© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Hong Kong Geriatrics Society and Hong Kong
Urological Association consensus on personalised management of male lower urinary tract symptoms in the era of multiple co-morbidities and polypharmacy
Peggy SK Chu, MB, BS, FRCS (Edin)1; Clarence LH Leung, MB, BS, FRCSEd (Urol)1; MH Cheung, MB, BS, FRCSEd (Urol)1; Sandy WS Woo, MB, BS, FHKCP2; TK Lo, MB, BS, FCSHK1; Tony NH Chan, MB, BS, FHKCP2; William KK Wong, MB, BS, FHKCP2
1 Hong Kong Urological Association, Hong Kong
2 The Hong Kong Geriatrics Society, Hong Kong
Corresponding author: Dr TK Lo (ltk616@ha.org.hk)
Abstract
Lower urinary tract symptoms (LUTS) are common
complaints of adult men. Benign prostatic hyperplasia
(BPH) represents the most common underlying
cause. As the incidence of BPH increases with age,
and pharmacological treatment is a major part of the
disease’s management, the majority of patients with
LUTS are managed by primary care practitioners.
There are circumstances in which specialist care
by urologists or geriatricians is required, such as
failure of medical treatment, adverse effects from
medical treatment, or complications from BPH.
Referral choices can be confusing to patients and
even practitioners in different specialties under such
circumstances. There is currently no local consensus
about the diagnosis, medical management, or
referral mechanism of patients with BPH. A
workgroup was formed by members of The Hong
Kong Geriatrics Society (HKGS) and the Hong Kong
Urological Association (HKUA) to review evidence
for the diagnosis and medical treatment of LUTS. A
consensus was reached by HKGS and HKUA on an algorithm for the flow of male LUTS care and the
use of uroselective alpha blockers, antimuscarinics,
beta-3 adrenoceptor agonists, and 5α-reductase
inhibitors in the primary care setting. This consensus
by HKGS and HKUA provides a new management
paradigm of male LUTS.
Introduction
In 2019, Hong Kong overtook Japan as the region
with the world’s longest life expectancy, with the
life expectancy of Hong Kong Chinese men at
82.38 years.1 Benign prostatic hyperplasia (BPH) is
the most common prostate problem for men older
than 50 years. The occurrence of lower urinary tract
symptoms (LUTS) due to BPH increases with age.
A 1984 autopsy study showed that the prevalence of
BPH rose with each decade after age 40, peaking at
88% in men in their 80s.2 Since the 1980s, medical
therapy has been prescribed for patients with
bothersome LUTS that negatively affects their quality
of life. Moreover, the number of co-morbid diseases
also increases with age. Co-morbidity increases from
10% at ages up to 19 years to 80% at ages 80 years and
older.3 Co-morbidity also leads to polypharmacy and
drug-drug interactions, which may result in serious
adverse effects.
There is no established local consensus
regarding the management of elderly male patients with LUTS. Thus, the Hong Kong Geriatrics Society
(HKGS) and the Hong Kong Urological Association
(HKUA) formed a working group with the aim
of providing insights to clinicians involved in the
medical management of male patients with LUTS
through a consensus article.
Diagnostic evaluation
The causes of male LUTS can be multifactorial.
Detailed history, appropriate questionnaires,
physical examination, and investigation not only
help clinicians to reach a diagnosis and identify some
alarming conditions (eg, prostate cancer and bladder
cancer) but also guide treatment options and give
prognostic information for patients’ counselling.
History
It is useful to determine the most predominant and
bothersome LUTS to guide their management, eg,
voiding symptoms (weak stream, intermittency, hesitancy, incomplete emptying) and storage
symptoms (urgency, frequency, nocturia). The
severity of symptoms can be categorised by the
International Prostate Symptoms Score as mild (0-7),
moderate (8-19), or severe (20-35). It is a validated
tool for the assessment of symptoms and quality of
life in patients with LUTS (online supplementary Appendix) and allows objective monitoring of
treatment response.
Focused histories of the presence of
neurological diseases, diabetes mellitus, medication,
drinking habits, and prior lower urinary tract
procedures are useful to identify causes of LUTS
other than BPH (eg, neurogenic bladder, polydipsia,
urethral stricture).
Referral to geriatricians should be considered
in elderly patients with history of postural
hypotension, delirium, dementia, frequent falling, or
polypharmacy, as these patients have a higher risk
of adverse effects from medical treatment of LUTS,
and comprehensive geriatric assessment may be
necessary.
Alarming symptoms should raise suspicion of
pathologies other than BPH, eg, gross haematuria
or unexplained dysuria may imply underlying
neoplastic or inflammatory causes, or bedwetting
may imply underlying chronic urinary retention with
overflow incontinence. Prompt referral to urologists
is preferable in the presence of such symptoms.
Physical examination
Digital rectal examination is used to assess prostate
size, consistency, the presence of prostatic nodules,
and anal tone. In addition, focused examination of
the abdomen, external genitalia, and lower limbs is
important. Palpable bladder, phimosis, penile mass, and abnormal neurological signs are important to
notice when considering referral to appropriate
specialists.
A rough estimation of prostate size by number
of finger breaths on digital rectal examination is
acceptable and may guide the use of 5α-reductase
inhibitors (5ARi). Imaging assessment by ultrasound
can be considered if more accurate assessment is
preferred.
Investigations
Most patients with LUTS have slow deterioration
of symptoms, and very few develop complications
over a 5-year period.4 In the primary care setting,
the aim of initial evaluation is to detect non-BPH
causes, and urinalysis should be included. Prostate-specific
antigen (PSA) can be measured after proper
counselling, and serum creatinine should be checked
when renal impairment is suspected. Numerous
additional investigations are also possible, such as
flow rate measurement, post-void residual urine
volume, renal ultrasonography, prostate sizing,
or urodynamic study. However, these additional
investigations are optional and need not be routinely
performed at the initial evaluation, as they are not
cost-effective. Selected patients with appropriate
indications (eg, LUTS with poor response to
medical treatment, presence of alarming symptoms,
impaired renal function) benefit the most from these
tests, and input from specialists is preferred in these
circumstances.
Urinalysis
Urinalysis (dipstick or sediment count) should be
included in the primary evaluation of any patients
presenting with LUTS to search for urinary tract
infections, microscopic haematuria, and diabetes
mellitus. If abnormal findings are detected, further
tests are recommended.5
Prostate-specific antigen
One of the differential diagnoses of male LUTS is
prostate cancer. Prostate-specific antigen is organ-specific
but not cancer-specific. There is substantial
overlap in values between men with benign and
malignant prostate disease. Hence, elevated PSA
levels should be interpreted with caution.
For patients with abnormal DRE, checking PSA
can increase the detection rate of prostate cancer.
However, for patients with normal DRE, PSA should
be checked only when the detection of prostate cancer
will cause the disease’s management to be modified.
In general, in patients with life expectancy of <10
years or with multiple co-morbidities, checking of
PSA to detect prostate cancer might not be beneficial
to the patient and should only be performed with
special justification after proper counselling.
