Implementation of secondary stroke prevention protocol for ischaemic stroke patients in primary care
Hong Kong Med J 2015 Apr;21(2):136–42 | Epub 16 Jan 2015
DOI: 10.12809/hkmj144236
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Implementation of secondary stroke prevention protocol for ischaemic stroke patients in primary care
YK Choi, FHKCFP, FHKAM (Family Medicine)1; JH Han, MD, PhD1; Richard Li, FHKCP, FHKAM (Medicine)2; Kenny Kung, FHKCFP, FHKAM (Family Medicine)3; Augustine Lam, FHKCFP, FHKAM (Family Medicine)4
1 Lek Yuen General Out-patient Clinic, Department of Family Medicine, New Territories East Cluster, Hong Kong
2 Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong
3 Department of Family Medicine and Primary Care, The University of Hong Kong, Hong Kong
4 Department of Family Medicine, New Territories East Cluster, Hong Kong
Corresponding author: Dr YK Choi (yuekwan@hotmail.com)
Abstract
Objective: To investigate the effectiveness of a secondary stroke prevention protocol in the general out-patient clinic.
Design: Cohort study with pre- and post-intervention
comparisons.
Setting: Two general out-patient clinics in Hong
Kong.
Patients: Ischaemic stroke patients who had long-term
follow-up in two clinics were recruited. The
patients of one clinic received the intervention
(intervention group) and the patients of the second
clinic did not receive the intervention (control
group). The recruitment period lasted for 6 months
from 1 September 2008 to 28 February 2009. The
pre-intervention phase data collection started within
this 6-month period. The protocol implementation
started at the intervention clinic on 1 April 2009.
The post-intervention phase data collection started
9 months after the protocol implementation, and ran
for 6 months from 1 January 2010 to 30 June 2010.
Main outcome measures: Clinical data before and
after the intervention, including blood pressure,
glycated haemoglobin level, low-density lipoprotein level and
prescription pattern, were compared between the
two groups to see whether there was enhancement
of secondary stroke management.
Results: A total of 328 patients were recruited into the intervention group and 249 into the control group; data of 256 and 210 patients from these groups were analysed, respectively.
After intervention, there were significant reductions in mean (± standard deviation) systolic
blood pressure (135.2 ± 17.5 mm Hg to 127.7 ±
12.2 mm Hg), glycated haemoglobin level (7.2 ± 1.0% to
6.5 ± 0.8%), and low-density lipoprotein level (3.4 ± 0.8
mmol/L to 2.8 ± 1.3 mmol/L) in the intervention
group (all P<0.01). There were no significant reductions in mean
systolic blood pressure, glycated haemoglobin level, or
low-density lipoprotein level in the control group. There
was a significant increase in statin use (P<0.01) in
both clinics.
Conclusion: Through implementation of a clinic
protocol, the standard of care of secondary stroke
prevention for ischaemic stroke patients could be
improved in a general out-patient clinic.
New knowledge added by this
study
- A standard secondary stroke prevention protocol can significantly improve the control of cardiovascular risk factors in ischaemic stroke patients.
- Implementation of such a programme is effective and feasible in local primary care.
- This study supports more widespread use of a secondary stroke prevention programme in the setting of a general out-patient clinic.
Introduction
Stroke is the second commonest cause of death
worldwide1 and the fourth leading cause of death
in Hong Kong.2 Stroke is also the commonest cause
of permanent disability in adults. Patients with
stroke are at high risk for recurrent stroke and other
major vascular events. As the ageing population is
increasing in most developed countries, stroke will
remain a major burden to patients’ families and
carers, the health care system, and the community.
According to local data from Hong Kong,
cerebrovascular disease was the principal diagnosis
for about 26 500 in-patient discharges and deaths in
all hospitals and accounted for 7.5% of all deaths in
2012.3 The mortality rate was significantly higher in
patients with stroke recurrence than in those without.4 5 Prevention of recurrent stroke
offers great potential for reducing the burden of this
disease.
Over 80% of all strokes are ischaemic stroke.
There are effective strategies for secondary
prevention of ischaemic stroke, which are
summarised as follows6:
(1) Modification of lifestyle risk factors (smoking,
alcohol consumption, obesity, physical
inactivity).
(2) Modification of vascular risk factors
(hypertension, hypercholesterolaemia, diabetes).
(3) Antiplatelet therapy for non-cardioembolic
ischaemic stroke.
(4) Anticoagulation for cardioembolic stroke.
(5) Intervention for symptomatic carotid stenosis.
As stroke patients need lifelong monitoring
and control of risk factors, family physicians play the
most important role in providing secondary stroke
prevention care. However, despite the availability
of evidence-based guidelines, studies show that
adherence to these preventive strategies by physicians
is poor.7 8 9 10 11 Local Hong Kong data about secondary
stroke prevention in primary care are largely lacking.
This study aimed to review the clinical effectiveness
of a secondary stroke prevention programme in a
general out-patient clinic (GOPC).
Methods
This was a cohort study of pre- and post-intervention
comparison between patients receiving or not
receiving the intervention to ascertain the effect of a
secondary stroke prevention programme on clinical
outcomes.
Clinic setting
The Lek Yuen GOPC was selected as the intervention
site where the secondary stroke prevention
programme was implemented. Another clinic, the
Ma On Shan GOPC, was selected as the control site,
where usual care was provided. Both clinics are large
public primary care clinics under the management
of the Department of Family Medicine of the New
Territories East Cluster of the Hospital Authority.
Both clinics are accredited Family Medicine
Training Centres with similar service throughput
annually, covering a population of around 600 000
and providing approximately 30 000 attendances
monthly.
Most of the stroke patients in the clinics are
referred from the public hospitals. The patients
usually have a history of minor stroke with good
functional recovery and are clinically stable.
Clinic protocol development and implementation
A protocol of secondary stroke prevention (Box)
was developed with reference to evidence-based
guidelines (mainly according to the American Heart
Association and American Stroke Association stroke
guidelines).12 13 14
Study design
The target population of the study was all the
ischaemic stroke patients with long-term follow-up
in the two clinics. The recruitment period lasted for 6
months from 1 September 2008 to 28 February 2009.
