Hong Kong Med J 2020 Feb;26(1):44–55 | Epub 12 Feb 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Echocardiography update for primary care physicians: a review
Jeffrey SK Chan, MB, ChB1; Gary Tse, MPH, PhD1; H Zhao, MD2; XX Luo, MB3; CN Jin, PhD4; Kevin Kam, MB, ChB, MRCP1; YT Fan, MD, PhD1,5; Alex PW Lee, MD, FRCP1,5
1 Division of Cardiology, Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong
2 Department of Cardiology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
3 Department of Ultrasonography, Shenzhen Hospital, Southern Medical University, Shenzhen, China
4 Department of Cardiology, The Second Affiliated Hospital of Zhejiang University, Zhejiang, China
5 Laboratory for Cardiac Imaging and 3D Printing, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Alex PW Lee (alexpwlee@cuhk.edu.hk)
Abstract
Echocardiography is a key evaluation tool for the diagnosis, prognosis, and guidance of interventional management of numerous cardiovascular conditions, including ischaemia, heart failure, and structural heart diseases. Recent technological advancements have also seen the exploration of artificial intelligence, intracardiac vortex imaging, and three-dimensional printing in echocardiography. With cardiovascular diseases increasing in prevalence worldwide, it is important for clinicians including general practitioners to have updated knowledge of appropriate use of echocardiography. As such, this article reviews the current literature and summarises the latest developments and the general clinical usage of echocardiography.
Introduction
Cardiovascular diseases are a major burden to healthcare globally and a leading cause of death in industrialised countries.1 In 2015, cardiovascular disorders accounted for 77 600 inpatient discharges in Hong Kong, including 6190 deaths, making them the third most common cause of death. The prevalence of cardiovascular disease is increasing and the age-standardised mortality is decreasing.2 Thus, patients with heart diseases are increasingly likely to present to primary care physicians as their first point of care or for a follow-up examination: up to 15% of coronary heart disease patients are treated exclusively by primary care physicians.3 Furthermore, a long-term follow-up study has proven the viability of general practitioners providing care for low-risk chronic heart failure patients.4 As such, clinical care for patients with heart diseases has become increasingly relevant to primary care physicians.
Echocardiography does not involve any radiation and may be performed as a point-of-care diagnostic tool in both outpatient and inpatient settings. Doppler echocardiography allows haemodynamic assessment of stroke volume, pressure gradients, valvular regurgitations, and intracardiac shunts. The treatment of patients with cardiovascular diseases almost invariably involves the use of echocardiography as a means of initial and follow-up investigation. Many patients undergo cardiac surgery and/or catheter-based interventions guided by perioperative or intraprocedural echocardiography. Point-of-care echocardiography in primary care settings improves outcomes in some patients,5 and may identify otherwise-undiagnosed valvular heart diseases.6
Echocardiographic technologies remain a vibrant field of research. The rapid advancement of echocardiographic technologies requires primary care physicians to have an updated understanding of its appropriate use and referral. According to the American College of Cardiology, appropriate echocardiography “likely contributes to improving patient’s clinical outcomes” and inappropriate echocardiography may be “potentially harmful to patients and generate unwarranted costs to the healthcare system”.7 Recent technological developments have expanded the use of echocardiography dramatically. Therefore, the aim of this study was to review the contemporary literature on clinical indications and emerging technologies of echocardiography and to provide a summary for primary care physicians.
Basic echocardiography and the echocardiographic report
Transthoracic echocardiography (TTE) is non-invasive, readily available, and is generally the initial imaging option. In contrast, transoesophageal echocardiography (TEE) requires insertion of a probe into the oesophagus and thus involves sedation and risks of complications such as major bleeding (<0.01%) and hoarseness of voice (12%).8 However, TEE offers better resolution, especially of the left heart, mitral and aortic valves, and the aorta. A routine echocardiography involves several methods of imaging, including two-dimensional (2D; previously known as B mode), Doppler, and M mode echocardiography. Two-dimensional echocardiography, the most commonly used and intuitive method, is used for visualisation and dimensional measurements of the cardiac structures. Two-dimensional echocardiography is often the initial method of imaging, by which cardiac structures are identified and other methods applied. The left ventricular ejection fraction (LVEF) is also obtainable from 2D echocardiography, with the normal value being 50% to 70%. This is mainly used in the classification of heart failure.
Colour Doppler imaging allows visualisation of the blood flow, the conventional colour scheme being red for flow towards the probe and blue for away. This allows intuitive and easy assessment of valvular assessments, where stenotic valves generally show turbulent flow and often thickened valve leaflets, whereas regurgitating valves show a regurgitant jet. Trace or mild regurgitation is common even in normal, healthy individuals. When an abnormal, turbulent flow is detected by colour Doppler imaging, continuous and pulse wave spectral Doppler techniques should be used to further evaluate the nature and severity of the abnormal flow.
Continuous and pulse wave Doppler imaging provides quantitative measurement of flow velocity, volume, and pressure gradient at operator-defined locations. These allow calculation of stroke volume and haemodynamic assessment of the heart valves. Tissue Doppler provides information about movement of cardiac tissues, such as the diastolic motion of the mitral annulus that reflects left ventricular diastolic function. By means of transmitral flow assessment (ie, E, A, E/A ratio) and tissue Doppler imaging of the mitral annulus (ie, e′, a′, s′, E/e′), filling pressure and diastolic function of the left ventricle can also be assessed.9 Interpretation of these parameters is complex and requires correlation with the clinical picture; in general, the normal E/A ratio is 0.8-2 but may be high in young, fit persons; a E/e′ (medial mitral annulus) ratio <8 generally indicates normal left ventricular filling pressure. Further details may be found in the joint recommendation of the European Association for Cardiovascular Imaging and the American Society of Echocardiography for diastolic echocardiographic assessment.10
Lastly, M mode tracks over time the movement of tissues along a straight line extending from the probe and has the advantage of high temporal resolution. The M mode is most commonly used for the assessment of right ventricular function by means of tricuspid annular plane systolic excursion, which is typically ≥17 mm. The M mode is also useful in the assessment of constrictive pericarditis, pericardial effusion and cardiac tamponade, hypertrophic obstructive cardiomyopathy, pulmonary hypertension, and left ventricular outflow tract obstruction.11 An echocardiographic report thus contains information about dimensions and functions of the heart chambers and the proximal aorta. Table 1 summarises the typical components of an echocardiographic report.12 A set of selected standard echocardiographic images is shown in Figure 1.
