Hong Kong Med J 2022 Apr;28(2):161–8 | Epub 11 Apr 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Update on the Recommendations on Breast
Cancer Screening by the Cancer Expert Working
Group on Cancer Prevention and Screening
Cancer Expert Working Group on Cancer Prevention and Screening (August 2018 to July 2021)
Thomas HF Tsang, MB, BS, FHKAM (Community Medicine)1; Ka-hing Wong, MB, BS, FHKAM (Medicine)2; Kate Allen, PhD3; Karen KL Chan, MBBChir, FHKAM (Obstetrics and Gynaecology)4; Miranda CM Chan, MB, BS, FHKAM (Surgery)5; David VK Chao,FRCGP, FHKAM (Family Medicine)6;
Annie NY Cheung, MD, FHKAM (Pathology)7; Cecilia YM Fan, MB, BS, FHKAM (Family Medicine)8; Edwin P Hui, MD (CUHK), FHKAM (Medicine)9; Dennis KM Ip, MD10; KO Lam, MB, BS, FHKAM (Radiology)11; CK Law, FHKCR, FHKAM (Radiology)12; WL Law, MS, FHKAM (Surgery)13;
Herbert HF Loong, MB, BS, FHKAM (Medicine)14; Kam-hung Wong, MB, ChB, FHKAM (Radiology)15; Martin CS Wong, MD, FHKAM (Family Medicine)16; Rebecca MW Yeung, FHKAM (Radiology)17; Anthony CH Ying, MB, BS, FHKAM (Radiology)18; Rita KW Ho, MB, BS, FHKAM (Community Medicine)19
1 Hong Kong College of Community Medicine, Hong Kong
2 Centre for Health Protection, Department of Health, Hong Kong
3 World Cancer Research Fund International, United Kingdom
4 The Hong Kong College of Obstetricians and Gynaecologists, Hong Kong
5 Hospital Authority (Surgery), Hong Kong
6 The Hong Kong College of Family Physicians, Hong Kong
7 The Hong Kong College of Pathologists, Hong Kong
8 Professional Development and Quality Assurance Service, Department of Health, Hong Kong
9 Hong Kong College of Physicians, Hong Kong
10 School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
11 Department of Clinical Oncology, The University of Hong Kong, Hong Kong
12 Hong Kong College of Radiologists, Hong Kong
13 The College of Surgeons of Hong Kong, Hong Kong
14 Department of Clinical Oncology, The Chinese University of Hong Kong, Hong Kong
15 Hong Kong Cancer Registry, Hospital Authority, Hong Kong
16 The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
17 Hospital Authority (Clinical Oncology), Hong Kong
18 The Hong Kong Anti-Cancer Society, Hong Kong
19 Centre for Health Protection, Department of Health, Hong Kong
Corresponding author: Dr Rita KW Ho (head_ncdb@dh.gov.hk)
Abstract
Breast cancer (BC) is the most common cancer
among women in Hong Kong. The Food and Health
Bureau commissioned The University of Hong Kong
(HKU) to conduct the Hong Kong Breast Cancer
Study (HKBCS) with the aim of identifying relevant
risk factors for BC in Hong Kong and developing a
locally validated BC risk assessment tool for Hong
Kong Chinese women. After consideration of
the most recent international and local scientific
evidence including findings of the HKBCS,
the Cancer Expert Working Group on Cancer
Prevention and Screening (CEWG) has reviewed
and updated its BC screening recommendations.
Existing recommendations were preserved for
women at high risk and slightly changed for women
at moderate risk. The following major updates have
been made concerning recommendations for other
women in the general population:
Women aged 44 to 69 with certain combinations
of personalised risk factors (including presence
of history of BC among first-degree relative,
a prior diagnosis of benign breast disease,
nulliparity and late age of first live birth, early age
of menarche, high body mass index and physical
inactivity) putting them at increased risk of BC
are recommended to consider mammography
screening every 2 years. They should discuss with
their doctors on the potential benefits and harms
before undergoing mammography screening.
A risk assessment tool for local women (eg, one
developed by HKU) is recommended to be used
for estimating the risk of developing BC with
regard to the personalised risk factors described
above.
Introduction
In Hong Kong, the Cancer Coordinating Committee,
chaired by the Secretary for Food and Health, was
established in 2001 to formulate strategies regarding
cancer prevention and control. The Cancer
Expert Working Group on Cancer Prevention and
Screening (CEWG), under the Cancer Coordinating
Committee, was formed in 2002 to regularly
review international and local evidence, then make
local recommendations on cancer prevention and
screening.