As the usual follow-up interval of long-term patients
is about 3 to 4 months, the 6-month recruitment
period included all stroke patients who have
regular follow-up. The pre-intervention phase data
collection started within this 6-month period. The
protocol implementation started at the intervention
clinic on 1 April 2009. The post-intervention phase
data collection started 9 months after the protocol
implementation, that is, for 6 months from 1 January
2010 to 30 June 2010.
Sampling
The clinical data were collected by reviewing the
medical records of all patients assigned with the
International Classification of Primary Care coding
of K90 (stroke/cerebrovascular accident) or K91
(cerebrovascular disease). Only those patients
diagnosed with ischaemic stroke and who had at
least two consecutive follow-up visits within the
recruitment period were included. Patients who had
a history of haemorrhagic stroke were excluded. In
order to exclude patients with sporadic follow-up,
only those patients with two consecutive follow-up
visits in the post-intervention phase were regarded
as eligible for data collection. Those patients
without two consecutive follow-up visits in the post-intervention
phase were classified as dropouts.
Protocol implementation
One month before initiation of the protocol, two
1-hour training sessions were arranged for medical
officers and nurses in the intervention clinic.
During the training sessions, the treatment goals
for secondary ischaemic stroke prevention and
the relevant clinical evidence were presented. The
workflow and applicability of the protocol were
also discussed. Medical officers were required to
have good documentation of all the lifestyle and
cardiovascular risk factors of the ischaemic stroke
patients and provide care according to the protocol.
Nurses were trained to be familiar with the treatment
goals and provide patient education and lifestyle
modification interventions in line with the doctors’
referrals. Allied health services such as a dietitian,
smoking cessation clinic, diabetes complication
screening programme, and patient empowerment
programmes for diabetic and hypertensive patients
were available in both the intervention and control
clinics. Doctors in the intervention clinic were
encouraged to refer appropriate patients to these
services. There was no additional consultation time
allocated to these patients. In order to monitor
progress, the electronic consultation notes were
reviewed monthly for each patient to assess
compliance with the protocol. If suboptimal care
was noted, an electronic reminder with appropriate