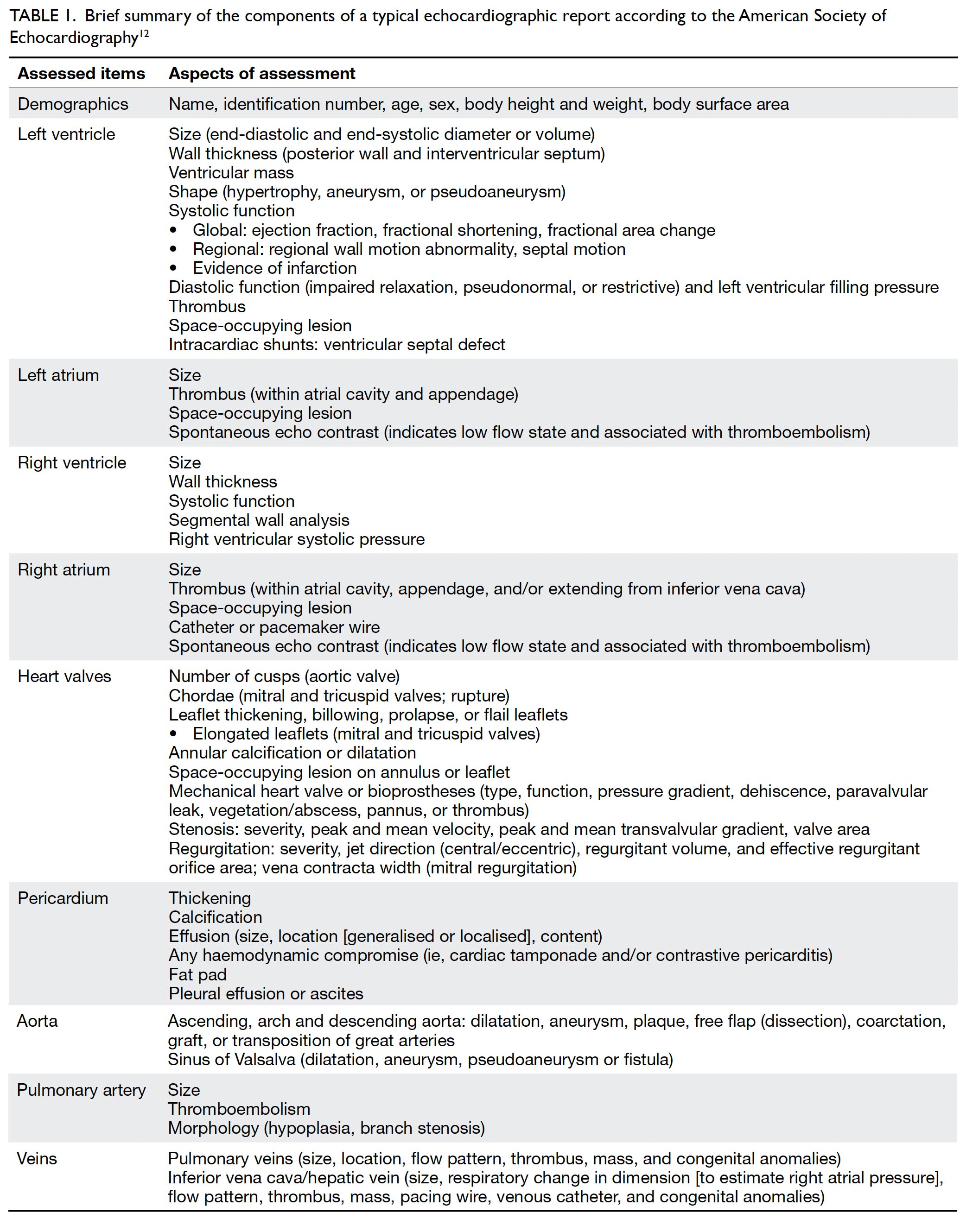
Table 1. Brief summary of the components of a typical echocardiographic report according to the American Society of Echocardiography12
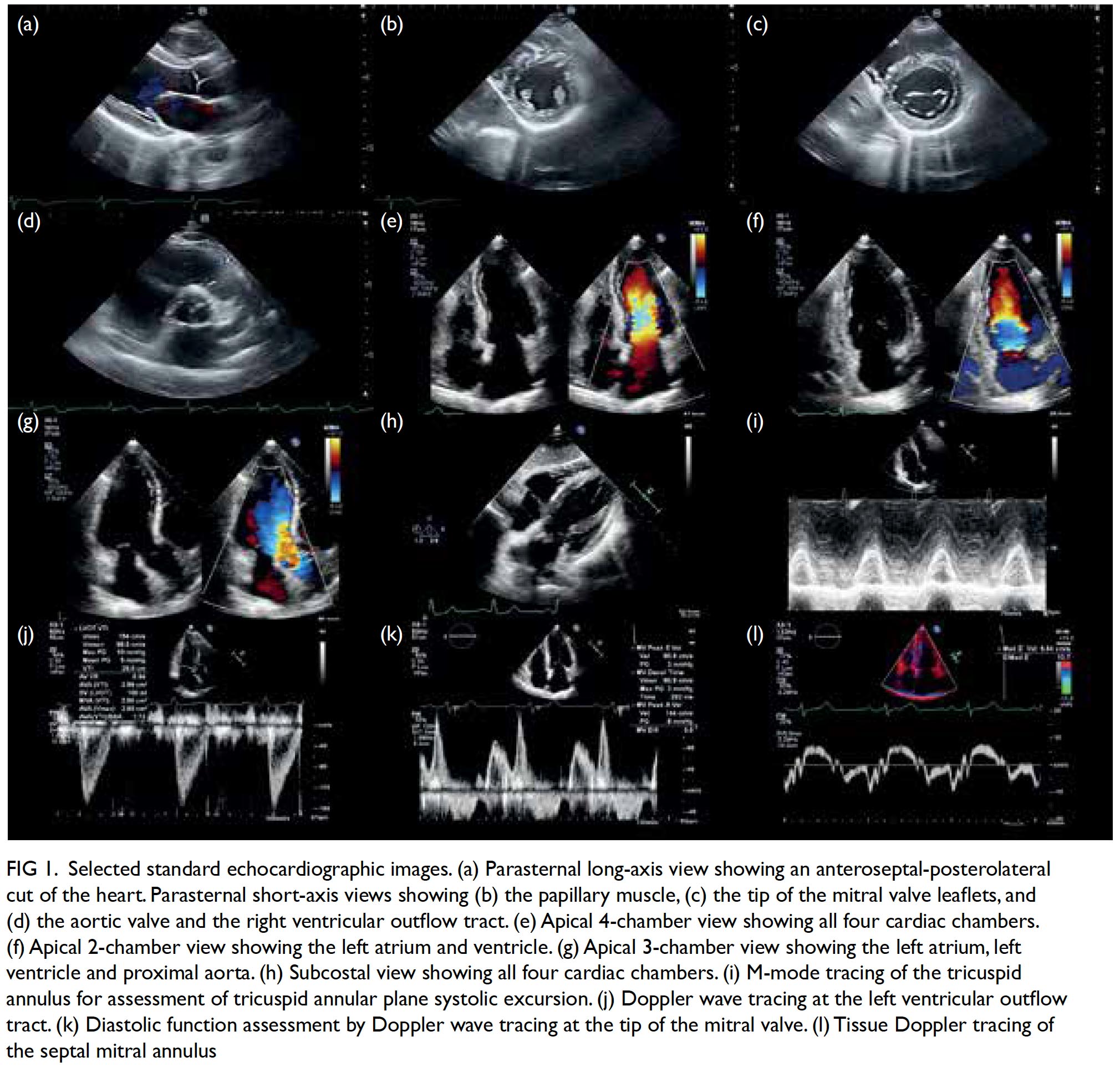
Figure 1. Selected standard echocardiographic images. (a) Parasternal long-axis view showing an anteroseptal-posterolateral cut of the heart. Parasternal short-axis views showing (b) the papillary muscle, (c) the tip of the mitral valve leaflets, and (d) the aortic valve and the right ventricular outflow tract. (e) Apical 4-chamber view showing all four cardiac chambers. (f) Apical 2-chamber view showing the left atrium and ventricle. (g) Apical 3-chamber view showing the left atrium, left ventricle and proximal aorta. (h) Subcostal view showing all four cardiac chambers. (i) M-mode tracing of the tricuspid annulus for assessment of tricuspid annular plane systolic excursion. (j) Doppler wave tracing at the left ventricular outflow tract. (k) Diastolic function assessment by Doppler wave tracing at the tip of the mitral valve. (l) Tissue Doppler tracing of the septal mitral annulus
Common clinical scenarios where echocardiography is indicated
The clinical indications for echocardiography are detailed in the appropriate use criteria from the European Association for Cardiovascular Imaging13 and the American College of Cardiology.7 This section summarises the key recommendations for the use of echocardiography in some common chief complaints encountered by clinicians. Key points are summarised in Table 2. In general, echocardiography is indicated for evaluating symptoms or conditions potentially of cardiac aetiology, including chest pain, shortness of breath, palpitations, transient ischaemic attack, stroke, or peripheral embolic event. Echocardiography is also considered appropriate when heart disease or structural abnormality are suspected on prior testing including but not limited to chest X-ray, electrocardiogram (ECG), or cardiac biomarkers, whether the patient is symptomatic or not. As a general principle, echocardiography is accepted as appropriate for initial diagnosis when there is a change in clinical status or when the results of the echocardiography are anticipated to change patient treatment. In contrast, routine testing when there is no change in clinical status or when test results are unlikely to lead to changes in treatment are generally considered inappropriate.