Breast cancer (BC) is the most common cancer
among women in Hong Kong. Although evidence
from other countries suggests that organised
mammography screening is effective for detecting
BC at an earlier stage and reducing mortality among
affected patients, there is a lack of information
concerning its usefulness and cost-effectiveness in
Hong Kong. While BC risk prediction models such
as the Gail model were developed in other areas for
estimation of an individual’s risk of BC, such models
have not been validated in Hong Kong.
To address the aforementioned evidence
gaps, the Hong Kong SAR Government previously
commissioned The University of Hong Kong to
conduct the Hong Kong Breast Cancer Study
(HKBCS) for the quantification of relevant BC risk
factors and development of a model for BC risk
stratification among women in Hong Kong. Based on the findings of the HKBCS and other relevant
studies, as well as epidemiological findings in Hong
Kong and other countries, the CEWG updated its
recommendations on BC screening; these updated
recommendations were endorsed by the Cancer
Coordinating Committee in June 2020. This article
focuses primarily on the revised CEWG screening
recommendations for women at average risk of
BC in the general population; it also discusses the
rationale for such recommendations.
Local epidemiology
In Hong Kong, 4761 invasive BC cases in women
were recorded in 2019; this constituted 27.4% of
all new cancer cases in women.1 The median age
at diagnosis was 58 years; 72% of patients had
stage I or II BC.1 In 2020, BC was the third leading
cause of cancer death in women (751 deaths).2 The
age-standardised incidence rate in 2019 and age-standardised
mortality rate in 2020 were 70.9 and 9.7
per 100 000 world standard population, respectively.2
Over the past three decades, the age-standardised
incidence rate has demonstrated an upward trend
while the age-standardised mortality rate did not
significantly change.2
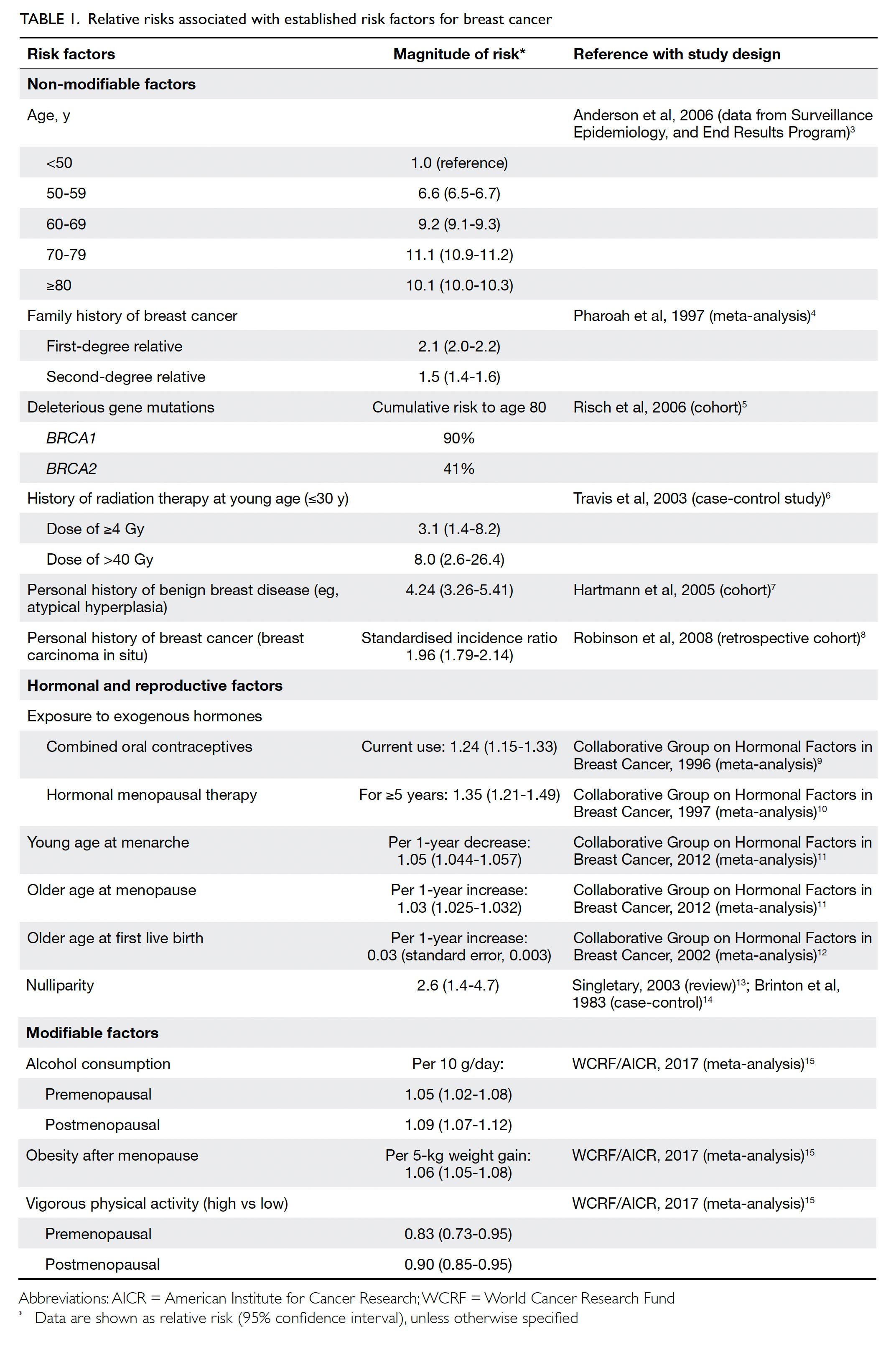
Risk factors and primary
prevention
Established risk factors for BC include family history
of BC, inheritance of certain gene mutations, history
of radiation therapy at a young age, personal history
of BC or benign breast diseases, hormonal and
reproduction factors, alcohol consumption, obesity
after menopause, and physical inactivity.3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 The
relative risks (RRs) associated with established risk
factors for BC are summarised in Table 1.3 4 5 6 7 8 9 10 11 12 13 14 15
Primary preventive measures are important
for lowering the risk of BC because some risk factors
are modifiable. These preventive measures include
regular physical activities, avoidance of alcohol
consumption, and the maintenance of a healthy body
weight and waist circumference.15 Moreover, women
are recommended to extend breastfeeding and give
birth at an earlier age to reduce their BC risk.12 15
Breast awareness
Breast awareness refers to a woman’s familiarity
with the normal look and feel of her breasts, which
facilitates prompt reporting of any abnormality to
doctors for early diagnosis and treatment. Delayed
pursuit of medical attention could lead to worse
survival in patients with BC; for example, the 5-year
survival rate was 7% higher among BC patients who