management advice was issued to the patient’s
electronic medical record. The consulting doctor
would then be able to provide the appropriate
management at the next follow-up. Throughout
the protocol implementation period, interim clinic
meetings were held quarterly to present the data for
protocol compliance with the medical and nursing
staff of the intervention clinic.
In the control clinic, no specific protocol was
applied. Medication prescription and adjustment
was based solely on the physicians’ discretion.
The drug formulary was the same in both clinics.
Statins were introduced to both clinic formularies
in July 2009. There were no training sessions for
doctors and nursing staff in the control clinic, and
no electronic reminders or interim meetings for
progress monitoring.
Data collection
Baseline characteristics on sex, age, chronic illness
status, chronic drug use, laboratory results, and blood
pressure (BP) values were extracted from the Clinical
Data Analysis and Reporting System. The latest
laboratory results and BP values within
the data collection period were taken as the study
data. Individual case records were also reviewed for
the following lifestyle parameters: smoking status,
alcohol consumption, body mass index (BMI), and
exercise and diet history.
Statistical analysis
All statistical analysis was performed using
the Statistical Package for the Social Sciences
(Windows version 20.0; SPSS Inc, Chicago [IL],
US). Continuous variables were expressed as mean
and standard deviation. Baseline comparisons were
made with the Student’s t test or the Chi squared test
as appropriate. The mean BP, glycated
haemoglobin (HbA1c) level, and low-density lipoprotein
(LDL) level before and after intervention were compared
by paired-samples t test in both the intervention and
control clinics.
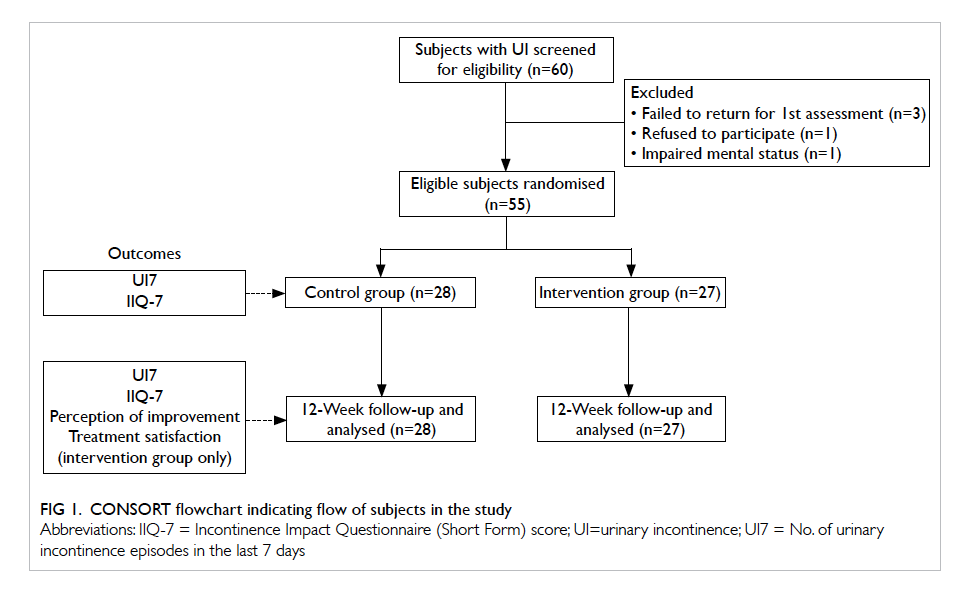
Results
In the intervention clinic, 328 patients were
recruited to the intervention group and 72 dropped
out. In the control clinic, 249 were recruited to the
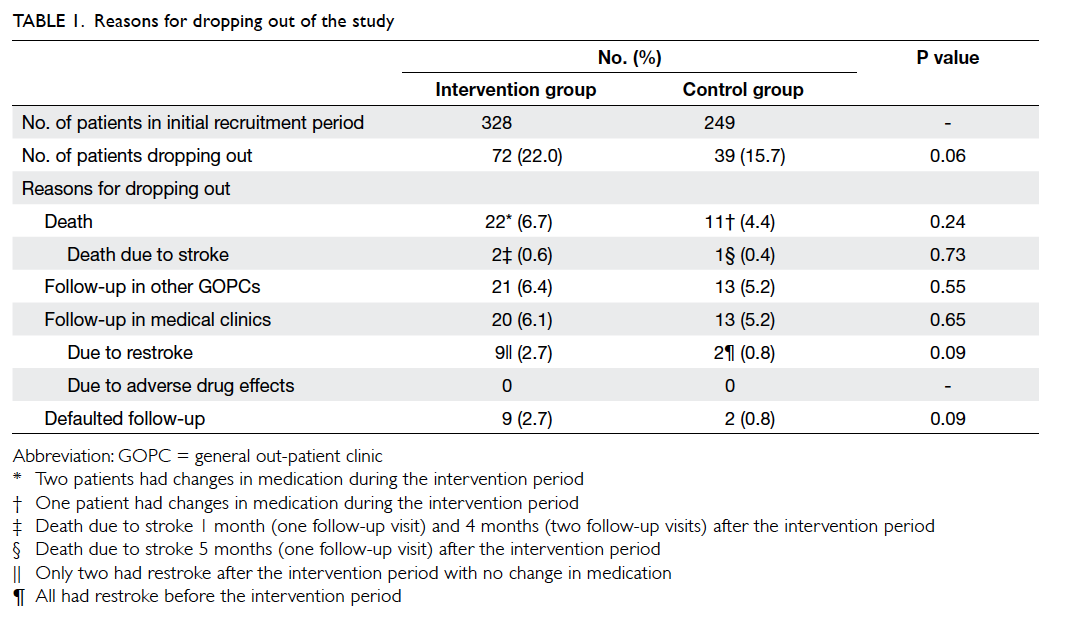
control group and 39 dropped out. The reasons for
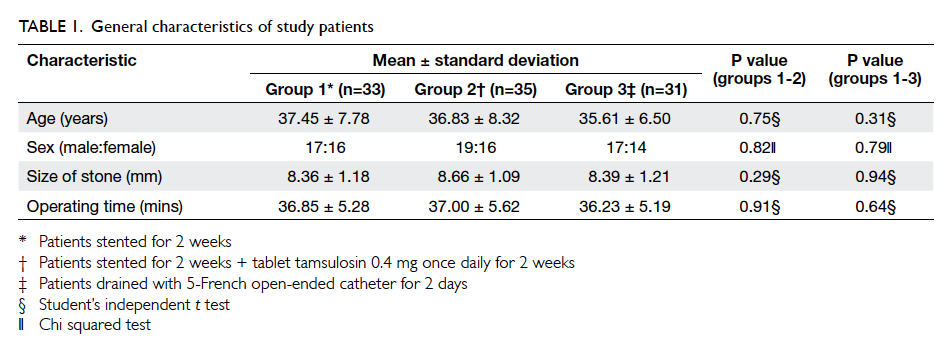
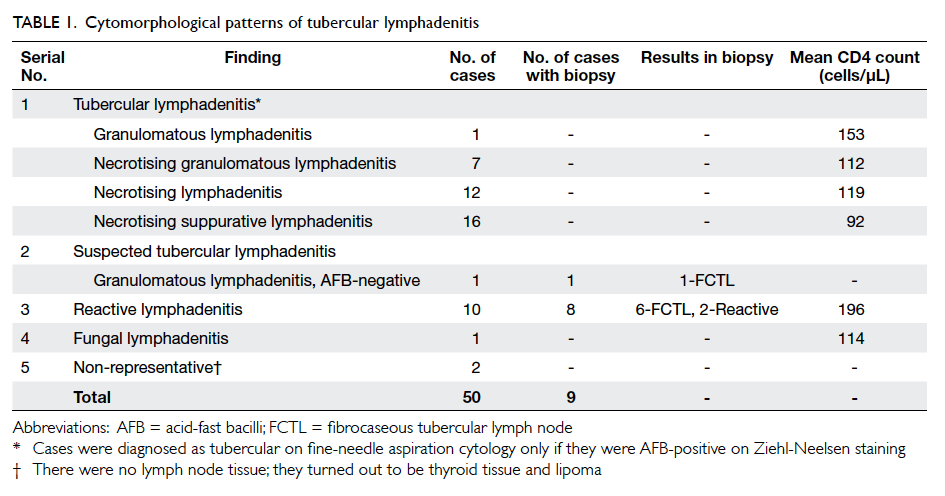
dropping out are shown in Table 1. More patients in the intervention group than in the control group
dropped out due to restroke (9 vs 2, respectively)
and death (22 vs 11, respectively), but these were not
statistically significant due to the small number of
patients. In both the intervention and control groups,
most of the patients who died had no medication
changes during the intervention period (Table 1).