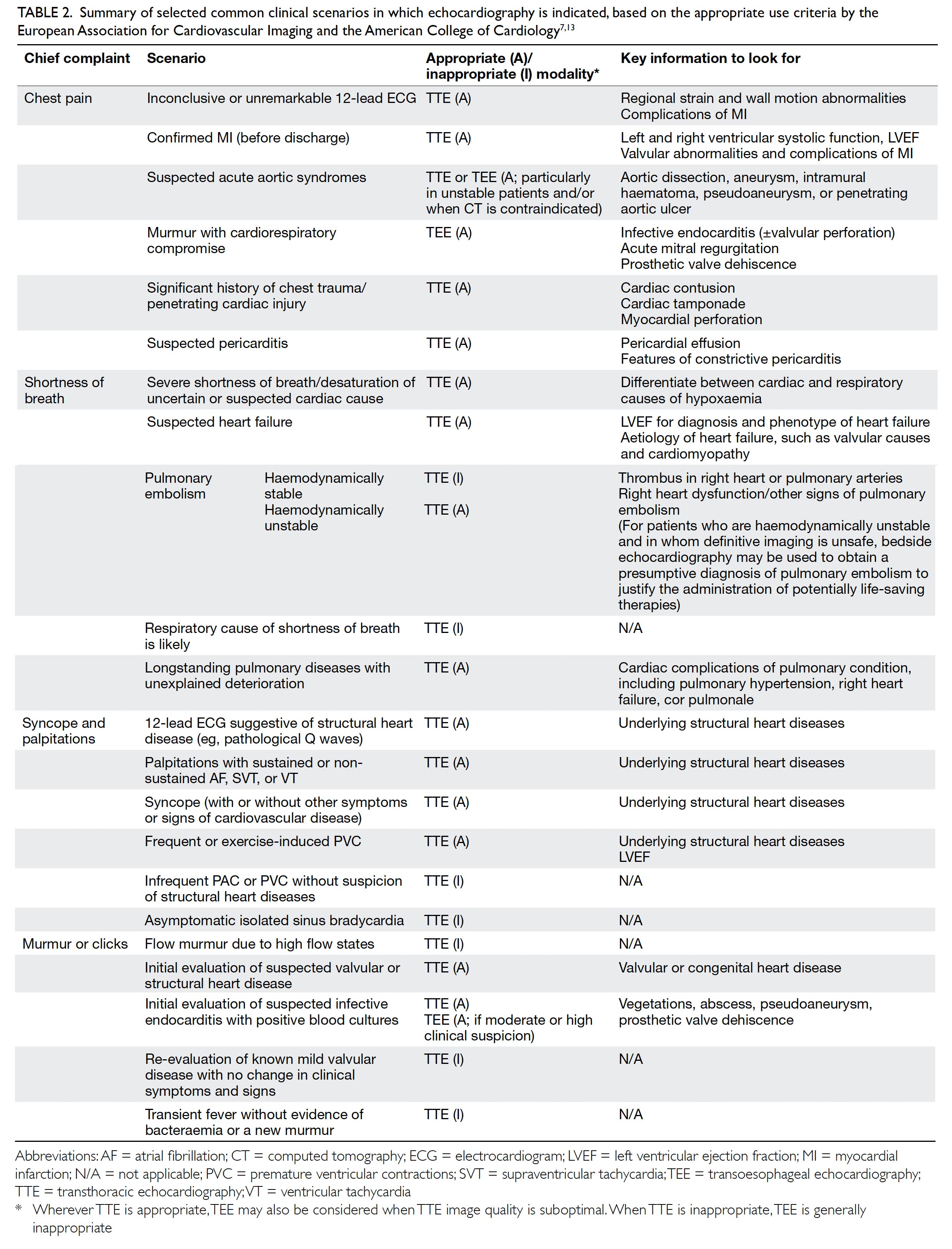
Table 2. Summary of selected common clinical scenarios in which echocardiography is indicated, based on the appropriate use criteria by the European Association for Cardiovascular Imaging and the American College of Cardiology7 13
Chest pain
The differential diagnoses of chest pain are broad, and reaching a definitive diagnosis is often difficult when relying on the clinical history and physical examination only. The most important diagnosis in patients presenting with acute chest pain is acute coronary syndrome (ACS). As such, 12-lead ECG and troponin assay are the first-line investigations for acute chest pain suggestive of ACS. Echocardiography aids or confirms ischaemic diagnoses, and is used to screen for non-ischaemic structural, aortic, and pericardial pathology in selected patients. For evaluation of acute chest pain with suspected myocardial ischaemia/infarction and non-diagnostic ECG, resting TTE is appropriate and can be performed while the patent has chest pain. The absence of regional wall motion abnormality on 2D TTE during chest pain virtually excludes acute myocardial infarction, with a sensitivity of 93% for any infarct and 100% for ST-elevation myocardial infarction.14 All patients with chest pain should have initial evaluation of ventricular function by TTE after ACS. After ACS, re-evaluation of ventricular function during the recovery phase is appropriate when results will guide therapy.15 For detecting other causes of chest pain, including acute aortic syndromes, valvular diseases, pulmonary embolism, and pericarditis, TTE is valuable and can be done in emergency settings. Although TTE can be used as a first-line test to assess suspected aortic pathologies, TEE allows clearer and more complete assessment of the thoracic aorta, with diagnostic accuracy equivalent to that of cardiac computed tomography (CT) scans or magnetic resonance imaging (MRI).16 Even though CT is the imaging modality of choice in acute aortic syndromes, TTE and TEE remain valuable for acute assessment and follow-up, with the advantage of not requiring radiation, and especially for hemodynamically unstable patients and situations where CT contrast is contraindicated (eg, severe renal impairment).17
Shortness of breath
Shortness of breath is a common symptom among patients presenting to primary and emergency care physicians. Differential diagnoses of breathlessness include cardiovascular disease, lung disease, anaemia, obesity, and deconditioning. In any setting, clinical history and examination are the first step in evaluation.13 When heart failure is suspected after initial clinical evaluation, TTE is mandatory to confirm or exclude the diagnosis; to quantify chamber volumes, systolic and diastolic function, and wall thickness; and to identify the aetiology of heart failure (eg, cardiomyopathy, valvular disease, or prior myocardial infarction). The TTE also serves to classify heart failure into heart failure with preserved, mid-range or reduced EF (Fig 2).18 Such classification guides management of the condition. For patients with heart failure with preserved or mid-range EF, assessment of diastolic function is important as diastolic dysfunction is known to be key in the pathogenesis. Although some patients have resting echocardiographic evidence of diastolic dysfunction, left ventricular filling pressure, which is the main haemodynamic parameter that explains exertional dyspnoea, may only be elevated during exertion. This may be demonstrated by diastolic stress echocardiography, providing additional haemodynamic information about the patient and aiding diagnosis and treatment.19