began treatment <3 months from symptom onset
than among patients who began treatment 3 to
6 months from symptom onset.18
Screening for women in the
general population
Importantly, BC screening is intended to detect BC in asymptomatic women before symptom onset; this
facilitates a better treatment outcome and improves
survival. Breast self-examination, clinical breast
examination, and mammography are the most
widely studied screening modalities for BC.
Breast self-examination and
clinical breast examination
In contrast to breast awareness, breast self-examination
refers to the regular and systematic
self-examination of a woman’s breasts. Meta-analysis
and two randomised controlled trials
(RCTs) in Shanghai and Russia showed that the
use of breast self-examination did not produce
significant differences in the size or stage of
BC, or in the number of BC deaths; however, it
generated false-positive findings, including more
benign lesions detected and unnecessary biopsies
performed.19 20 21 Thus, international health agencies
including the International Agency for Research on
Cancer (IARC), the American Cancer Society, and
the US Preventive Services Task Force (USPSTF)
recommend against teaching women breast self-examination
as a screening modality for BC17 22 23 24;
these agencies encourage women to become more
aware of breast changes and promptly seek medical
advice regarding changes.17 24 25 With respect to
clinical breast examination, three RCTs showed
that this screening modality could detect smaller
lesions and earlier stages of BC.26 27 28 However,
there is inadequate evidence that clinical breast
examination screening reduces BC mortality among
asymptomatic women.17 21 22 23 24
Mammography screening
Evidence from other countries suggests that
organised mammography screening programmes
are effective in detecting tumours at an early stage
and reducing BC deaths, with the greatest benefit
observed among women aged 50 to 69 years.17 22 23 24 29 30 31 32 33
Mammography screening was associated with
an approximately 20% reduction in BC mortality
among women of all ages at average risk after
13 years of follow-up, as reported in meta-analyses
of RCTs (RR=0.80-0.82), a meta-analysis of cohort
studies (RR=0.75), and modelling studies (median
RR=0.85).22 29 When compared with women aged
<50 years, mammography screening for women
aged ≥50 years was associated with slightly greater
BC mortality reduction (14%-23% vs 15%), mostly
because of greater mortality reduction among
women aged 60 to 69 years (31%-32%).29
A systematic review by the USPSTF reported the effects of mammography screening in different
age-groups. Fair-quality evidence from a meta-analysis
of mammography trials showed that the RRs
for BC mortality were 0.92 (95% confidence interval
[CI]=0.75-1.02) among women aged 39 to 49 years,
0.86 (95% CI=0.68-0.97) among women aged 50 to
59 years, 0.67 (95% CI=0.54-0.83) among women
aged 60 to 69 years, and 0.80 (95% CI=0.51-1.28)
among women aged 70 to 74 years; the mortality
benefit generally increased with age.30 Similarly, the
Canadian Task Force on Preventive Health Care
reviewed the benefit of mammography screening for
average-risk women aged 40 to 74 years; screening
resulted in a modest reduction in BC mortality, with