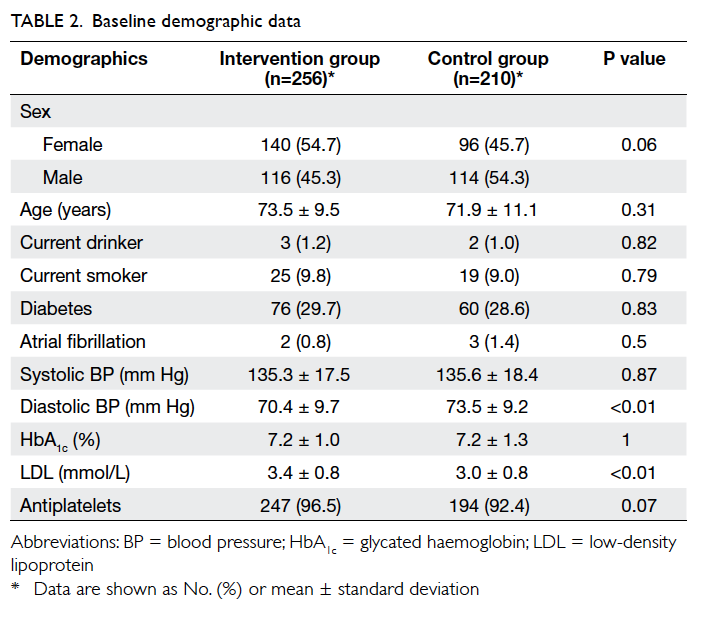
A total of 256 patients in the intervention
group and 210 in the control group were recruited for
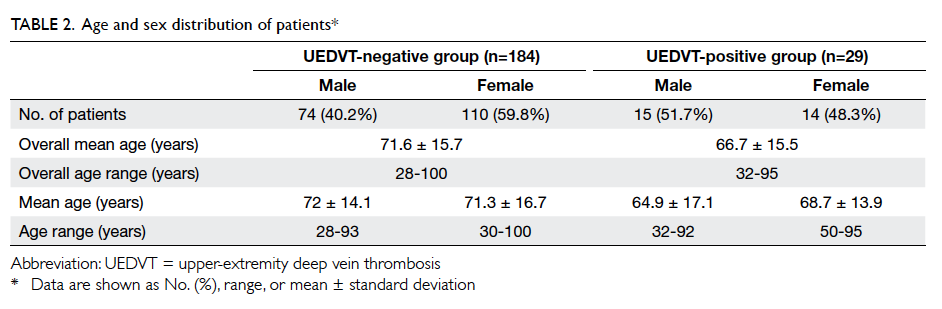
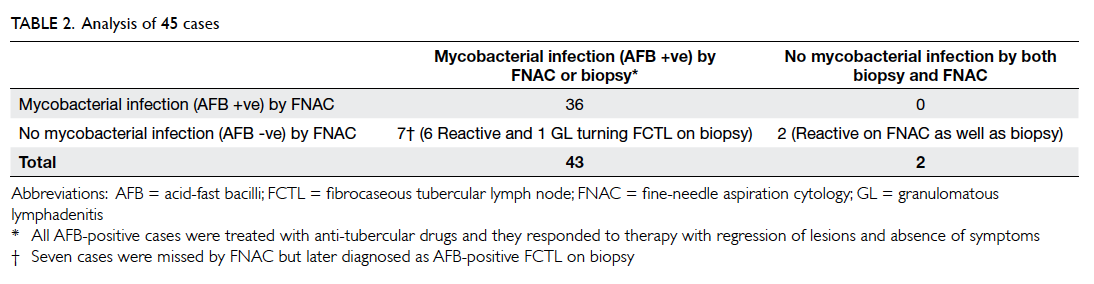
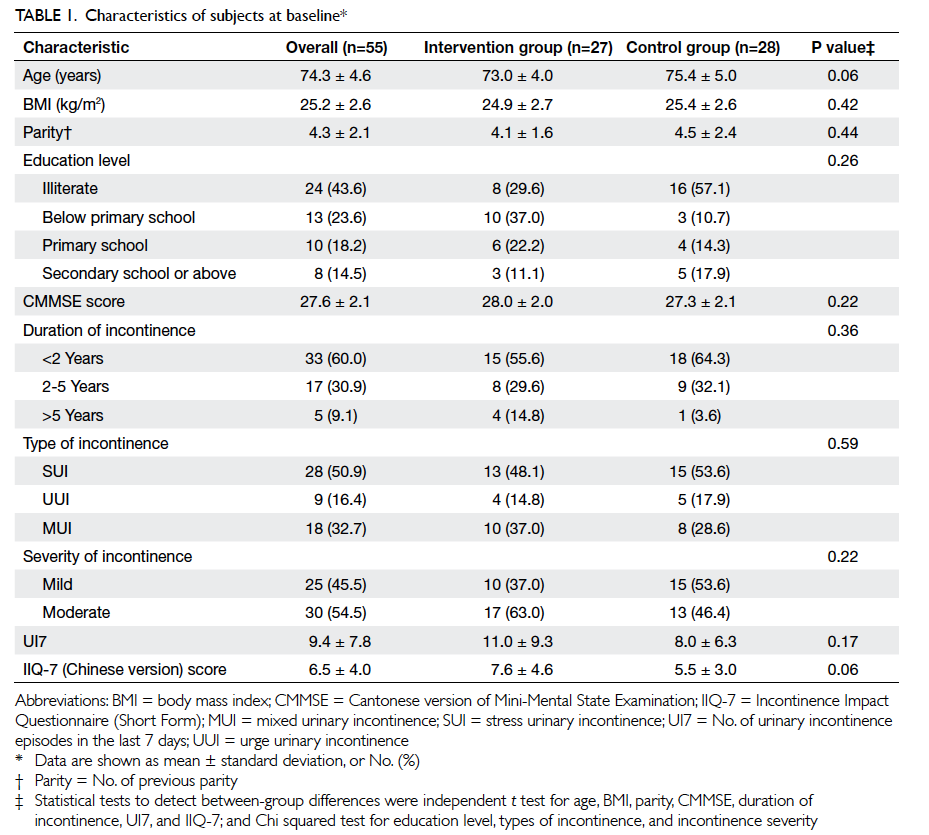
data analysis. At baseline, there were no significant
differences in the demographic and cardiovascular
risk factor profiles between the two groups, except
that patients in the intervention group had a higher
mean LDL level and a lower mean diastolic BP (Table 2).