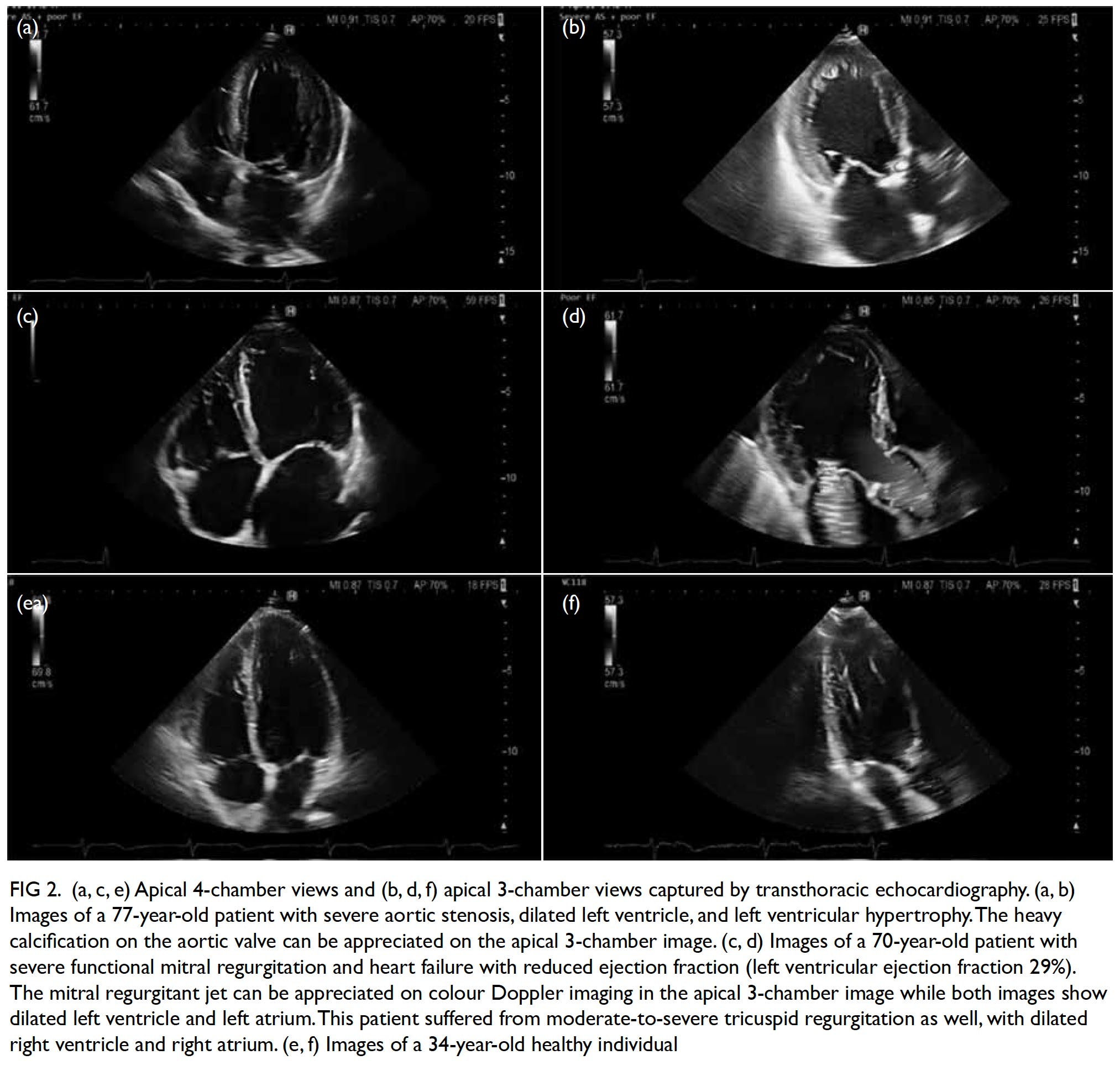
Figure 2. (a, c, e) Apical 4-chamber views and (b, d, f) apical 3-chamber views captured by transthoracic echocardiography. (a, b) Images of a 77-year-old patient with severe aortic stenosis, dilated left ventricle, and left ventricular hypertrophy. The heavy calcification on the aortic valve can be appreciated on the apical 3-chamber image. (c, d) Images of a 70-year-old patient with severe functional mitral regurgitation and heart failure with reduced ejection fraction (left ventricular ejection fraction 29%). The mitral regurgitant jet can be appreciated on colour Doppler imaging in the apical 3-chamber image while both images show dilated left ventricle and left atrium. This patient suffered from moderate-to-severe tricuspid regurgitation as well, with dilated right ventricle and right atrium. (e, f) Images of a 34-year-old healthy individual
Patients who have undergone chemotherapy or radiotherapy are at risk of developing heart failure, and baseline echocardiography with regular follow-up examinations are required. The precise algorithm depends on the cardiotoxic agent prescribed. Traditionally, LVEF has been used as the main marker of cardiac function and cancer therapeutics- related cardiac dysfunction is defined as a drop in LVEF of ≥5% in symptomatic patients or a drop in LVEF of ≥10% to an LVEF of <53% in asymptomatic patients.20 However, significant damage has occurred when a drop in LVEF is detected. Earlier detection can potentially reverse myocardial dysfunction before irreparable damage occurs.21 Thus, global longitudinal strain assessment is recommended for these patients (see section “Advanced and emerging imaging technologies”).20
In the acute setting of respiratory failure where patients present with acute shortness of breath and hypoxaemia of uncertain aetiology, or with hypotension of uncertain or suspected cardiac aetiology, TTE is useful to confirm or exclude cardiac diseases. However, if pulmonary or other noncardiac causes of respiratory distress are established, TTE is unnecessary. Right ventricular function can be evaluated by TTE to determine prognosis and predict recurrence of pulmonary embolism.7 13 However, CT, ventilation-perfusion imaging, and pulmonary angiography remain the imaging modalities of choice in the diagnosis of patients with suspected pulmonary embolism.22 In particular, the triple-rule-out CT—which uses contrast enhancement to evaluate the coronary arteries, aorta, and pulmonary arteries—may be used reliably to detect or exclude pulmonary embolism.23 24 TTE is not sensitive nor specific for diagnosing acute pulmonary embolism. Conversely, in a haemodynamically compromised patient with high clinical probability of pulmonary embolism, unequivocal signs of right ventricular pressure overload and/or right-heart/pulmonary artery thrombi on bedside TTE may support a presumptive diagnosis of pulmonary embolism and may justify emergency reperfusion treatment if immediate CT angiography is not feasible. Although sudden-onset dyspnoea is the most common presenting complaint in pulmonary embolism (80%), chest pain is also common (49%) and a low threshold of clinical suspicion should be maintained.25
For patients with known chronic pulmonary diseases presenting with acute deterioration, careful physical exam and TTE can identify complicating factors such as pulmonary hypertension and right heart failure (cor pulmonale).