the lowest absolute benefit among women aged
<50 years.33
Biennial mammography screening is
recommended for some women in some developed
countries such as Australia, Canada, the US, and
European countries.24 33 34 The IARC has evaluated
the effectiveness of biennial mammography
screening in some of these countries; approximately
40% reduction in BC mortality was observed among
women aged 50 to 69 years who had undergone
screening.17 23 Additionally, a significant reduction
in advanced BC was observed among women aged
≥50 years who underwent screening (RR=0.62,
95% CI=0.46-0.83), but not among women aged 39
to 49 years.30
Although the benefit of using mammography
as a tool for BC screening is evident, there are
limitations concerning its use as a screening
modality.17 22 23 24 29 30 31 32 33 35 Possible adverse outcomes
related to such use of mammography include
overdiagnosis and overtreatment. For example,
women with a diagnosis of ductal carcinoma in situ
often rapidly undergo radical treatment although
they may live with this non-invasive condition in
the absence of diagnosis and subsequent treatment.
Estimates of the rate of overdiagnosis varied widely,
depending on study designs and methodologies.
Observational studies generally led to estimated
overdiagnosis rates of 0% to 54%, while the rates
estimated on the basis of RCT data ranged from
11% to 22%.32 35 36 A pooled analysis of 13 European
studies also reported wide variation, such that crude
estimates of overdiagnosis ranged from 0% to 54%;
these estimates were reduced to 1% to 10% after
adjustment for BC risk and lead-time bias.17 29
Mammography screening could also cause
false-positive findings which lead to recall for
unnecessary, additional imaging and subsequent
invasive procedures (mostly biopsies). The USPSTF
systematic review of mammography screening
revealed that the 10-year cumulative false-positive
and biopsy rates were higher for annual screening
than for biennial screening (61% vs 42% and 7% vs
5%, respectively); these rates were also higher among women aged 40 to 49 years and women with dense
breasts.35 The IARC Working Group estimated
that the cumulative risk of false-positive recall in
organised screening programmes was approximately
20% for women who underwent mammography
screening 10 times between the ages of 50 and
70 years, where fewer than 5% of all false-positive
mammography screening results led to an invasive
procedure.17 23 Women may experience anxiety while
waiting for the results of mammography screening
or upon recall for further investigations. Women
with false-positive mammography results generally
experienced short-term negative psychological
consequences, although such effects could be
mitigated via clear communication with their
physicians.17 23 25
Radiation-induced BC is also a concern for
women. Systematic reviews estimated that the risk
of death from mammography-related radiation-induced
BC ranged from 1 to 11 per 100 000
women, depending on age and screening interval;
however, such risk is outweighed by the ability of
mammography to prevent BC deaths.17 23 35
Concerning the frequency of mammography
screening, no RCTs have directly compared the
benefits of annual to biennial screening in women