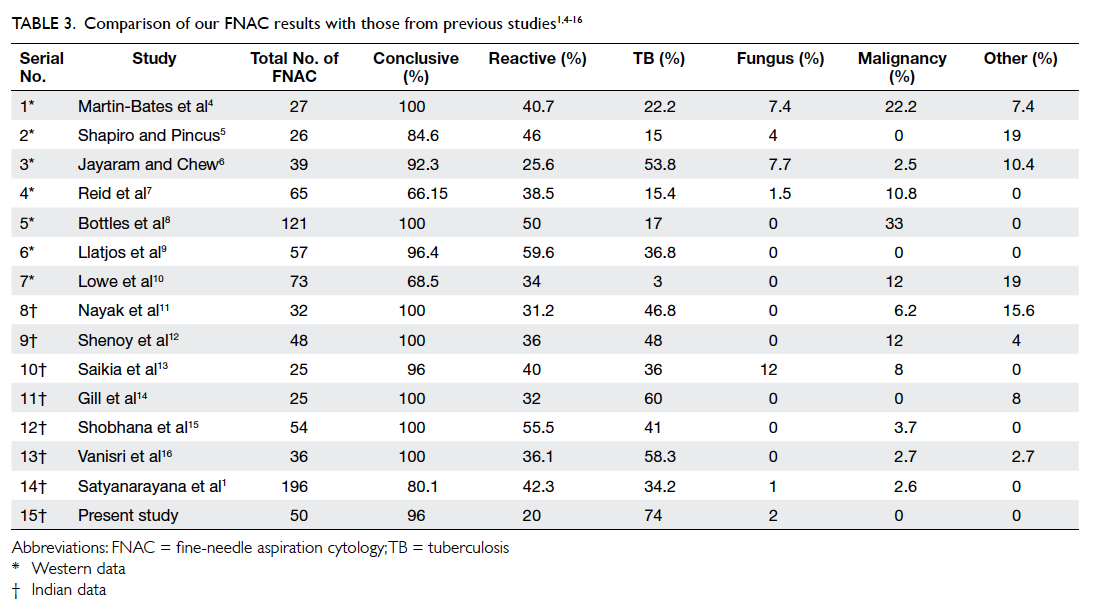
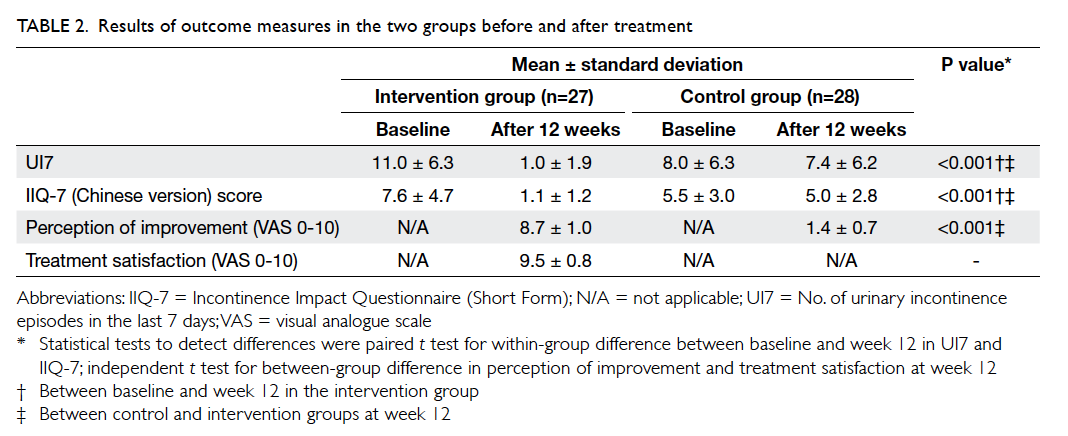
After the intervention period, significant
improvements in systolic BP, HbA1c and
LDL levels were observed in the intervention group
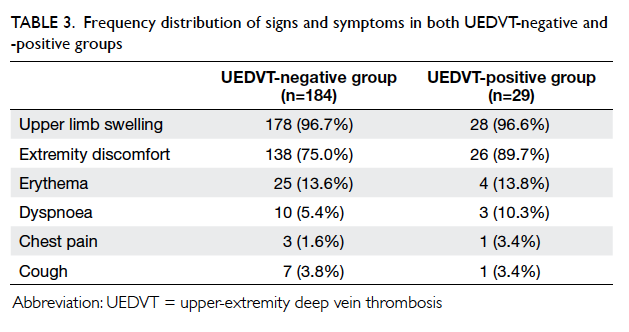
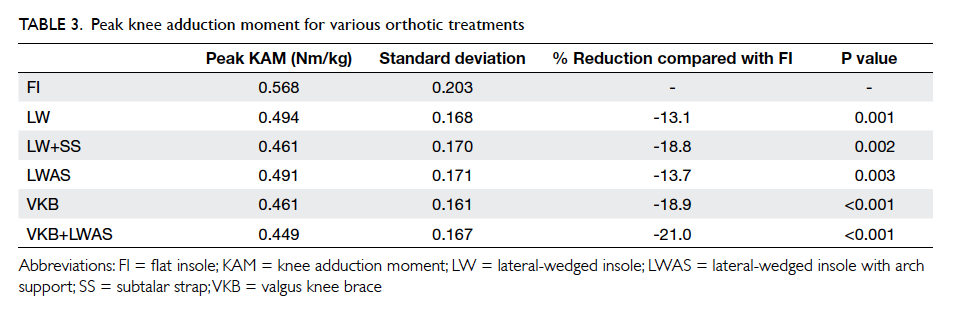
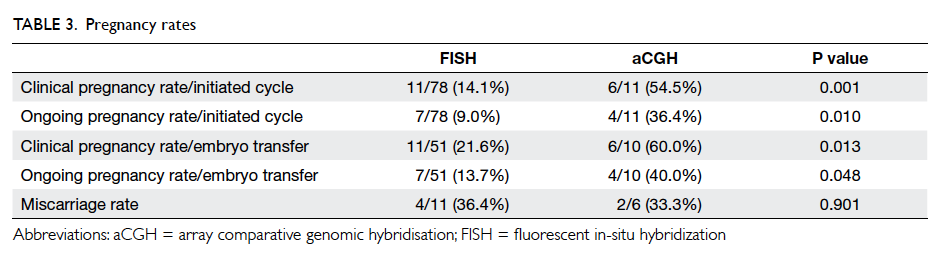
(Table 3). There were significant improvements in
all lifestyle modification parameters (alcohol and
smoking status, obtaining exercise and diet history, and BMI
measurement) in the intervention group (P<0.01),
and the control group had improvements in smoking
status (P<0.01) and BMI measurement (P<0.05)
[Table 3].