Syncope, dizziness, and cardiac source of embolism
The differential diagnoses for syncope or dizziness include vasovagal and orthostatic syncope, arrhythmias, cardiac structural abnormalities, and neurological causes.26 A TTE is indicated when clinical symptoms, signs, or ECG findings are consistent with a structural cardiac diagnosis known to cause syncope/dizziness, such as aortic stenosis (Fig 2), left ventricular dysfunction, hypertrophic cardiomyopathy, intracardiac tumours, or pericardial effusions. A normal TTE in this setting is still an important finding that helps to direct the clinician in diagnosis and treatment.7 13
In patients with suspected cardioembolic stroke, echocardiography especially TEE is useful for identifying intracardiac thrombi in the left atrial appendage (typically in patients with atrial fibrillation) and aortic atherosclerosis. In younger patients with cryptogenic stroke, patent foramen ovale, right-to-left interatrial shunt, and/ or atrial septal aneurysm should be looked for on echocardiography with agitated saline contrast at rest and on Valsalva manoeuvre. TEE is also indicated in patients with high clinical suspicion of septic emboli associated with infective endocarditis.27
Palpitation and abnormal electrocardiogram findings
Patients presenting with palpitation should be evaluated with a careful history and physical examination followed by a 12-lead ECG and, where appropriate, ambulatory ECG monitor (eg, Holter, loop recorder). If frequent ventricular premature complexes, atrial fibrillation, supraventricular, or ventricular tachycardia are diagnosed, TTE is appropriate to identify and evaluate underlying structural heart abnormalities.7 13 28 However, it is inappropriate to routinely order TTE for evaluation of infrequent atrial premature complexes, ventricular premature complexes without other evidence of heart disease, or asymptomatic isolated sinus bradycardia.13
Patients with new right bundle branch block should be assessed with a careful history focusing on potential causes of right ventricular strain (eg, pulmonary embolism, pulmonary hypertension, obstructive sleep apnoea). Echocardiographic examination should be performed if there is suspicion of pulmonary disease potentially impacting the right ventricle. Asymptomatic isolated chronic right bundle branch block generally does not require further evaluation or treatment. In contrast, left bundle branch block needs to be assessed carefully, mostly by history taking and physical examination for any associated disorders, including but not limited to hypertension, coronary artery disease, heart failure, myocarditis, valvular heart disease, and cardiomyopathies. Typically, an echocardiographic examination for LVEF is indicated. A subset of patients may be indicated for further evaluation by stress testing with imaging, depending on the clinical contexts.29
Murmur or clicks
Murmur or clicks on auscultation may suggest the presence of valvular or congenital heart diseases. Initial evaluation with TTE is appropriate when there is a reasonable suspicion of valvular (Fig 2) or structural heart disease.7 Conversely, echocardiography is inappropriate for routine evaluation of murmur when there are no other symptoms or signs of valvular or structural heart disease. An innocent murmur is typically a short and soft systolic ejection murmur, with normal S1 and S2, normal cardiac impulse, and no evidence for any haemodynamic abnormality. Hand grip usually decreases the intensity of an innocent murmur but increases that of a regurgitant murmur. Initial evaluation of suspected infective endocarditis with positive blood cultures or a new murmur with TTE is appropriate. Initial evaluation with TEE for suspected endocarditis is appropriate if pretest probability is moderate or high (eg, staphylococcal bacteraemia, fungaemia, prosthetic heart valve, or intracardiac device).30 31 However, echocardiography should not be routinely ordered for evaluation of transient fever without evidence of bacteraemia or a new murmur.
Re-evaluation of known valvular heart disease with echocardiography is considered appropriate where there is any change in clinical status or cardiac examination, or to guide therapy (eg, detecting asymptomatic ventricular dysfunction that would prompt early surgical or transcatheter valve intervention). Routinely repeating echocardiography is inappropriate in patients with no or mild valve disease on prior echocardiography with no change in clinical symptoms or cardiac exam. In patients with exercise-induced symptoms but only moderate valvular dysfunction on resting echocardiography, an exercise or pharmacological stress echocardiography may be necessary to reveal the true severity of valve dysfunction during exertion.28 A TEE is indicated in some patients where TTE is inconclusive, or when intervention is planned.32
Screening asymptomatic patients for hereditary structural heart diseases
This is a complex topic and a full discussion is beyond the scope of this review. Thus, this subsection shall focus on the two most common congenital diseases affecting the heart with the highest inheritability: bicuspid aortic valve (BAV) and Marfan syndrome. Although BAV is not always syndromic, 9% of first-degree relatives of BAV patients also have BAV,33 and echocardiographic screening in these patients detects a substantial number of BAV cases.34 In most cases, a single TTE is sufficient to diagnose BAV. Once diagnosed, the patient should be referred for specialist cardiology treatment and follow-up.