of any age; however, observational studies found no
differences between biennial and annual screening in
women aged >=50 years.24 29 30 A modelling study from
the US estimated that women screened biennially
from age 50 to age 74 avoided a median of seven BC
deaths versus no screening, whereas women screened
annually from age 40 to age 74 avoided additional
three deaths; however, annual screening yielded 1988
more false-positives and 11 more overdiagnoses
per 1000 women screened, indicating that biennial
screening is a more cost-effective strategy for average-risk
populations of women.37 Guidelines from other
regions (eg, the World Health Organization, USPSTF,
and most developed countries) generally recommend
biennial mammography screening for women at
average risk of BC.24 34 38
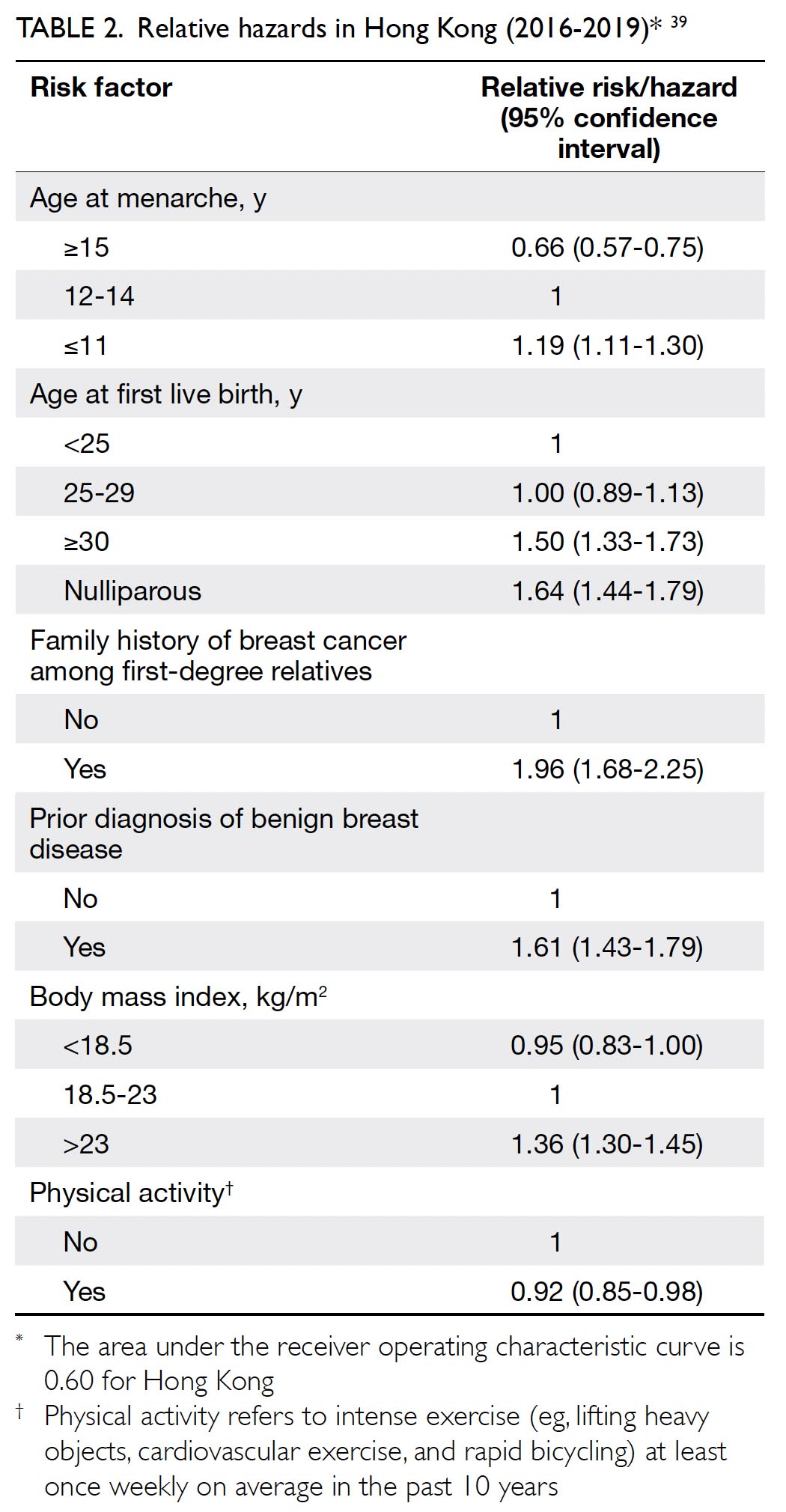
Previously, the CEWG considered the
available scientific evidence to be insufficient for
recommendations regarding population-based
mammography screening among women at average
risk in Hong Kong. Recently, the University of Hong
Kong research team completed a territory-wide
case-control study (HKBCS) involving 3501 BC
cases and 3610 controls.39 The study estimated the
risk of BC in women based on a list of parameters
including age, age at menarche, age at first live birth,
family history of BC among first-degree relatives,
prior benign breast disease diagnosis, body mass
index, and physical activity (Table 2).39 The RRs of
these identifiable risk factors were incorporated to
develop a risk prediction model (ie, personalised
risk assessment tool) applicable to the Chinese population in Hong Kong, with the aim of guiding
mammography screening and improving the cost-effectiveness
of mass screening. The HKBCS found
that while the relative reduction in BC mortality
was similar between risk-based screening and
conventional age-based screening, it would be
more cost-effective to provide risk-based biennial
mammography screening to Hong Kong Chinese
women aged 44 to 69 years who had an increased
risk of BC according to the newly developed risk
assessment tool.39 Targeted screening in women
at increased risk of BC would reduce the potential
for harm related to unnecessary biopsy or other
invasive tests conducted to confirm false-positive
mammography findings; it would also optimise the
use of scarce healthcare resources. Women with
high risk (eg, BRCA1/2 mutation carriers) and
moderate risk, as defined by the CEWG, should follow the respective CEWG recommendations on
BC screening (Table 3).40
Other imaging techniques
Compared with conventional two-dimensional
mammography, digital breast tomosynthesis (also
known as three-dimensional mammography)
lowers recall rates for false-positives and detects
more cancers; however, it exposes women to more
radiation.17 23 24 30 41 42 Thus far, it remains unclear
whether digital breast tomosynthesis can provide to patients by detecting clinically significant
cancers, rather than causing overdiagnosis. Current
international guidelines do not support the use of
digital breast tomosynthesis as a screening tool and
future research in this area is warranted.17 23 24 30 33
Ultrasonography, as an adjunct to mammography
in women with radiologically dense breasts, may
depict small BCs not visible on mammography,