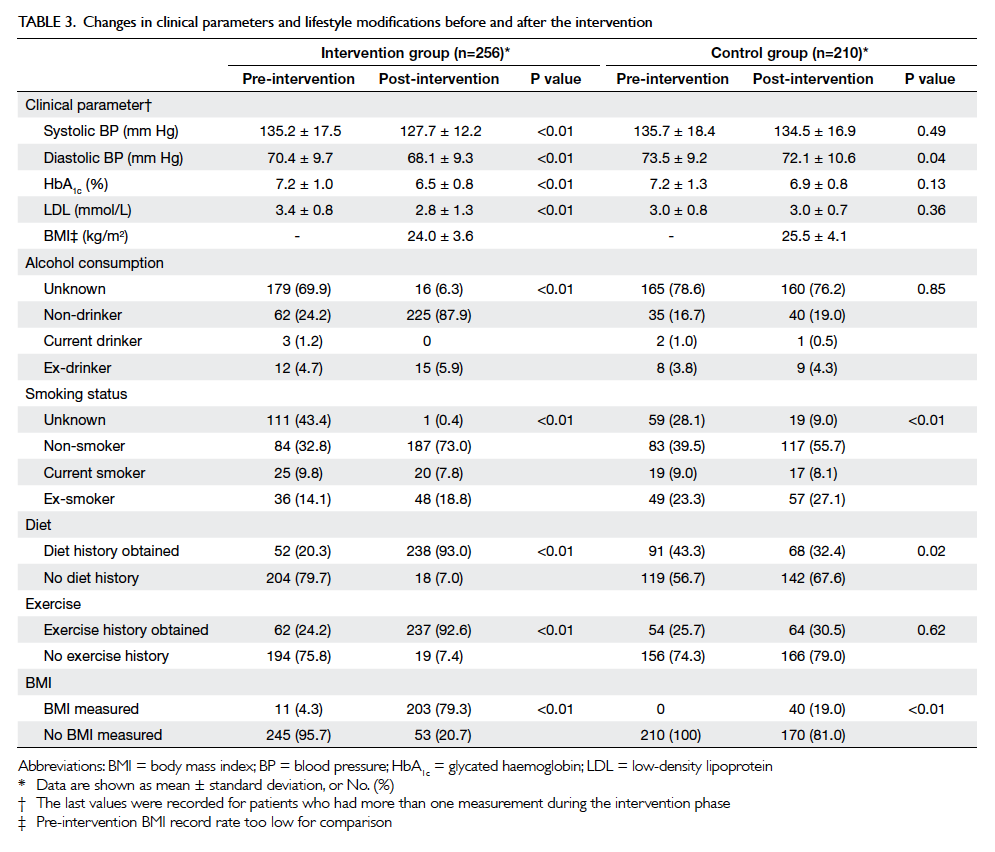
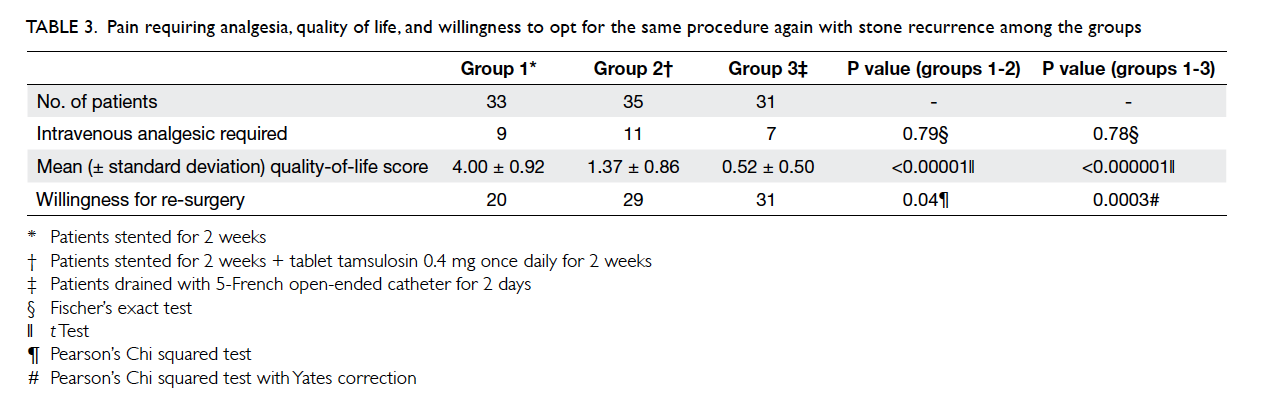
Table 3. Changes in clinical parameters and lifestyle modifications before and after the intervention
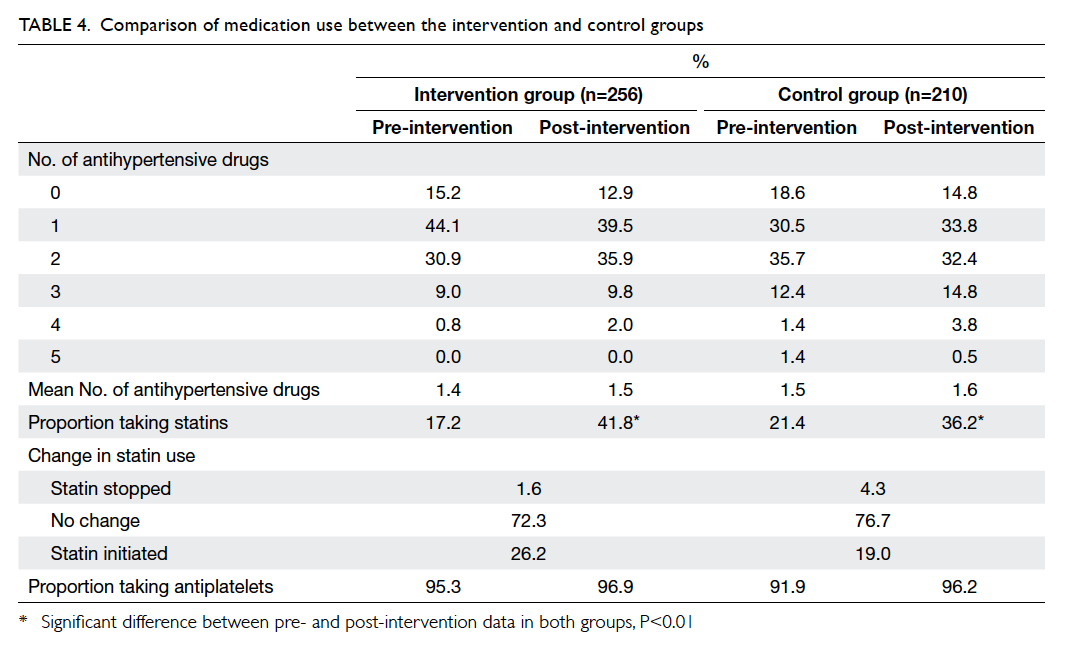
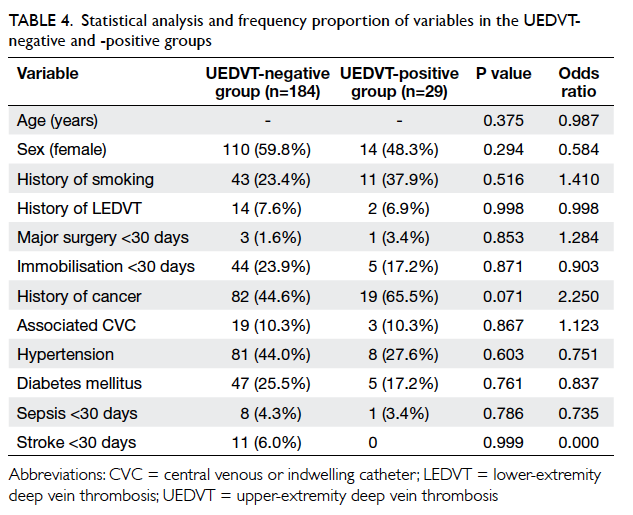
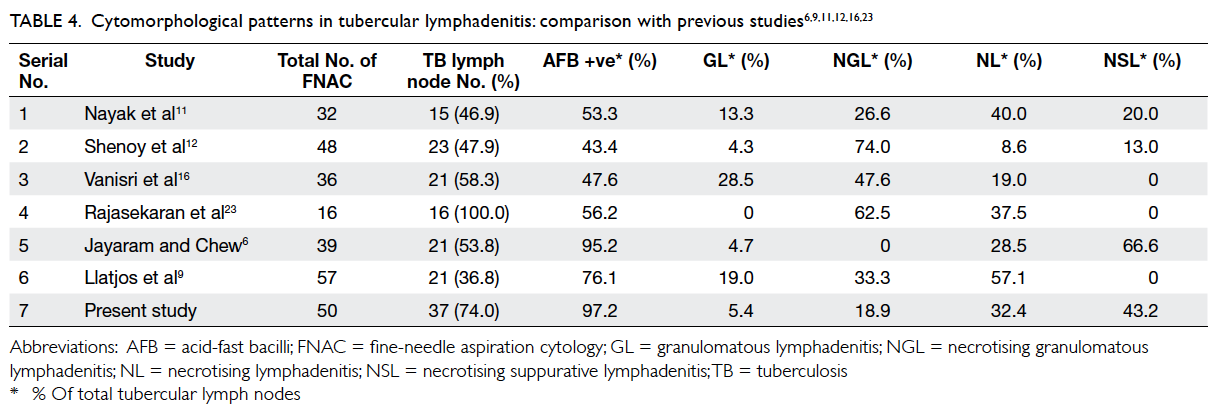
There was no significant increase in the number
of antihypertensive drugs prescribed in either group
(Table 4). Approximately 96% of patients were taking
an antiplatelet after the intervention period in both
clinics (Table 4) and the antiplatelet was always
aspirin. The proportion of patients prescribed statins
increased significantly in both groups since the
introduction of simvastatin to the GOPC formulary
in 2009. However, the overall proportion of statin
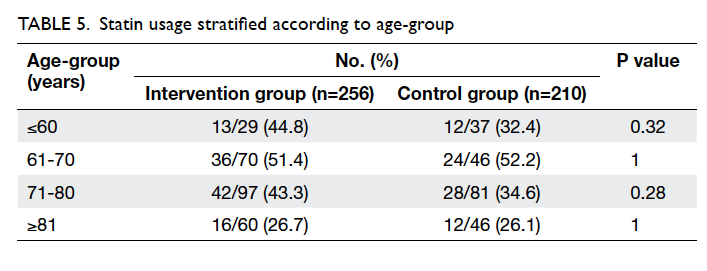
use was still below 50%. Statins were less frequently
prescribed to patients older than 80 years (Table 5).
Statins were stopped for 1.6% of patients in the
intervention group and 4.3% in the control group
(Table 4). Statins were discontinued because of
dyspepsia for all patients in the intervention group.
The reasons for stopping statins for the control
group were dyspepsia, myalgia, mild liver function
derangement, hypotension, hypoglycaemia, and
drug-induced hepatitis. Only two patients in the
control group required emergency admission for
hypoglycaemia during the intervention period.
There was no restroke in the intervention group
and four restrokes in the control group during the
intervention period.
Discussion
This study showed that the implementation of
a secondary stroke prevention programme in
GOPCs could improve control of cardiovascular
risk factors, including BP, HbA1c and
LDL levels among ischaemic stroke patients. We observed
an improvement of BP control in the
intervention group, although there was no significant
increase in the number of antihypertensives used.
However, since simvastatin was introduced into the
GOPC drug formulary in 2009, the use of statins
increased in both the control and intervention
clinics, although the effect of LDL reduction was
only observed in the intervention group. This
result implies that the improvement in outcome
for this group is due to more than just the effects of
medications. Lifestyle modifications may provide
additional benefits.
Although the BP and HbA1c level
in the intervention group were comparable with
