
cellular
membrane, it results in the release of intracellular
contents whenever tumour cells are electroporated.
Ball et al14 reported four
instances of hyperkalaemia in
21 patients treated with IRE, but without significant
sequelae. Early postoperative arterial blood gas
sampling and electrocardiographic monitoring
during the procedure may help to prevent the lethal consequence of
severe hyperkalaemia.
Premature termination of the procedure for
technical reasons has also been reported, but detailed
explanations were not given.11
This complication
subjects patients to further IRE treatments, but
under more controlled conditions.
Postoperative complications
The postoperative complications of IRE have
been
reported in several studies. Postoperative pain was
of primary concern for most of the operations.
About half of the patients who underwent IRE
had some degree of postoperative pain but those
treated with either IRE or radiofrequency ablation
reported similar pain scores.15
Notably, there have
been no reported instance of vascular or biliary
complications after IRE for periductal tumours or
tumours abutting major vessels. This means that for
tumours in difficult locations, it is a promising local
ablative treatment modality compared with other
local ablative therapies. Besides, hitherto there has
not been any mortality directly related to IRE. One
study reported that the mortality rate at 30 days was
0%.9 However, another study
available in
abstract form reported one fatality 1 month after the
operation, though no other details were provided.16
As of 2012, IRE has been performed 158 times in
106 patients with liver tumours, with no attributed
mortality.7 At the time of
writing, a prospective
multicentre phase II study on the efficacy and safety
profile of the NanoKnife System (AngioDynamics)
for early-stage HCC has just been completed and the
outcome of this study is eagerly awaited.
Conclusion
The tumour ablative effect of IRE appears
promising.
In particular, it seems effective for small tumours
(<3 cm), periductal tumours, and tumours abutting
major hepatic vessels, where conventional local
ablative treatments for such difficult tumour
locations could be risky and less effective.
References
1. Rubinsky B. Irreversible
electroporation in medicine. Technol Cancer Res Treat
2007;6:255-60.
2. Benevento R, Santoriello A, Perna G, Canonico S. Electrochemotherapy of cutaneous metastases from breast cancer in elderly patients: a preliminary report. BMC Surg 2012;12 Suppl 1:S6. CrossRef
3. Campana LG, Valpione S, Falci C, et al. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat 2012;134:1169-78. CrossRef
4. Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol 2012;19:192-8. CrossRef
5. Davalos RV, Mir IL, Rubinsky B.
Tissue ablation with irreversible electroporation. Ann Biomed Eng
2005;33:223-31. CrossRef
6. Rubinsky B, Onik G, Mikus P.
Irreversible electroporation: a new ablation modality—clinical
implications. Technol Cancer Res Treat 2007;6:37-48.
7. Charpentier KP. Irreversible
electroporation for the ablation of liver tumors: are we there
yet? Arch Surg 2012;147:1053-61. CrossRef
8. Maor E, Ivorra A, Leor J,
Rubinsky B. The effect of irreversible electroporation on blood
vessels. Technol Cancer Res Treat 2007;6:307-12.
9. Thomson KR, Cheung W, Ellis SJ,
et al. Investigation of the safety of irreversible electroporation
in humans. J Vasc Interv Radiol 2011;22:611-21. CrossRef
10. Cannon R, Ellis S, Hayes D,
Narayanan G, Martin RC 2nd. Safety and early efficacy of
irreversible electroporation for hepatic tumors in proximity to
vital structures. J Surg Oncol 2013;107:544-9. CrossRef
11. Kingham TP, Karkar AM,
D’Angelica MI, et al. Ablation of perivascular hepatic malignant
tumors with irreversible electroporation. J Am Coll Surg
2012;215:379-87. CrossRef
12. Cheung W, Kavnoudias H, Roberts
S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation
for unresectable hepatocellular carcinoma: initial experience and
review of safety and outcomes. Technol Cancer Res Treat
2013;12:233-41.
13. Tiong L, Maddern GJ.
Systematic review and meta-analysis of survival and disease
recurrence after radiofrequency ablation for hepatocellular
carcinoma. Br J Surg 2011;98:1210-24. CrossRef
14. Ball C, Thomson KR, Kavnoudias
H. Irreversible electroporation: a new challenge in “out of
operating theater” anesthesia. Anesth Analg 2010;110:1305-9. CrossRef
15. Narayanan G, Froud T, Lo K,
Barbery KJ, Perez-Rojas E, Yrizarry J. Pain analysis in patients
with hepatocellular carcinoma: irreversible electroporation versus
radiofrequency ablation—initial observations. Cardiovasc Intervent
Radiol 2013;36:176-82. CrossRef
16. Narayanan G, Yrizarry J,
Perez-Rojas E, et al. Safety and efficacy of irreversible
electroporation in the treatment of primary HCC [abstract]. J Vasc
Interv Radiol 2011;22:S63-S64. CrossRef