while increasing false-positive recall.43 44 Systematic
reviews conducted by Cochrane, IARC, and USPSTF
have concluded that there is insufficient evidence to
support the use of ultrasonography in asymptomatic women as a routine screening tool to decrease BC
mortality.17 23 24 25
Revised recommendation
In accordance with local data and the latest scientific
evidence, the CEWG has revised its BC screening
recommendations for women in Hong Kong, as
summarised below40:
1. Breast self-examination is not recommended as a screening tool for BC for asymptomatic women. Women are recommended to be breast aware (be familiar with the normal look and feel of their breasts) and seek medical attention promptly if suspicious symptoms arise.
2. There is insufficient evidence to recommend clinical breast examination or ultrasonography as a screening tool for BC for asymptomatic women.
3. It is recommended that risk-based approach should be adopted for BC screening.
4. While the BC screening recommendations for (a) women at high risk remain status quo, those for (b) women at moderate risk and (c) other women at general population are revised. Details of recommendations for women at different risk profiles are listed in Table 3.40
1. Breast self-examination is not recommended as a screening tool for BC for asymptomatic women. Women are recommended to be breast aware (be familiar with the normal look and feel of their breasts) and seek medical attention promptly if suspicious symptoms arise.
2. There is insufficient evidence to recommend clinical breast examination or ultrasonography as a screening tool for BC for asymptomatic women.
3. It is recommended that risk-based approach should be adopted for BC screening.
4. While the BC screening recommendations for (a) women at high risk remain status quo, those for (b) women at moderate risk and (c) other women at general population are revised. Details of recommendations for women at different risk profiles are listed in Table 3.40
Author contributions
All authors have made substantial contributions to the concept
or design, acquisition of data, analysis or interpretation of
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
As editors of this journal, DVK Chao, HHF Loong, and MCS Wong were not involved in the peer review process of this article. The other authors have no conflicts of interest to disclose.
Declaration
An earlier version of this article was published online at the
website of the Centre for Health Protection in January 2021:
Cancer Expert Working Group on Cancer Prevention and
Screening (CEWG). Recommendations on Prevention and
Screening for Breast Cancer–For Health Professionals. Centre
for Health Protection; January 2021. https://www.chp.gov.hk/
files/pdf/breast_cancer_professional_hp.pdf
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
References
1. Hong Kong Cancer Registry, Hospital Authority, Hong
Kong SAR Government. Female breast cancer in 2019. Available from: https://www3.ha.org.hk/cancereg/pdf/factsheet/2019/breast_2019.pdf. Accessed 1 Apr 2022.
2. Department of Health, Census and Statistics Department,
Hong Kong SAR Government. Breast cancer. Available
from: https://www.chp.gov.hk/en/healthtopics/content/25/53.html. Accessed 1 Apr 2022.