Serum creatinine
Assessment of renal function should be considered in
patients with high risk of renal impairment (eg, those
with multiple co-morbidities and polypharmacy).
Treatment
The majority of patients with LUTS have slow
progression of symptoms, with fewer than 2%
developing urinary retention and fewer than 10%
requiring BPH surgery over a 5-year period.4
Patients not bothered by their symptoms can be
safely managed conservatively with education and
lifestyle changes.6 Examples of lifestyle changes
include reduction of fluid intake before bedtime to
lessen nocturia, avoidance of caffeinated beverages
or alcohol to reduce frequency and urgency, urethral
milking to prevent post-micturition dribbling, and
optimising the timing of medication, especially
diuretics.
In addition to education and lifestyle changes,
medical treatment can be considered for patients
with bothersome symptoms. Voiding symptoms
can be regarded as the manifestation of underlying
bladder outlet obstruction resulting from BPH,
which underpins the rationale of using alpha-1
adrenoceptor antagonists (α1-blockers). Storage
symptoms can be attributed to either underlying
obstruction-induced change in bladder function
or overactive bladder without bladder outlet
obstruction. The choice of agent depends on the
predominant type of symptoms (ie, voiding vs
storage symptoms). For patients with predominant
voiding symptoms, the first-line medical treatment
is α1-blockers, which have been shown to improve
both voiding and storage symptoms.7 For patients
with predominant storage symptoms or residual
storage symptoms after a trial of α1-blockers,
antimuscarinics and beta-3 adrenoceptor agonist (β3
agonist) can be considered. For patients with large
prostate (eg, >40 cc), 5ARi can be used to reduce the
prostate size, improving symptoms and preventing
disease progression in terms of acute urinary
retention and future need of BPH surgery. It is
important to consider adverse effects before starting
medical treatment, especially in older patients with
multiple co-morbidities and polypharmacy.
Surgical treatment can be considered for
patients who develop BPH complications (eg, urinary
retention, bladder stones, obstructive uropathy,
recurrent urinary tract infection, haematuria) or
symptoms refractory to medical treatment. However,
surgery is associated with potential morbidities and
mortality, especially in frail geriatric patients.
Frailty is a syndrome characterised by reduced
physiological reserve and increased vulnerability
to adverse outcomes. Even minor stressor events
such as surgery can trigger disproportionate worsening of health status in frail elderly people.
The most frequently used model to identify frailty
is the phenotype described by Fried et al8 in 2001,
which comprises five variables: unintentional
weight loss, self-reported exhaustion, low energy
expenditure, slow gait speed, and weak grip
strength. The definition of polypharmacy has no
universally agreed cut-off point with regard to the
number of medications. Different researchers have
arbitrarily chosen various cut points. In the late
1990’s, the United States Centers for Medicare and
Medicaid Services implemented a quality indicator
measure that targets patients taking nine or more
concurrent medications. An alternative definition of
polypharmacy is the use of more medications than
are medically necessary.9
After commencement of medical therapy,
apart from the monitoring of treatment response
and adverse drug reactions, it is also crucial to review
medical conditions and identify the new occurrence
of geriatric red flags (eg, frailty, polypharmacy)
as patients age. The consensus algorithm on male
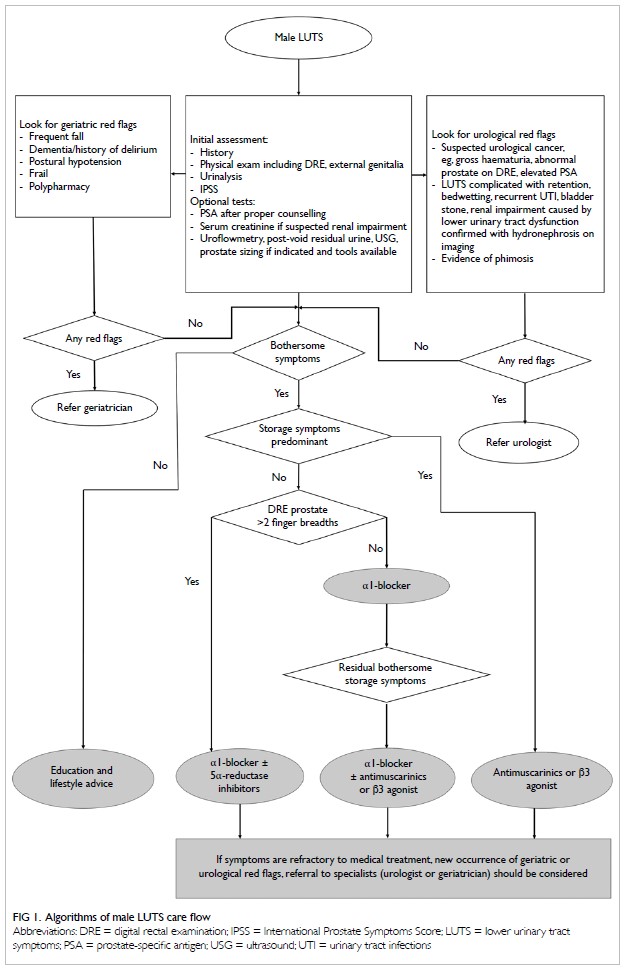
LUTS care flow in the primary care setting by HKGS
and HKUA is outlined in Figure 1.
Alpha-1 adrenoceptor antagonists
The use of α1-blockers has been shown to be
effective at reducing LUTS associated with BPH.5
The α1-blockers relax smooth muscle tone at the
bladder neck and prostate by blocking the action
of endogenously released noradrenaline.10 They are
usually considered as the first-line therapy for male
LUTS because of their good efficacy on symptomatic
relief but do not alter the natural progression of the
disease.
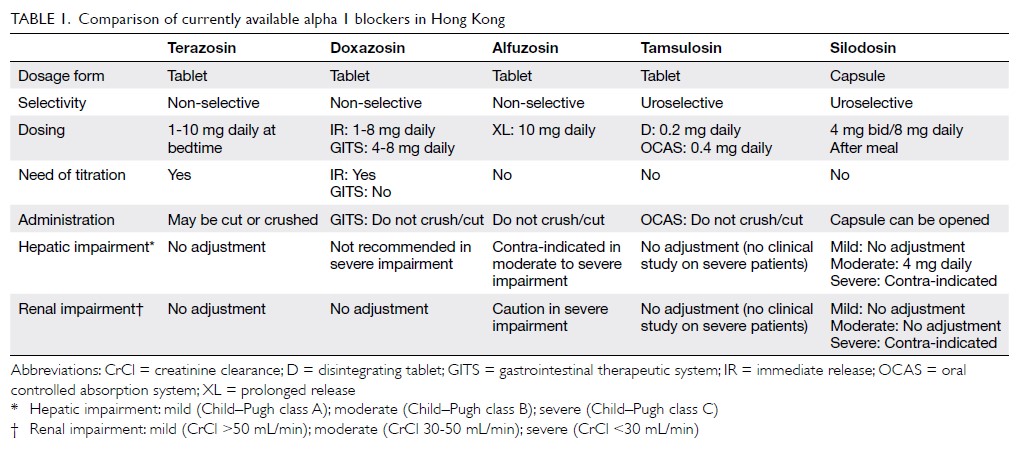
Currently available α1-blockers include
prazosin, terazosin, doxazosin, alfuzosin, tamsulosin
and silodosin. They have different uroselectivity,
pharmacokinetic properties, and formulations
(Table 1). Prazosin is a short-acting drug that requires
multiple dosing schedules and was the earliest drug
to be used for treatment of BPH. However, the
2003 American Urological Association Guidelines
concluded that there was insufficient support for
recommending prazosin as a treatment option for
LUTS secondary to BPH.11 These and the European
Association of Urology Guidelines regard prazosin
as a nonstandard treatment.