recent recommendations (BP <140/90 mm Hg for
patients without diabetes; BP <130/80 mm Hg and
HbA1c level of <7% for patients with diabetes), the mean
LDL levels remained well above the recommended
target of 1.9 mmol/L. Only about half of the
patients were taking statins. There is a suggestion
that doctors may not prescribe or maximise statin
therapy because treatment may be considered futile,
especially among older people whose life expectancy
is limited.15 This trend was observed in both the
control and intervention sites in this study (Table 4). The percentage of patients taking statins was
relatively low in this study as some doctors may have
concerns about the possible side-effects. However,
no severe adverse effects of statins were noted in
the intervention group despite the more aggressive
treatment approach.
The implementation of secondary stroke
prevention protocol has raised doctors’ awareness
of lifestyle modification for patients with ischaemic
stroke. This was reflected by the significant
increase in the use of lifestyle modifications in the
intervention group. We encouraged doctors to
provide appropriate advice on lifestyle modification
when lifestyle risk factors were identified during the
consultation. However, due to heavy patient loads in
the GOPC, no additional time can be allocated for
medical consultations. During clinic meetings, our
staff expressed difficulty in providing quality lifestyle
education due to limited consultation time. The lack
of additional resources for lifestyle education was a
main shortcoming of this programme.
Certain subgroups of ischaemic stroke patients
are not well represented by this study, for example,
those with atrial fibrillation. Atrial fibrillation is
one of the major risk factors for recurrent stroke.16
However, as warfarin was not available in the drug
formulary of the GOPCs during the study period,
most patients with atrial fibrillation were not
referred to these clinics. Only a few patients with
atrial fibrillation were identified in our study and
all of them had a contra-indication for warfarin. At
the time of writing, warfarin has become available
in the GOPCs and several novel anticoagulants have
been introduced as self-finance items. The use of
anticoagulants in GOPCs is an important aspect of
secondary stroke prevention that warrants further
investigation.