3. Anderson WF, Pfeiffer RM, Dores GM, Sherman ME.
Comparison of age distribution patterns for different
histopathologic types of breast carcinoma. Cancer
Epidemiol Biomarkers Prev 2006;15:1899-905. Crossref
4. Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA.
Family history and the risk of breast cancer: a systematic
review and meta-analysis. Int J Cancer 1997;71:800-9. Crossref
5. Risch HA, McLaughlin JR, Cole DE, et al. Population
BRCA1 and BRCA2 mutation frequencies and cancer
penetrances: a kin-cohort study in Ontario, Canada. J Natl
Cancer Inst 2006;98:1694-706. Crossref
6. Travis LB, Hill DA, Dores GM, et al. Breast cancer
following radiotherapy and chemotherapy among young
women with Hodgkin disease. JAMA 2003;290:465-75. Crossref
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast
disease and the risk of breast cancer. N Engl J Med
2005;353:229-37. Crossref
8. Robinson D, Holmberg L, Møller H. The occurrence of
invasive cancers following a diagnosis of breast carcinoma
in situ. Br J Cancer 2008;99:611-5. Crossref
9. Collaborative Group on Hormonal Factors in Breast Cancer.
Breast cancer and hormonal contraceptives: collaborative
reanalysis of individual data on 53 297 women with breast
cancer and 100 239 women without breast cancer from 54
epidemiological studies. Lancet 1996;347:1713-27. Crossref
10. Collaborative Group on Hormonal Factors in Breast
Cancer. Breast cancer and hormone replacement therapy:
collaborative reanalysis of data from 51 epidemiological
studies of 52,705 women with breast cancer and 108,411
women without breast cancer. Lancet 1997;350:1047-59. Crossref
11. Collaborative Group on Hormonal Factors in Breast
Cancer. Menarche, menopause, and breast cancer risk:
individual participant meta-analysis, including 118 964
women with breast cancer from 117 epidemiological
studies. Lancet Oncol 2012;13:1141-51. Crossref
12. Collaborative Group on Hormonal Factors in Breast
Cancer. Breast cancer and breastfeeding: collaborative
reanalysis of individual data from 47 epidemiological
studies in 30 countries, including 50 302 women with
breast cancer and 96 973 women without the disease.
Lancet 2002;360:187-95. Crossref
13. Singletary SE. Rating the risk factors for breast cancer. Ann
Surg 2003;237:474-82. Crossref
14. Brinton LA, Hoover R, Fraumeni JF Jr. Reproductive factors
in the aetiology of breast cancer. Br J Cancer 1983;47:757-62. Crossref
15. World Cancer Research Fund International/American
Institute for Cancer Research. Diet, Nutrition, Physical
Activity and Breast Cancer. 2017. Available from: https://www.wcrf.org/wp-content/uploads/2021/02/Breast-cancer-report.pdf. Accessed 26 Feb 2021.
16. International Agency for Research on Cancer. World
Health Organization. List of classifications by cancer
sites with sufficient or limited evidence in humans, IARC
Monographs Volumes 1 to 129. Available from: https://monographs.iarc.fr/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf. Accessed 4 Jan 2021.
17. International Agency for Research on Cancer. World Health Organization. IARC Handbooks of Cancer
Prevention, Volume 15. Breast Cancer Screening. France:
World Health Organization; 2016.
18. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with
breast cancer: a systematic review. Lancet 1999;353:1119-26. Crossref
19. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-57. Crossref
20. Semiglazov VF, Manikhas AG, Moiseenko VM, et al.
Results of a prospective randomized investigation [Russia
(St. Petersburg)/WHO] to evaluate the significance of self-examination
for the early detection of breast cancer [in
Russian]. Vopr Onkol 2003;49:434-41.
21. Kösters JP, Gøtzsche PC. Regular self-examination or
clinical examination for early detection of breast cancer.
Cochrane Database Syst Rev 2003;(2):CD003373. Crossref
22. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer
screening for women at average risk: 2015 guideline update
from the American Cancer Society. JAMA 2015;314:1599-614. Crossref
23. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast
cancer screening—viewpoint of the IARC working group.
N Engl J Med 2015;372:2353-8. Crossref
24. Siu AL, US Preventive Services Task Force. Screening
for breast cancer: U.S. Preventive Services Task
Force recommendation statement. Ann Intern Med
2016;164:279-96. Crossref
25. American Cancer Society. American Cancer Society
screening recommendations for women at average
breast cancer risk. Available from: https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early0detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html. Accessed 4
Jul 2021.