Although different α1-blockers have similar
efficacy in improving symptoms and uroflow
at appropriate doses, uroselective agents (α-1A
blockers) and long-acting preparations appeared
to be better tolerated. The differences between the
tolerability of various α1-blockers can be explained
by the differences in the expression and distribution
of receptor subtypes (alpha 1A and 1B) in the body
(Fig 2).
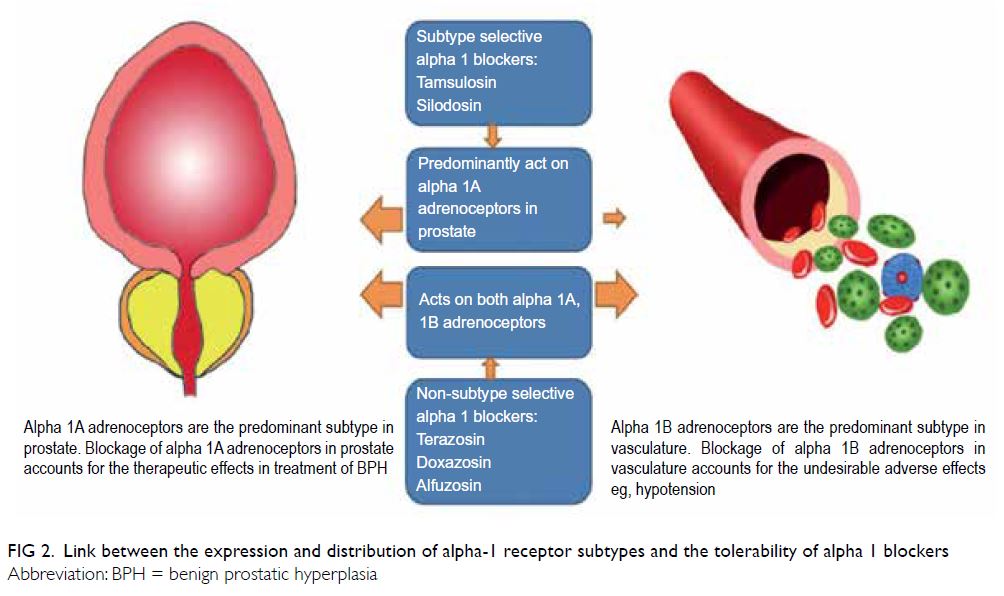
Figure 2. Link between the expression and distribution of alpha-1 receptor subtypes and the tolerability of alpha 1 blockers
Major adverse effects of α1-blocker use
include dizziness, asthenia, postural hypotension,
and syncope, which can result in falling (odds ratio
[OR]=1.14) and fractures (OR=1.16), especially in
elderly people,12 the majority of whom cannot tolerate
these drugs at the higher adult dose range. Studies
have consistently demonstrated that uroselective
agents including tamsulosin and silodosin have the
least effect on blood pressure and the lowest risk of
developing vascular-related events.7 13 14 However, a
minor but significant change in blood pressure and heart rate was observed with tamsulosin, whereas no
significant change was demonstrated with silodosin
compared with placebo in a randomised controlled
trial.15
There have been reports concerning the
association of α1-blockers (especially tamsulosin)
with intraoperative floppy iris syndrome,16 leading
to a high rate of complications during cataract
surgery, and it is suggested that ophthalmologists
be reminded so that they can take precautions.
Sexually active patients should be informed of the adverse effect of abnormal ejaculation, which was
another adverse reaction more commonly related
with tamsulosin (OR=8.58) and silodosin (OR=32.5),
and patients should be informed of the potential
implications.17
Patients who are naïve to α-blockers may
develop postural hypotension, known as the “first
dose phenomenon,” which is more pronounced with
non-selective α1-blockers during the first 8 weeks of
treatment. However, it should not be overlooked with
uroselective agents, and special precautions should
be taken, especially in elderly patients. In addition,
swallowing difficulties are not uncommon in elderly
patients, in whom modified release preparations are
inappropriate.
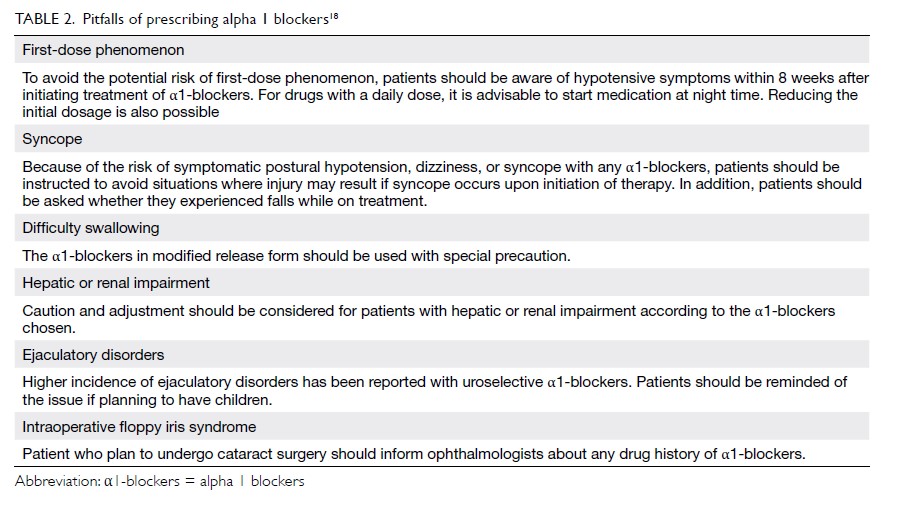
The pitfalls of prescribing α1-blockers are
summarised in Table 2.18 Their most troublesome
adverse effect is postural hypotension. The
situation is even more complicated if the patient
has concomitant hypertension or is taking multiple
medications with hypotensive effects for various
indications. It is estimated that more than 25% of
men aged >60 years have concomitant BPH and
hypertension,19 which poses a significant challenge
in the prescription of α1-blockers. Non-selective
α1-blockers have been available as antihypertensive
agents for over 40 years. They reduce blood pressure
by blocking postsynaptic alpha (mainly alpha-1B)
receptors, thereby inhibiting noradrenaline release
that induces vasoconstriction, resulting in dilatation
of arterioles and venules. Among all α1-blockers,
prazosin, terazosin, and doxazosin are approved for
the management of hypertension, whereas alfuzosin,
tamsulosin, and silodosin have minimal effect on
blood pressure.