In contrast, Marfan syndrome is an autosomal dominant disorder of the fibrillin gene on chromosome 15. Its main effects on the cardiovascular system include aortic aneurysms (especially thoracic) and aortic regurgitation. The diagnosis of Marfan syndrome is made with the Ghent nosology, a set of diagnostic criteria last revised in 2010 that includes clinical, laboratory, and imaging criteria.35 The imaging criteria includes significant aortic root dilatation (Z score of ≥2). The European Society of Cardiology recommends at-risk relatives without a definite diagnosis of Marfan syndrome to be screened every 5 years until a definitive diagnosis is established or excluded.17 Once diagnosed, yearly echocardiography is required for the stable patient, as well as baseline and at least 5-yearly cardiac MRI scan. If aneurysmal changes are detected beyond the aortic root, MRI scan should be repeated at least yearly.36
Advanced and emerging imaging technologies
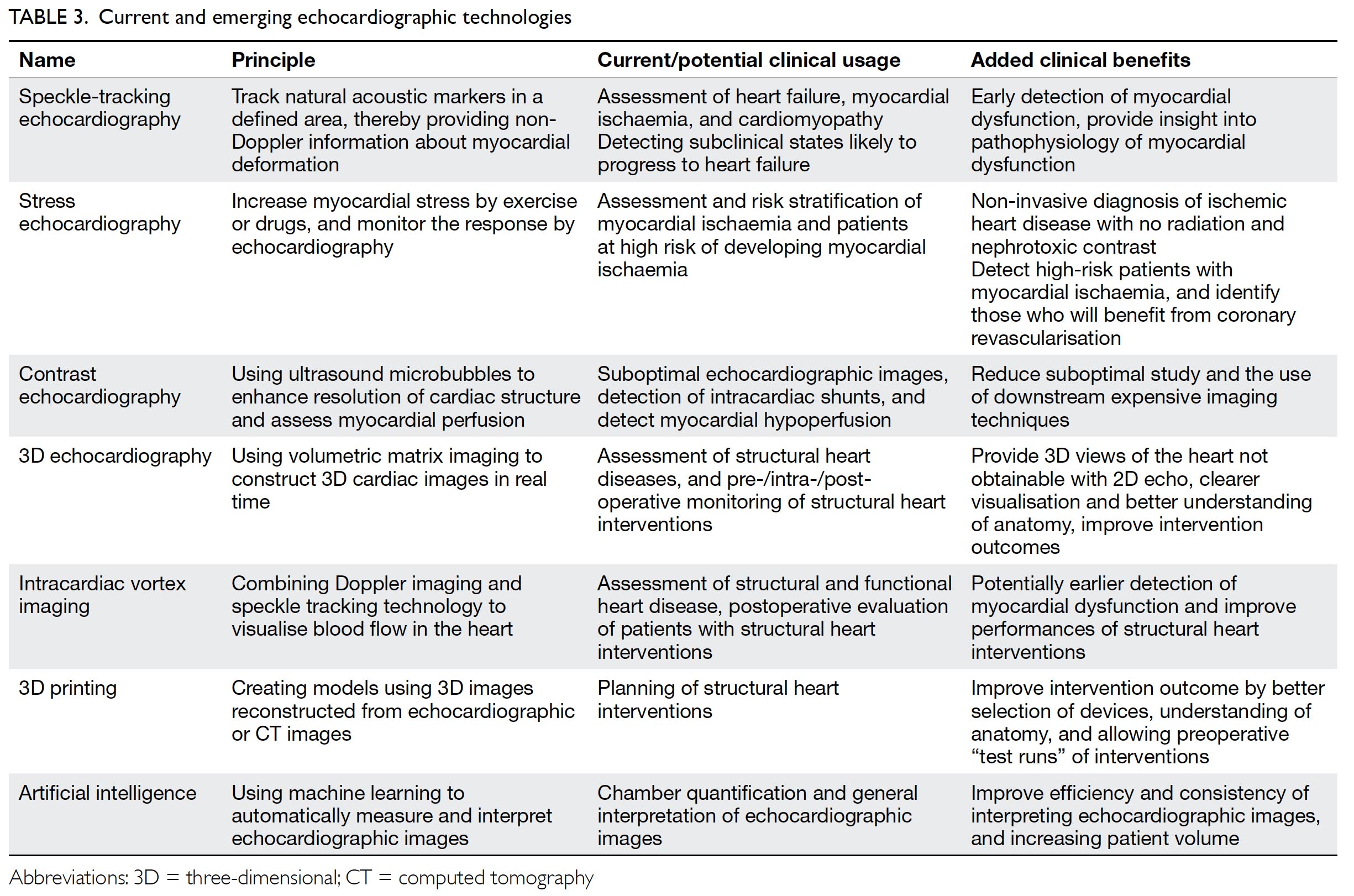
This section summarises advanced echocardiographic technologies that are relevant to common cardiovascular diseases, as well as emerging imaging technologies (Table 3).
Stress echocardiography is valuable for evaluation of suspected coronary artery disease. Myocardial ischaemia causes reduced systolic wall thickening and excursion during stress, constituting stress-induced regional wall motion abnormalities (Fig 3). These may precede angina and ST-T changes on ECG. The sensitivity of exercise and dobutamine stress echocardiography for detection of coronary artery disease are 85% and 80% with specificity of 77% and 86%, respectively.37 Using cardiac MRI as reference standard, three-dimensional (3D) echocardiography provides more accurate and reproducible left ventricle volume and ejection fraction measurements than 2D echocardiography.38 39 40 41 42 The American Society of Echocardiography recommends the use of 3D echocardiography in chamber quantification when such technique is available in the echocardiography laboratory.43 Three-dimensional TEE has brought new insights to the mechanisms of mitral regurgitation,44 45 and has become essential in guiding transcatheter structural interventions such as transcatheter edge-to-edge mitral46 or tricuspid47 repair, transcatheter aortic valve implantation,48 and left atrial appendage occlusion (Fig 4).49 50 51 More recently, 3D printing has been used to create 3D models from 3D TEE images for preprocedural simulation.52 53 54 Speckle-tracking echocardiography allows measurement of myocardial strain (deformation; Fig 3).55 56 Assessment of the global longitudinal strain is useful for detection of chemotherapy-related cardiotoxicity in cancer patients, better than the assessment of LVEF.20