26. Pisani P, Parkin DM, Ngelangel C, et al. Outcome of
screening by clinical examination of the breast in a trial in
the Philippines. Int J Cancer 2006;118:149-54. Crossref
27. Mittra I, Mishra GA, Singh S, et al. A cluster randomized,
controlled trial of breast and cervix cancer screening in
Mumbai, India: methodology and interim results after
three rounds of screening. Int J Cancer 2010;126:976-84. Crossref
28. Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical
breast examination: preliminary results from a cluster
randomized controlled trial in India. J Natl Cancer Inst
2011;103:1476-80. Crossref
29. Myers ER, Moorman P, Gierisch JM, et al. Benefits and
harms of breast cancer screening: a systematic review.
JAMA 2015;314:1615-34. Crossref
30. Nelson HD, Fu R, Cantor A, Pappas M, Daeges, Humphrey L.
Effectiveness of breast cancer screening: systematic review
and meta-analysis to update the 2009 U.S. Preventive
Services Task Force Recommendation. Ann Intern Med
2016;162:244-55. Crossref
31. Gøtzsche PC, Jørgensen KJ. Screening for breast cancer
with mammography. Cochrane Database Syst Rev
2013;(6):CD001877. Crossref
32. Marmot MG, Altman DG, Cameron DA, Dewar JA,
Thompson SG, Wilcox M. The benefits and harms of breast
cancer screening: an independent review. Br J Cancer 2013;108:2205-40. Crossref
33. Klarenbach S, Sims-Jones N, Lewin G, et al. Recommendations on screening for breast cancer in
women aged 40-74 years who are not at increased risk for
breast cancer. CMAJ 2018;190:E1441-51. Crossref
34. Ebell MH, Thai TN, Royalty KJ. Cancer screening
recommendations: an international comparison of high
income countries. Public Health Rev 2018;39:7. Crossref
35. Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M,
Humphrey. Harms of breast cancer screening: systematic
review to update the 2009 U.S. Preventive Services Task
Force Recommendation. Ann Intern Med 2016;164:256-67. Crossref
36. Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA.
Twenty five year follow-up for breast cancer incidence
and mortality of the Canadian National Breast Screening
Study: randomised screening trial. BMJ 2014;348:g366. Crossref
37. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative
modeling of the benefits and harms associated with
different U.S. Breast Cancer Screening Strategies. Ann
Intern Med 2016;164:215-25. Crossref
38. World Health Organization. WHO position paper on
mammography screening. 2014. Available from: https://apps.who.int/iris/bitstream/handle/10665/137339/9789241507936_eng.pdf;jsessionid=D2DD6C57F1C3720A905DDD98E28EA589?sequence=1. Accessed 4 Jan 2021.
39. Health and Medical Research Fund. Commissioned Study
to The University of Hong Kong. Preventing breast cancer
in Hong Kong Chinese women through personalised
risk stratification and characterization: an epidemiologic
modeling study and the development of a biorepository
of cases and controls. Final Report. Available from:
https://rfs1.fhb.gov.hk/app/fundedsearch/projectdetail.xhtml?id=1903. Accessed 4 Jan 2021.
40. Centre for Health Protection, Hong Kong SAR
Government. Cancer Expert Working Group on Cancer
Prevention and Screening (CEWG). Recommendations
on Prevention and Screening for Breast Cancer for health
professionals. January 2021. Available from: https://www.chp.gov.hk/files/pdf/breast_cancer_professional_hp.pdf.
Accessed 29 Jan 2021.
41. Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D
digital mammography with tomosynthesis for population
breast-cancer screening (STORM): a prospective
comparison study. Lancet Oncol 2013;14:583-9. Crossref
42. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer
screening using tomosynthesis in combination with digital
mammography. JAMA 2014;311:2499-507. Crossref
43. Berg WA, Blume JD, Cormack JB, et al. Combined screening
with ultrasound and mammography vs mammography
alone in women at elevated risk of breast cancer. JAMA
2008;299:2151-63. Crossref
44. Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity
of mammography and adjunctive ultrasonography to
screen for breast cancer in the Japan Strategic Anti-cancer
Randomized Trial (J-START): a randomised controlled
trial. Lancet 2016;387:341-8. Crossref
45. Gartlehner G, Thaler K, Chapman A, et al. Mammography
in combination with breast ultrasonography versus
mammography for breast cancer screening in
women at average risk. Cochrane Database Syst Rev
2013;(4):CD009632. Crossref