Because certain α1-blockers are approved
treatments for hypertension, they are a reasonable
choice for treatment of hypertensive men with
LUTS. However, with advances in hypertensive
treatment over past decades, the role of α1-blockers
in this context has changed, especially after the
introduction of uroselective agents. A consensus
was reached regarding revision of the use of
available safety data on α1-blockers in patients with
hypertension (Table 3).20 21 22 23 24 25 26 27 28 29
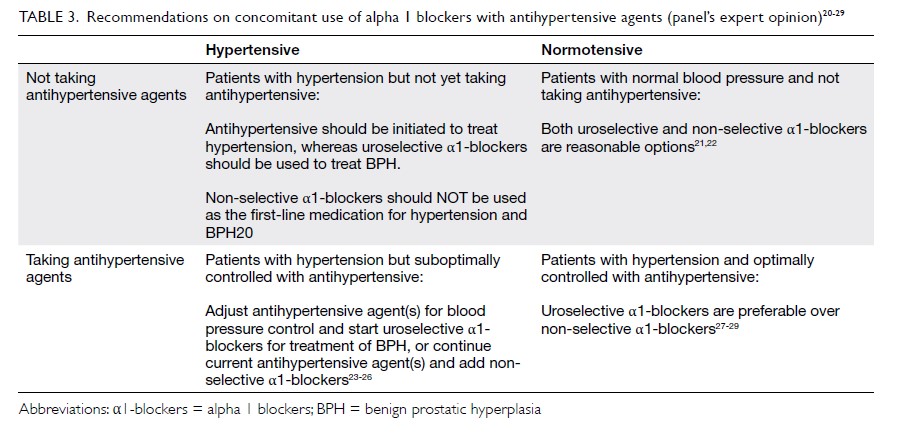
Table 3. Recommendations on concomitant use of α1-blockers with antihypertensive agents (panel’s expert opinion)20 21 22 23 24 25 26 27 28 29
Patients with hypertension but not yet taking
antihypertensive
For hypertensive LUTS patients who are not taking
any antihypertensives, we do not recommend the
use of non-selective α1-blockers for treatment
of BPH and hypertension together (first-line
treatment of hypertension). This recommendation
is based on the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
Study.20 That study showed that compared with
chlorthalidone (diuretics), the use of doxazosin is
associated with significantly higher risk of stroke,
congestive heart failure, peripheral vascular disease,
angina, and cardiovascular disease requiring
coronary revascularisation. Multiple guidelines (The
Eighth Joint National Committee (JNC8) guideline30,
the European Society of Cardiology/the European
Society of Hypertension guideline31, the American
College of Cardiology/American Heart Association
guideline32 and Hypertension Canada’s 2017
guideline for diagnosis33) do not recommend α1-blockers as the first-line therapy for hypertension.
Therefore, we recommended treating LUTS with uroselective agents, and hypertension should be
treated with another class of antihypertensives
according to existing hypertension guidelines.
Patients with hypertension that
is suboptimally controlled with antihypertensive
For hypertensive LUTS patients who have
suboptimal blood pressure control but are taking
antihypertensive treatment, the addition of non-selective
α1-blockers (doxazosin gastrointestinal
therapeutic system [GITS] and terazosin) for
treatment of hypertension as second- or third-line
agents seems to be a reasonable option for achieving
optimal blood pressure control.23 24 25 26 However,
postural hypotension is a significant concern in the
treatment group. Therefore, although non-selective
α1-blockers are effective at reducing blood pressure
as add-on therapy, the risks and benefits of this
approach should be balanced and individualised.
This is the case especially when other classes of
antihypertensives may have additional benefits in
certain patients in whom treatment of LUTS with
uroselective agents and hypertension separately
with another class of antihypertensive agents might
be advisable.
Patients with normal blood pressure and not
taking antihypertensive
Among normotensive male patients aged >40 years
with LUTS who were not taking antihypertensives,
a multicentre study showed that doxazosin GITS
improved the International Prostate Symptoms
Score and the Quality of Life index with minimal effect on blood pressure.21 Another review of
α1-blockers’ effects on blood pressure showed
no significant changes in blood pressure in
normotensive patients irrespective of the type of
α1-blockers used (tamsulosin, alfuzosin, doxazosin
GITS, or terazosin).22 Therefore, it is reasonable to
consider either non-selective or uroselective α1-blockers in this group.
Patients with hypertension that is optimally
controlled with antihypertensive
The safety data on non-selective α1-blockers
in normotensive LUTS patients who are taking
antihypertensives with optimal blood pressure
control are largely based on post-hoc analysis of
randomised controlled trials assessing standard
versus intensive blood pressure control, which
involves non-selective α1-blockers as third- or
fourth-line antihypertensives. In the Action to
Control Cardiovascular Risk in Diabetes trial,
which involved 10 251 high-risk participants
with type 2 diabetes mellitus at 77 centres, non-selective
α1-blockers were significantly associated
with postural hypotension, which was associated
with higher mortality and rates of heart failure
and hospitalisation.27 Another European study,
the Systolic Blood Pressure Intervention trial,
which involved 9361 patients with increased
cardiovascular risk but without diabetes, found that
non-selective α1-blockers are associated with higher
risk of syncope and falling, although no significant
hypotensive events were demonstrated.28 Therefore,
we recommend the use of uroselective agents for
management of LUTS in this group of patients.
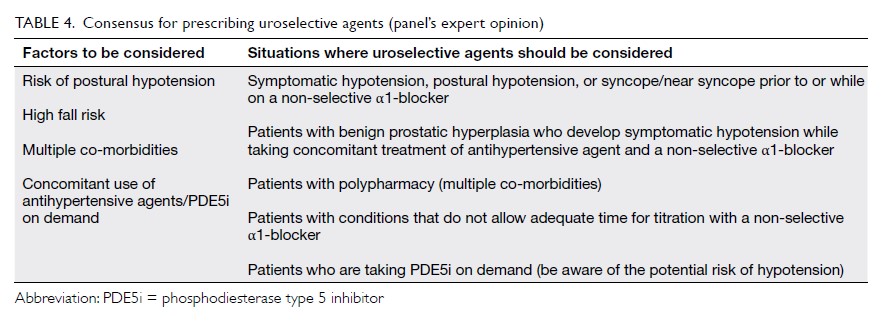
Apart from the risk of postural hypotension, the selection between non-selective and uroselective
agents should also be based on other factors,
including the risk of falling, polypharmacy, co-morbidities,
and specific situations where the use of
uroselective agents is advisable (Table 4).
Nevertheless, α1-blockers are effective
in relieving LUTS and improving quality of
life for patients with BPH. However, treatment
decisions should be individualised and based on
comprehensive assessment of patients with different
needs, especially in frail elderly patients, as they tend
to have accumulated co-morbidities, disabilities, and
polypharmacy that often interact with each other.