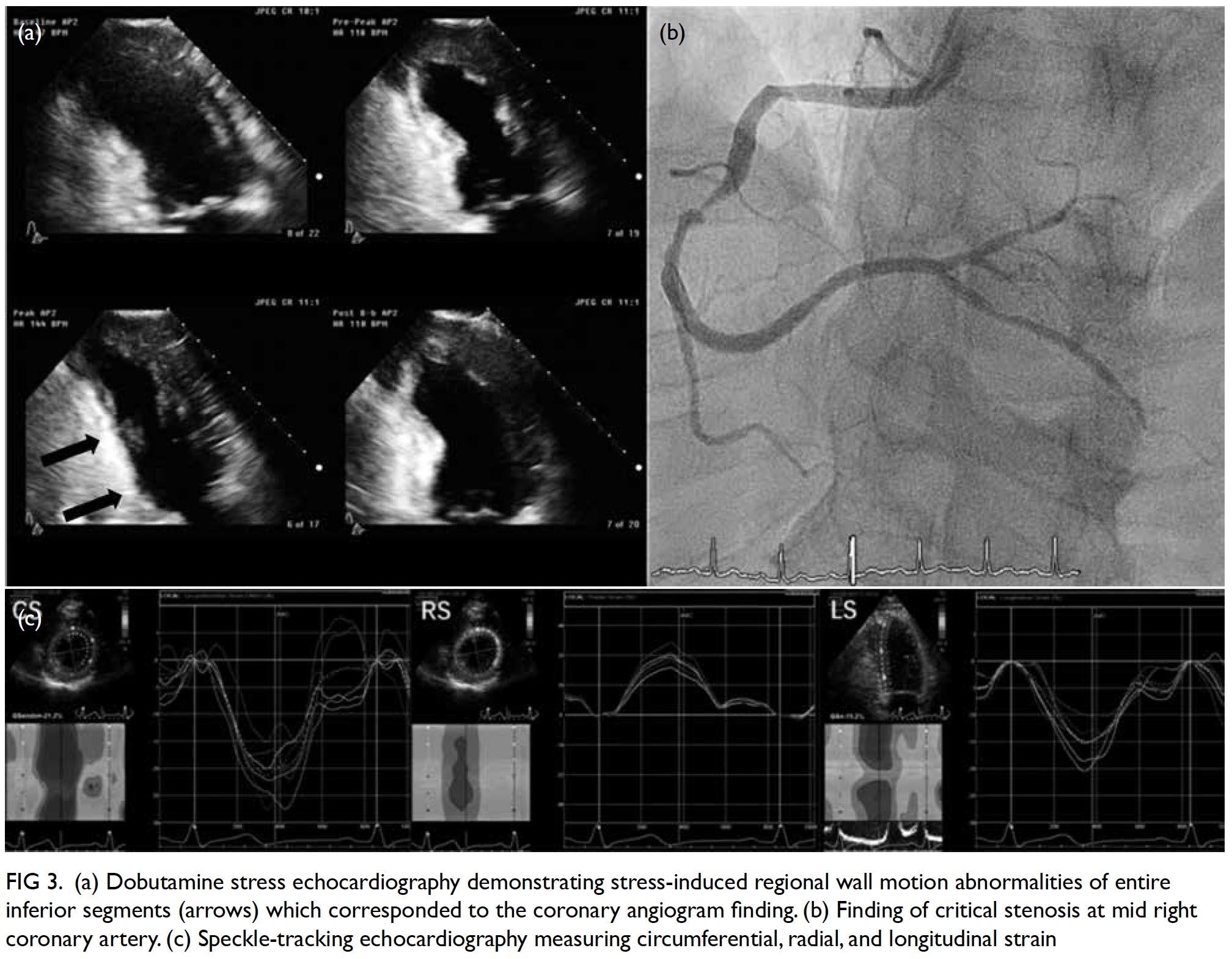
Figure 3. (a) Dobutamine stress echocardiography demonstrating stress-induced regional wall motion abnormalities of entire inferior segments (arrows) which corresponded to the coronary angiogram finding. (b) Finding of critical stenosis at mid right coronary artery. (c) Speckle-tracking echocardiography measuring circumferential, radial, and longitudinal strain
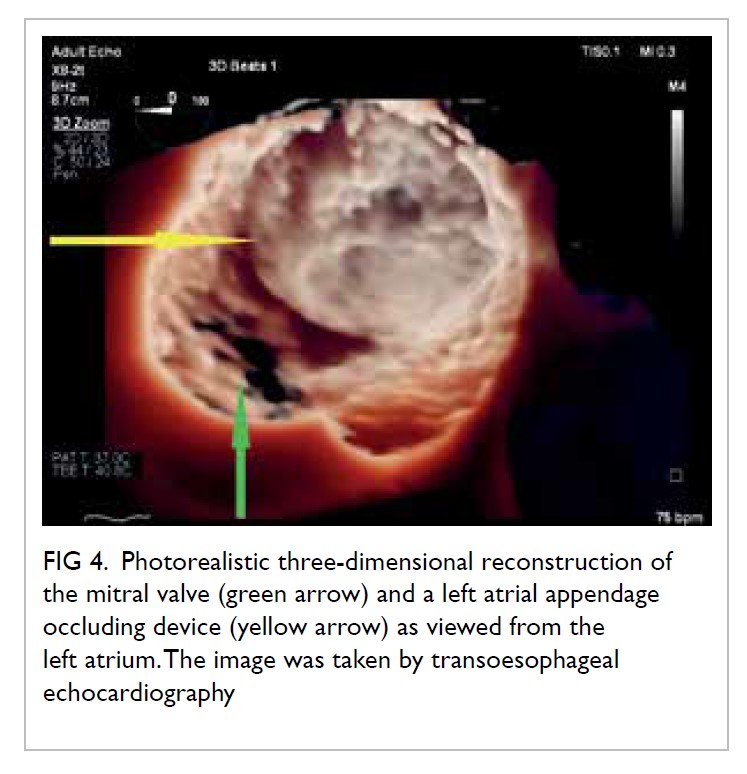
Figure 4. Photorealistic three-dimensional reconstruction of the mitral valve (green arrow) and a left atrial appendage occluding device (yellow arrow) as viewed from the left atrium. The image was taken by transoesophageal echocardiography.
More recently, echocardiography has been used
to visualise intracardiac blood flow.57 Intracardiac vortex patterns of various pathological conditions have been shown to differ from those of healthy patients, including heart failure,58 dilated and hypertrophic cardiomyopathy,59 coronary heart disease,60 and valvular diseases.61
Conclusion
Echocardiography is essential in a wide range of clinical scenarios. Appropriate use of echocardiography improves clinical outcomes by increasing diagnostic accuracy, providing non-invasive or minimally invasive assessment of disease status and risk stratification, and enabling real-time monitoring and guidance of interventional procedures. Technological advancements have extended echocardiography beyond 2D imaging, allowing advanced structural visualisation and functional measurement of the heart. Echocardiography remains a vibrant field of research, and recent developments are promising clinically. Echocardiography will continue to grow as a core clinical investigation for a wide spectrum of disorders, and its relevance to primary care physicians cannot be overstated.
Author contributions
All authors are responsible for writing the article. APW Lee had critical revision for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Funding/support
This work was funded by the Hong Kong Special Administrative Region Government Health and Medical Research Fund (Ref 05160976).
Disclosure of potential conflicts of interest
APW Lee receives speaker honorarium and research grant from Philips Healthcare.
References
1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. Crossref
2. Centre for Health Protection. Heart diseases. Available from: https://www.chp.gov.hk/en/healthtopics/ content/25/57.html. Accessed 11 Mar 2019.