Implementation of evidence-based guidelines
into routine clinical practice is complicated.17 18
Physicians usually have concerns about the
applicability of new trial data to individual patients,
and it takes time for them to change their practice.
Apart from considering the best available evidence,
we also need to take into account the practical
barriers in the clinical practice setting. The heavy
workload in the clinic, shortage of consultation time,
and limited scope of the drug formulary may impose
difficulty in introducing an evidence-based protocol
to local GOPCs.
From the experience of this study, a dedicated
training session for clinic staff is necessary
before the implementation of any new protocol.
Additional review sessions are needed to audit
clinicians’ compliance with the protocol. Review of
the GOPC drug formulary, for example, to include
greater choices of statins and antiplatelets, may
be helpful to improve the care of stroke patients.
Lifestyle modification is an important aspect for
secondary stroke prevention, but time constraints
in busy GOPCs are always an issue. A designated
nurse clinic for patient education and annual risk
factor monitoring should be introduced. For better
utilisation of resources, it is beneficial to recruit
community partners from allied health services to
provide a structured secondary stroke prevention
programme for patient empowerment and
engagement.
In our study, approximately 5% to 6% of
patients were lost to other GOPCs and medical
clinics (Table 1). This may introduce some bias. In addition, differences between the two clinics such
as proportions of health care workers, doctors’
qualifications, and differences in the socio-economic
groups of the patients are possible confounders that
might introduce bias. The intervention group had a
higher rate of dropouts due to death and restroke
although this was not statistically significant. As
most of these patients had no change in medications
during the intervention period (Table 1), the higher death and restroke rates were unlikely to be related to
any adverse effects from the implementation of the
protocol. However, we do not have data on the rates
of stroke recurrence, adverse events, and mortality
over a longer period, which are the most important
outcomes for effective secondary stroke prevention.
Furthermore, we may need to take into account the
Hawthorne effect when looking at the effectiveness
of the protocol implementation, in that physicians
perform better simply because they are aware that
they are in a study rather than because of the nature
of the protocol.19 20 This is an unavoidable bias in
clinical research.
Conclusion
This study demonstrates that through
implementation of a standardised treatment
protocol, the standard of care of secondary stroke
prevention for ischaemic stroke patients could
be improved in local GOPCs. However, due to
the relatively small sample size in this study, this
preliminary result should be interpreted with
caution and further studies involving more primary
care clinics are required to test its clinical value.
References
1. The top ten causes of death. (Fact sheet No 310/July 2013).
Geneva: World Health Organization; 2013.
2. Centre for Health Protection. Vital statistics: death rates
by leading causes of death, 2001-2012. Hong Kong:
HKSAR Government.
3. Centre for Health Protection. Health topics: non
communicable diseases and risk factors: cerebrovascular
disease. Available from: http://www.chp.gov.hk/en/content/9/25/58.html. Accessed 31 Mar 2014.
4. Cheung CM, Tsoi TH, Hon SF, et al. Outcomes after first-ever
stroke. Hong Kong Med J 2007;13:95-9.
5. Tsoi TH, Huang CY, Hon SF, et al. Trends in stroke types
and mortality in Chinese. Stroke 2004;35:e256.
6. Wong HC, Mok CT. Update on secondary stroke
prevention. Hong Kong Pract 2007;29:271-6.
7. Wang Y, Wu D, Wang Y, Ma R, Wang C, Zhao W. A survey
on adherence to secondary ischemic stroke prevention.
Neurol Res 2006;28:16-20. Crossref
8. Whitford DL, Hickey A, Horgan F, O’Sullivan B, McGee H,
O’Neill D. Is primary care a neglected piece of the jigsaw in
ensuring optimal stroke care? Results of a national study.
BMC Fam Pract 2009;10:27. Crossref
9. Ovbiagele B, Drogan O, Koroshetz WJ, Fayad P, Saver JL.
Outpatient practice patterns after stroke hospitalization
among neurologists. Stroke 2008;39:1850-4. Crossref
10. Rudd AG, Lowe D, Hoffman A, Irwin P, Pearson M.
Secondary prevention for stroke in the United Kingdom:
results from the National Sentinel Audit of Stroke. Age
Ageing 2004;33:280-6. Crossref
11. Xu G, Liu X, Wu W, Zhang R, Yin Q. Recurrence after
ischemic stroke in Chinese patients: impact of uncontrolled
modifiable risk factors. Cerebrovasc Dis 2007;23(2-3):117-20. Crossref
12. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in
patients with ischemic stroke or transient ischemic attack:
a statement for healthcare professionals from the American
Heart Association/American Stroke Association Council
on Stroke: co-sponsored by the Council on Cardiovascular
Radiology and Intervention: the American Academy
of Neurology affirms the value of this guideline. Stroke
2006;37:577-617. Crossref
13. Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA
recommendations for the prevention of stroke in
patients with stroke and transient ischemic attack. Stroke
2008;39:1647-52. Crossref
14. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke
in patients with stroke or transient ischemic attack: a
guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke
2011;42:227-76. Crossref
15. Walker DB, Jacobson TA. Initiating statins in the elderly:
the evolving challenge. Curr Opin Endocrinol Diabetes
Obes 2008;15:182-7. Crossref
16. Li R, Cheng S, Mok M, et al. Atrial fibrillation is an
independent risk factor of poor stroke outcome and
mortality in Chinese ischaemic stroke patients. Cerebrovasc
Dis 2013;36(Supp 1):74.
17. Cranney M, Warren E, Barton S, Gardner K, Walley T.
Why do GPs not implement evidence-based guidelines? A
descriptive study. Fam Pract 2001;18:359-63. Crossref
18. Foy R, Eccles M, Grimshaw J. Why does primary care need
more implementation research? Fam Pract 2001;18:353-5. Crossref
19. Wickström G, Bendix T. The “Hawthorne effect”—what
did the original Hawthorne studies actually show? Scand J
Work Environ Health 2000;26:363-7. Crossref
20. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M,
Fisher P. The Hawthorne effect: a randomised, controlled
trial. BMC Med Res Methodol 2007;7:30. Crossref