Antimuscarinics
Antimuscarinics are commonly used as
pharmacological treatments for overactive bladder.
This class of drug can also be used in predominant
or mixed storage LUTS. These drugs increase
bladder capacity and reduce urgency by blockading
the muscarinic receptor during bladder storage.34
Antimuscarinics have shown a modest benefit
over placebo in reducing urgency incontinence in
women.35 36 The efficacy of all the antimuscarinics
is similar.37 However, there is lack of head-to-head
comparison, and not all antimuscarinics
have been tested in elderly men. These drugs
often require higher doses to achieve the optimal
effects, and we recommend starting with the lowest
dose and titrating up as needed if the patient has
insufficient response and minimal adverse effects.
Antimuscarinics should be avoided if the patient
has clinically palpable bladder. These drugs can be
associated with increased post-void residual urine
volume after therapy, but acute retention is rare.5
Follow-up is recommended at 4 to 6 weeks to assess
therapeutic response and determine whether a
change in medication is necessary. Men should be
advised to discontinue medication if they develop
voiding difficulty, urinary infection, or worsening LUTS after initiation of therapy.
All antimuscarinics exert peripheral
anticholinergic effects that may limit drug tolerability
and dose escalation.35 Common adverse events
include dry mouth (up to 16%), constipation (up to
4%), dizziness (up to 5%), micturition difficulty (up
to 2%), blurred vision for near objects, tachycardia,
drowsiness, and worsened cognitive function.5 Up to
two-thirds of patients discontinue these medications
beyond 1 year.38 Constipation and compensatory
fluid intake for dry mouth may exacerbate urinary
incontinence. Patients with dementia are more
vulnerable to the adverse effects of antimuscarinics.39 40
Antimuscarinics should be avoided in patients
with uncontrolled tachyarrhythmia, myasthenia
gravis, and narrow angle-closure glaucoma. The
adverse effects of antimuscarinics can be explained
by the distribution of muscarinic acetylcholine
receptor subtypes throughout the body (Fig 3). The differences in tolerability between antimuscarinics
can be explained by their differences in selectivity
for receptor subtypes and tissue penetration.
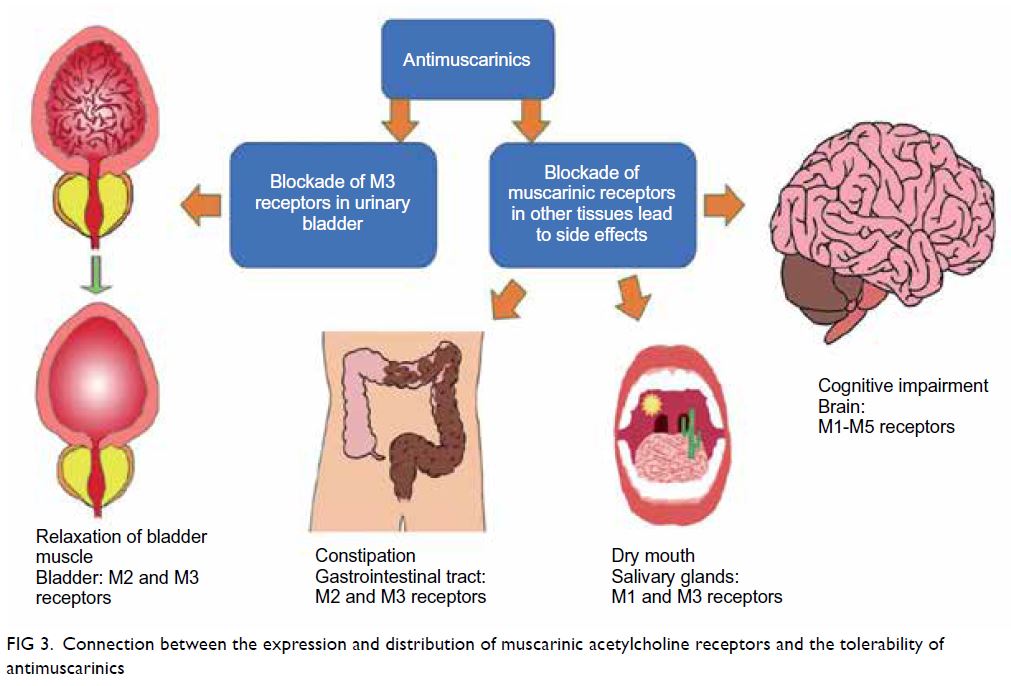
Figure 3. Connection between the expression and distribution of muscarinic acetylcholine receptors and the tolerability of antimuscarinics
Antimuscarinics may have additive adverse
effects when combined with other medications that
have strong anticholinergic effects. They should be
used with caution or preferably avoided if elderly
patients are concomitantly taking other medications
with high anticholinergic potency, eg, first-generation
H1 antihistamines (chlorpheniramine,
hydroxyzine, diphenhydramine), anti-Parkinson’s
drugs (benztropine, trihexyphenidyl), spasmolytics
(atropine, hyoscine), anti-emetics (promethazine),
muscle relaxants, antipsychotics (chlorpromazine,
fluphenazine, trifluoperazine, clozapine), and tricyclic
antidepressants (amitriptyline, clomipramine,
doxepin, imipramine, nortriptyline).41 42 43
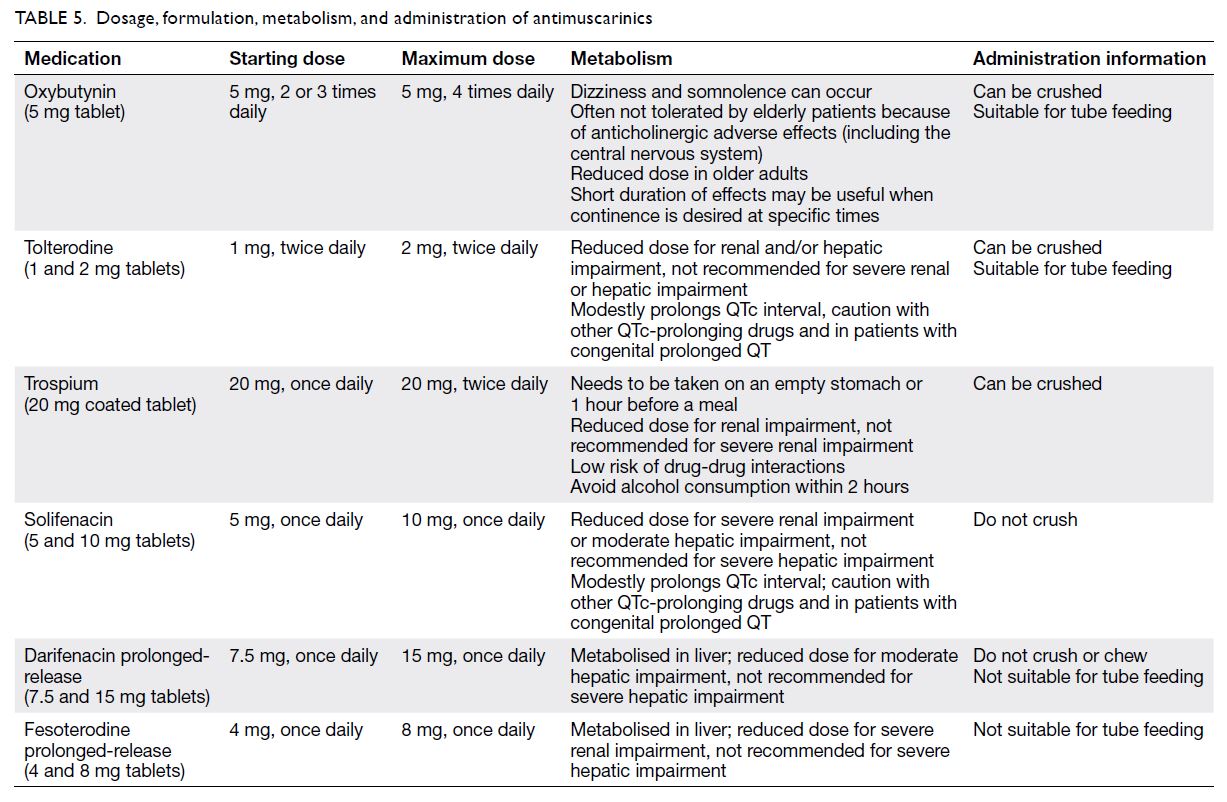
The antimuscarinics registered in Hong
Kong include oxybutynin, solifenacin, tolterodine,
trospium, darifenacin, and fesoterodine. Solifenacin,
darifenacin, and trospium may have less impact on
the central nervous system (Table 5).