3. Sigurdsson EL, Jónsson JS, Thorgeirsson G. Medical treatment and secondary prevention of coronary heart disease in general practice in Iceland. Scand J Prim Health Care 2002;20:10-5. Crossref
4. Luttik ML, Jaarsma T, van Geel PP, et al. Long-term follow-up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH-2 study. Eur J Heart Fail 2014;16:1241-8. Crossref
5. Senni M, Rodeheffer RJ, Tribouilloy CM, et al. Use of echocardiography in the management of congestive heart failure in the community. J Am Coll Cardiol 1999;33:164-70. Crossref
6. d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515-22. Crossref
7. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/ SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. J Am Soc Echocardiogr 2011;24:229-67. Crossref
8. Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. Crossref
9. Anavekar NS, Oh JK. Doppler echocardiography: a contemporary review. J Cardiol 2009;54:347-58. Crossref
10. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. Crossref
11. Feigenbaum H. Role of M-mode technique in today’s echocardiography. J Am Soc Echocardiogr 2010;23:240-57. Crossref
12. Gardin JM, Adams DB, Douglas PS, et al. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr 2002;15:275-90.Crossref
13. Steeds RP, Garbi M, Cardim N, et al. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: a report of literature and current practice review. Eur Heart J Cardiovasc Imaging 2017;18:1191-204. Crossref
14. Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction. A prospective study using two-dimensional echocardiography. Circulation 1991;84(3 Suppl):I85-92.
15. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. Crossref
16. Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med 2006;166:1350-6. Crossref
17. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases
of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. Crossref
18. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and
chronic heart failure: The Task Force for the diagnosis
and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with
the special contribution of the Heart Failure Association
(HFA) of the ESC. Eur Heart J 2016;37:2129-200. Crossref
19. Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging 2019;pii:S1936-878X(19)30444-9.
20. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911-39. Crossref
21. Cardinale D, Colombo A, Lamantia G, et al. Anthracycline- induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213- 20. Crossref
22. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69,3069a-
3069k. Crossref
23. Wnorowski AM, Halpern EJ. Diagnostic yield of triple- rule-out CT in an emergency setting. AJR Am J Roentgenol 2016;207:295-301. Crossref
24. Schertler T, Frauenfelder T, Stolzmann P, et al. Triple rule- out CT in patients with suspicion of acute pulmonary embolism: findings and accuracy. Acad Radiol 2009;16:708- 17. Crossref
25. Miniati M, Cenci C, Monti S, Poli D. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One 2012;7:e30891. Crossref
26. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883-948. Crossref
27. Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac
source of embolism. J Am Soc Echocardiogr 2016;29:1-42. Crossref
28. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease.
Eur Heart J 2017;38:2739-91. Crossref
29. Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay. J Am Coll Cardiol 2019;74:e51-e156. Crossref
30. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075-128. Crossref
31. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014;129:e521-643. Crossref
32. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. Circulation 2019;139:e698-e800. Crossref
33. Freeze SL, Landis BJ, Ware SM, Helm BM. Bicuspid aortic valve: a review with recommendations for genetic counseling. J Genet Couns 2016;25:1171-8. Crossref
34. Cozijnsen L, van der Zaag-Loonen HJ, Braam RL, et al. Yield of family screening in patients with isolated bicuspid aortic valve in a general hospital. Int J Cardiol 2018;255:55-8. Crossref
35. Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. Crossref
36. Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. Crossref
37. Beleslin BD, Ostojic M, Djordjevic-Dikic A, et al. Integrated evaluation of relation between coronary lesion features and stress echocardiography results: the importance of coronary lesion morphology. J Am Coll Cardiol 1999;33:717-26. Crossref
38. Thavendiranathan P, Liu S, Verhaert D, et al. Feasibility, accuracy, and reproducibility of real-time full-volume 3D transthoracic echocardiography to measure LV volumes and systolic function: a fully automated endocardial contouring algorithm in sinus rhythm and atrial fibrillation. JACC Cardiovasc Imaging 2012;5:239-51. Crossref
39. Grewal J, Majdalany D, Syed I, Pellikka P, Warnes CA. Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2010;23:127-33. Crossref
40. Jacobs LD, Salgo IS, Goonewardena S, et al. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J 2006;27:460-8. Crossref
41. Corsi C, Lang RM, Veronesi F, et al. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation 2005;112:1161-70. Crossref
42. Kühl HP, Schreckenberg M, Rulands D, et al. High-resolution transthoracic real-time three-dimensional echocardiography: quantitation of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004;43:2083-90. Crossref
43. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quentification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovasular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. Crossref
44. Lee AP, Hsiung MC, Salgo IS, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation 2013;127:832-41. Crossref
45. Tang Z, Fan YT, Wang Y, Jin CN, Kwok KW, Lee AP. Mitral annular and left ventricular dynamics in atrial functional mitral regurgitation: a three-dimensional and speckle- tracking echocardiographic study. J Am Soc Echocardiogr 2019;32:503-13. Crossref
46. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307-18. Crossref
47. Cheung GS, Kam KK, Lam YY, Lee AP. Transcatheter tricuspid valve edge-to-edge repair for severe tricuspid regurgitation in a Chinese patient. Heart Asia 2018;10:e010997. Crossref
48. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705. Crossref
49. Yu CM, Khattab AA, Bertog SC, et al. Mechanical antithrombotic intervention by LAA occlusion in atrial fibrillation. Nat Rev Cardiol 2013;10:707-22. Crossref
50. Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2011;32:2189-214. Crossref
51. Lee AP, Lam YY, Yip GW, Lang RM, Zhang Q, Yu CM. Role of real time three-dimensional transesophageal echocardiography in guidance of interventional procedures in cardiology. Heart 2010;96:1485-93. Crossref
52. So KC, Fan Y, Sze L, et al. Using multimaterial 3-dimensional printing for personalized planning of complex structural heart disease intervention. JACC Cardiovasc Interv 2017;10:e97-e98. Crossref
53. Fan Y, Yang F, Cheung GS, et al. Device sizing guided by echocardiography-based three-dimensional printing is associated with superior outcome after percutaneous left atrial appendage occlusion. J Am Soc Echocardiogr 2019;32:708-19.e1. Crossref
54. Fan Y, Kwok KW, Zhang Y, Cheung GS, Chan AK, Lee AP. Three-dimensional printing for planning occlusion procedure for a double-lobed left atrial appendage. Circ Cardiovasc Interv 2016;9:e003561. Crossref
55. Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17:1021-9. Crossref
56. Blessberger H, Binder T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart 2010;96:716-22. Crossref
57. Sengupta PP, Korinek J, Belohlavek M, et al. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol 2006;48:1988-2001. Crossref
58. Poh KK, Lee LC, Shen L, et al. Left ventricular fluid dynamics in heart failure: echocardiographic measurement and utilities of vortex formation time. Eur Heart J Cardiovasc Imaging 2012;13:385-93. Crossref
59. Tang C, Zhu Y, Zhang J, et al. Analysis of left ventricular fluid dynamics in dilated cardiomyopathy by echocardiographic particle image velocimetry. Echocardiography 2018;35:56-63. Crossref
60. Zhang H, Ren X, Song J, et al. Intraventricular isovolumic relaxation flow patterns studied by using vector flow mapping. Echocardiography 2016;33:902-9. Crossref
61. Dyverfeldt P, Kvitting J, Carlhäll CJ, et al. Hemodynamic aspects of mitral regurgitation assessed by generalized phase-contrast MRI. J Magn Reson Imaging 2011;33:582-8. Crossref