Beta-3 adrenoceptor agonist
Beta-3 agonist is a new class of pharmacological
treatment used to relieve storage symptoms (urgency,
urinary frequency, and urge urinary incontinence)
associated with overactive bladder. It acts by binding
to the β3 adrenergic receptors on the bladder smooth
muscle causing bladder relaxation during the storage
phase. Mirabegron is currently the only approved β3
agonist for treatment of overactive bladder.
ln a phase III clinical trial, mirabegron 50 mg
daily resulted in a 50% reduction in the number of
urgency episodes per 24 hours and a 128% increase in
the mean volume voided per micturition compared
with placebo.44 Unlike antimuscarinics, it has
better tolerability with less dry mouth. In the study,
mirabegron’s incidence of dry mouth was similar to
that of placebo.45
Mirabegron has no influence on bladder
contraction during the voiding phase. In the clinical
trial, the incidence rate of acute urinary retention
was the lowest in mirabegron-treated patients
compared with the tolterodine and placebo groups
(0.1%, 0.6%, and 0.2%, respectively).44 The same trial
showed that mirabegron did not increase intraocular
pressure, and it is therefore not contra-indicated in
patients with glaucoma.
Regarding cardiovascular safety, the review
and real-world data on mirabegron did not show
any increased risk compared with conventional
antimuscarinics or in those with coexisting
cardiovascular disease.46 47 The European Association
of Urology guideline recommends β3 agonist as
a first-line medication for men with moderate-to-severe LUTS who have predominantly bladder
storage symptoms.48
Beta-3 agonist can be considered when
antimuscarinic adverse effects and high
anticholinergic burden are concerns, especially in
elderly adults with multiple co-morbidities and
cognitive impairment. Several studies have shown
that mirabegron is safe and effective in older
patients.49 50 51
The recommended dosage of mirabegron is
50 mg daily. For patients with renal impairment
(estimated glomerular filtration rate 15-29 mL/min/1.73 m2), Child–Pugh class B liver impairment, and
those aged ≥80 years with multiple co-morbidities,
25 mg daily should be considered. Mirabegron is not
recommended in patients with poorly controlled
hypertension (systolic blood pressure >180 mm Hg
or diastolic blood pressure >110 mm Hg), severe
renal impairment (estimated glomerular filtration
rate <15 mL/min/1.73 m2), or Child–Pugh class C
liver impairment. The most common adverse effects
reported were hypertension, nasopharyngitis, and
headache but the overall adverse event rates were
similar to those with placebo.52
5α-reductase inhibitors
5α-reductase is responsible for conversion of
testosterone to dihydrotestosterone, which has
an important role in prostate growth and the
development of BPH.53 There are two isoforms of
5α-reductase: type 1—the predominant enzyme in
extraprostatic tissue such as skin and liver; and type
2—the predominant enzyme in prostate (>90%),
which is critical to development of BPH.
The 5ARi drugs inhibit conversion of
testosterone to dihydrotestosterone, inducing
apoptosis and atrophy of prostatic epithelial cells.54
It results in reduction of prostate volume and hence
relief of bladder outflow obstruction. There are two
types of 5ARi: finasteride, which acts only on type
2 5α-reductase, and dutasteride, which acts on both
types. Meta-analysis has shown no differences in
efficacy or safety among these two drugs.55 56 There
are a few registered 5ARi drugs: Proscar (finasteride
5 mg), Avodart (dutasteride 0.5 mg), and Duodart
(combination of dutasteride 0.5 mg and tamsulosin
0.4 mg).
Long-term 5ARi treatment in patients with
moderate to severe LUTS and prostate volume
>40 cc has been shown to reduce the symptoms
score, risk of urinary retention, and risk of BPH-related
surgery. In a landmark study, patients taking
finasteride had improvement in symptoms and
uroflow, their prostate size reduced by 20%, their
risk of acute urinary retention reduced by 57%, and
their risk of BPH-related surgery reduced by 55%
compared with placebo after 4 years of treatment.56
There are some practical tips for prescribing
5ARi. First, the patient should have an enlarged
prostate >40 cc on ultrasound imaging. If ultrasound
is not readily available, it is acceptable to start 5ARi
treatment when the prostate size is greater than two
finger breadths on DRE. Second, it is important to
inform the patient that 5ARi have a slow onset of
action (3-6 months), as time is required for prostate
volume reduction. Continuous long-term treatment
should be expected. Third, the effects of 5ARis on PSA
levels should be explained to patients. The PSA level
is expected to be reduced by 50% after 6 to 12 months
of treatment,56 and therefore, good drug compliance
is required for proper interpretation of the PSA
level in prostate cancer screening. A persistent PSA
rise from the nadir in a patient on long-term 5ARi
treatment is an indicator for prostate biopsy, and
urological referral should be considered.57 Finally,
although some studies have suggested a higher
incidence of high-grade prostate cancer in patients
taking long-term 5ARi, no causal relationship has
been proven, and there is no difference in long-term
survival.58 The common adverse effects are
sexual dysfunction, such as decreased libido, erectile
dysfunction, and ejaculatory problems in around 4% to 8% and breast enlargement and tenderness in 1% of patients.56
Conclusion
Male LUTS is a common presentation to primary
care practitioners. Focused history and physical
examination are essential to differentiate BPH
from other causes of male LUTS and to guide its
management. Patients with minimal symptoms can
be managed conservatively, and pharmacological
treatments can be considered if symptoms are
bothersome. For patients with symptoms refractory
to pharmacological treatments or who have
complications (eg, urinary retention, obstructive
uropathy), surgical intervention can be performed
after assessment by urologists. With an ageing
population, geriatricians are adopting an increasing
role in the management of male patients with
LUTS in the era of multiple co-morbidities and
polypharmacy, as these patients are at higher risk
of adverse effects from pharmacological treatments
and are not optimal for surgical intervention. A
consensus has been reached by the HKGS and HKUA
regarding the diagnosis, evaluation, management,
and referral mechanism for LUTS in the primary
care setting. With collaboration between primary
care practitioners, geriatricians and urologists, we
hope that more holistic care can be provided to male
patients with LUTS in Hong Kong.
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgements
We thank Dr CH Cheng for drawing the images in Figures 2 and 3; and Dr CW Man for his assistance in editing the
manuscript.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
1. United Nations Development Programme. Human
Development Report 2019. Beyond income, beyond
averages, beyond today: inequalities in human development
in the 21st century. 2019. Available from: http://hdr.undp.org/en/content/human-development-report-2019. Accessed 7 Jul 2020.
2. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development
of human benign prostatic hyperplasia with age. J Urol
1984;132:474-9. Crossref
3. van den Akker M, Buntinx F, Metsemakers JF, Roos S,
Knottnerus JA. Multimorbidity in general practice:
prevalence, incidence, and determinants of co-occurring
chronic and recurrent diseases. J Clin Epidemiol
1998;51:367-75. Crossref
4. Ball AJ, Feneley RC, Abrams PH. The natural history of untreated “prostatism”. Br J Urol 1981;53:613-6. Crossref
5. Gravas S, Cornu JN, Gacci M, et al. EAU Guidelines on management of non-neurogenic male LUTS. Arnhem, The Netherlands: EAU Guidelines Office; 2020.
6. Brown CT, Yap T, Cromwell DA, et al. Self management for men with lower urinary tract symptoms: randomised
controlled trial. BMJ 2007;334:25. Crossref
7. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in
patients with lower urinary tract symptoms suggestive of
benign prostatic obstruction. Eur Urol 1999;36:1-13. Crossref
8. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci
Med Sci 2001;56:M146-56. Crossref
9. Tjia J, Velten SJ, Parsons C, Valluri S, Briesacher BA. Studies to reduce unnecessary medication use in frail older
adults: a systematic review. Drugs Aging 2013;30:285-307. Crossref
10. Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate.
Br J Pharmacol 2006;147 Suppl 2:S88-119. Crossref
11. AUA Practice Guidelines Committee. AUA guideline
on management of benign prostatic hyperplasia (2003).
Chapter 1: Diagnosis and treatment recommendations. J
Urol 2003;170:530-47. Crossref
12. Welk B, McArthur E, Fraser LA, et al. The risk of fall
and fracture with the initiation of a prostate-selective
α antagonist: a population based cohort study. BMJ
2015;351:h5398. Crossref
13. Djavan B, Chapple C, Milani S, Marberger M. State of the
art on the efficacy and tolerability of alpha1-adrenoceptor
antagonists in patients with lower urinary tract symptoms
suggestive of benign prostatic hyperplasia. Urology
2004;64:1081-8. Crossref
14. Morgia G. Does the use of silodosin to treat benign
prostatic hyperplasia really offer something new? Eur Urol
2011;59:353-5. Crossref
15. Chapple CR, Montorsi F, Tammela TL, et al. Silodosin
therapy for lower urinary tract symptoms in men with
suspected benign prostatic hyperplasia: results of an
international, randomized, double-blind, placebo- and
active-controlled clinical trial performed in Europe. Eur
Urol 2011;59:342-52. Crossref
16. Storr-Paulsen A, Nørregaard JC, Børme KK, Larsen AB,
Thulesen J. Intraoperative floppy iris syndrome (IFIS): a
practical approach to medical and surgical considerations
in cataract extractions. Acta Ophthalmol 2009;87:704-8. Crossref
17. Gacci M, Ficarra V, Sebastianelli A, et al. Impact of medical
treatments for male lower urinary tract symptoms due
to benign prostatic hyperplasia on ejaculatory function:
a systematic review and meta-analysis. J Sex Med
2014;11:1554-66. Crossref
18. United States Department of Veterans Affairs. Pharmacy
Benefits Management Services, Medical Advisory Panel,
VISN Pharmacist Executives. Alfuzosin, silodosin, tamsulosin/clinically uroselective alpha1-adrenergic
blockers: recommendations for use. 2010. Available
from: https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/Alpha_Blockers_(Clinically_Uroselective_Alfuzosin_Silodosin_Tamsulosin)_Clinical_Recommendations.pdf. Accessed 27 Nov 2019.
19. Boyle P, Napalkov P. The epidemiology of benign
prostatic hyperplasia and observations on concomitant
hypertension. Scand J Urol Nephrol Suppl 1995;168:7-12. Crossref
20. ALLHAT Collaborative Research Group [editorial]. Major
cardiovascular events in hypertensive patients randomized
to doxazosin vs chlorthalidone: the antihypertensive and
lipid-lowering treatment to prevent heart attack trial
(ALLHAT). JAMA 2000;283:1967-75. Crossref
21. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of
cardiovascular events with an antihypertensive regimen of
amlodipine adding perindopril as required versus atenolol
adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure
Lowering Arm (ASCOT-BPLA): a multicentre randomised
controlled trial. Lancet 2005;366:895-906. Crossref
22. Lowe FC, Olson PJ, Padley RJ. Effects of terazosin therapy
on blood pressure in men with benign prostatic hyperplasia
concurrently treated with other antihypertensive
medications. Urology 1999;54:81-5. Crossref
23. Chung BH, Hong SJ. Long-term follow-up study to evaluate
the efficacy and safety of the doxazosin gastrointestinal
therapeutic system in patients with benign prostatic
hyperplasia with or without concomitant hypertension.
BJU Int 2006;97:90-5. Crossref
24. Lee SH, Park KK, Mah SY, Chung BH. Effects of α-blocker
‘add on’ treatment on blood pressure in symptomatic
BPH with or without concomitant hypertension. Prostate
Cancer Prostatic Dis 2010;13:333-7. Crossref
25. Black HR, Keck M, Meredith P, Bullen K, Quinn S, Koren A.
Controlled-release doxazosin as combination therapy
in hypertension: the GATES study. J Clin Hypertens
(Greenwich) 2006;8:159-66. Crossref
26. de Alvaro F, Hernández-Presa MA, ASOCIA Study.
Effect of doxazosin gastrointestinal therapeutic system
on patients with uncontrolled hypertension: the ASOCIA
Study. J Cardiovasc Pharmacol 2006;47:271-6. Crossref
27. Fleg JL, Evans GW, Margolis KL, et al. Orthostatic
hypotension in the ACCORD (Action to Control
Cardiovascular Risk in Diabetes) blood pressure trial:
prevalence, incidence, and prognostic significance.
Hypertension 2016;68:888-95. Crossref
28. SPRINT Research Group; Wright JT Jr, Williamson JD, et al.
A randomized trial of intensive versus standard blood-pressure
control. N Engl J Med 2015;373:2103-16. Crossref
29. Hiremath S, Ruzicka M, Petrcich W, et al. Alpha-blocker use and the risk of hypotension and hypotension-related
clinical events in women of advanced age. Hypertension
2019;74:645-51. Crossref
30. James PA, Oparil S, Carter BL, et al. 2014 evidence-based
guideline for the management of high blood pressure
in adults: report from the panel members appointed to
the Eighth Joint National Committee (JNC 8). JAMA
2014;311:507-20. Crossref
31. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH
Guidelines for the management of arterial hypertension:
The Task Force for the management of arterial
hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for
the management of arterial hypertension of the European
Society of Cardiology and the European Society of
Hypertension. J Hypertens 2018;36:1953-2041. Crossref
32. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/
AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection,
Evaluation, and Management of High Blood Pressure in
Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines. Hypertension 2018;71:e13-e115. Crossref
33. Leung AA, Daskalopoulou SS, Dasgupta K, et al.
Hypertension Canada’s 2017 Guidelines for Diagnosis, Risk
Assessment, Prevention, and Treatment of Hypertension
in Adults. Can J Cardiol 2017;33:557-76. Crossref
34. Finney SM, Andersson KE, Gillespie JI, Stewart LH.
Antimuscarinic drugs in detrusor overactivity and the
overactive bladder syndrome: motor or sensory actions?
BJU Int 2006;98:503-7. Crossref
35. Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane
RL. Benefits and harms of pharmacologic treatment for
urinary incontinence in women: a systematic review. Ann
Intern Med 2012;156:861-74. Crossref
36. Reynolds WS, McPheeters M, Blume J, et al. Comparative
effectiveness of anticholinergic therapy for overactive
bladder in women: a systematic review and meta-analysis.
Obstet Gynecol 2015;125:1423-32. Crossref
37. Qaseem A, Dallas P, Forciea MA, et al. Nonsurgical
management of urinary incontinence in women: a
clinical practice guideline from the American College of
Physicians. Ann Intern Med 2014;161:429-40. Crossref
38. Brostrøm S, Hallas J. Persistence of antimuscarinic drug use. Eur J Clin Pharmacol. 2009;65:309-14. Crossref
39. Coupland CA, Hill T, Dening T, Morriss R, Moore M,
Hippisley-Cox J. Anticholinergic drug exposure and the
risk of dementia: a nested case-control study. JAMA Intern
Med 2019;179:1084-93. Crossref
40. Fox C, Richardson K, Maidment ID, et al. Anticholinergic
medication use and cognitive impairment in the older
population: the medical research council cognitive function
and ageing study. J Am Geriatr Soc 2011;59:1477-83. Crossref
41. Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J
Clin Pharmacol 2013;69:1485-96. Crossref
42. Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of
anticholinergic risk scales and associations with adverse
health outcomes in older people. J Am Geriatr Soc
2015;63:85-90. Crossref
43. American Geriatrics Society 2015 Beers Criteria Update
Expert Panel. American Geriatrics Society 2015 updated
Beers criteria for potentially inappropriate medication use
in older adults. J Am Geriatr Soc 2015;63:2227-46. Crossref
44. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy,
safety, and tolerability. Neurourol Urodyn 2014;33:17-30. Crossref
45. Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in
patients with overactive bladder: results from a randomised
European-Australian phase 3 trial. Eur Urol 2013;63:283-
95. Crossref
46. Rosa GM, Ferrero S, Nitti VW, Wagg A, Saleem T,
Chapple CR. Cardiovascular safety of β3-adrenoceptor
agonists for the treatment of patients with overactive bladder syndrome. Eur Urol 2016;69:311-23. Crossref
47. Katoh T, Kuwamoto K, Kato D, Kuroishi K. Real-world
cardiovascular assessment of mirabegron treatment
in patients with overactive bladder and concomitant
cardiovascular disease: results of a Japanese post-marketing
study. Int J Urol 2016;23:1009-15. Crossref
48. Burkhard FC, Bosch JL, Cruz F, et al. EAU Guidelines on
urinary incontinence in adults. Arnhem, The Netherlands:
EAU Guidelines Office; 2016.
49. Wagg A, Staskin D, Engel E, Herschorn S, Kristy RM,
Schermer CR. Efficacy, safety, and tolerability of mirabegron
in patients aged ≥65yr with overactive bladder wet: a phase
IV, double-blind, randomised, placebo-controlled study
(PILLAR). Eur Urol 2020;77:211-20. Crossref
50. Makhani A, Thake M, Gibson W. Mirabegron in the
treatment of overactive bladder: safety and efficacy in thehttps://doi.org/10.2147/CIA.S174402
very elderly patient. Clin Interv Aging 2020;15:575-81. Crossref
51. Lee YK, Kuo HC. Safety and therapeutic efficacy of
mirabegron 25 mg in older patients with overactive
bladder and multiple comorbidities. Geriatr Gerontol Int
2018;18:1330-3. Crossref
52. Tubaro A, Batista JE, Nitti VW, et al. Efficacy and safety of
daily mirabegron 50 mg in male patients with overactive
bladder: a critical analysis of five phase III studies. Ther Adv Urol 2017;9:137-54. Crossref
53. Carson C 3rd, Rittmaster R. The role of dihydrotestosterone
in benign prostatic hyperplasia. Urology 2003;61(4 Suppl
1):2-7. Crossref
54. Rittmaster RS, Norman RW, Thomas LN, Rowden G.
Evidence for atrophy and apoptosis in the prostates of men
given finasteride. J Clin Endocrinol Metab 1996;81:814-9. Crossref
55. Jun JE, Kinkade A, Tung AC, Tejani AM. 5α-reductase
inhibitors for treatment of benign prostatic hyperplasia: a
systematic review and meta-analysis. Can J Hosp Pharm
2017;70:113-9. Crossref
56. Roehrborn CG, Boyle P, Bergner D, et al. Serum prostatespecific
antigen and prostate volume predict long-term
changes in symptoms and flow rate: results of a four-year,
randomized trial comparing finasteride versus placebo.
PLESS Study Group. Urology 1999;54:662-9. Crossref
57. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC,
Roehrborn CG. The interpretation of serum prostate
specific antigen in men receiving 5alpha-reductase
inhibitors: a review and clinical recommendations. J Urol
2006;176:868-74. Crossref
58. Thompson IM Jr, Goodman PJ, Tangen CM, et al. Longterm
survival of participants in the prostate cancer
prevention trial. N Engl J Med 2013;369:603-10. Crossref