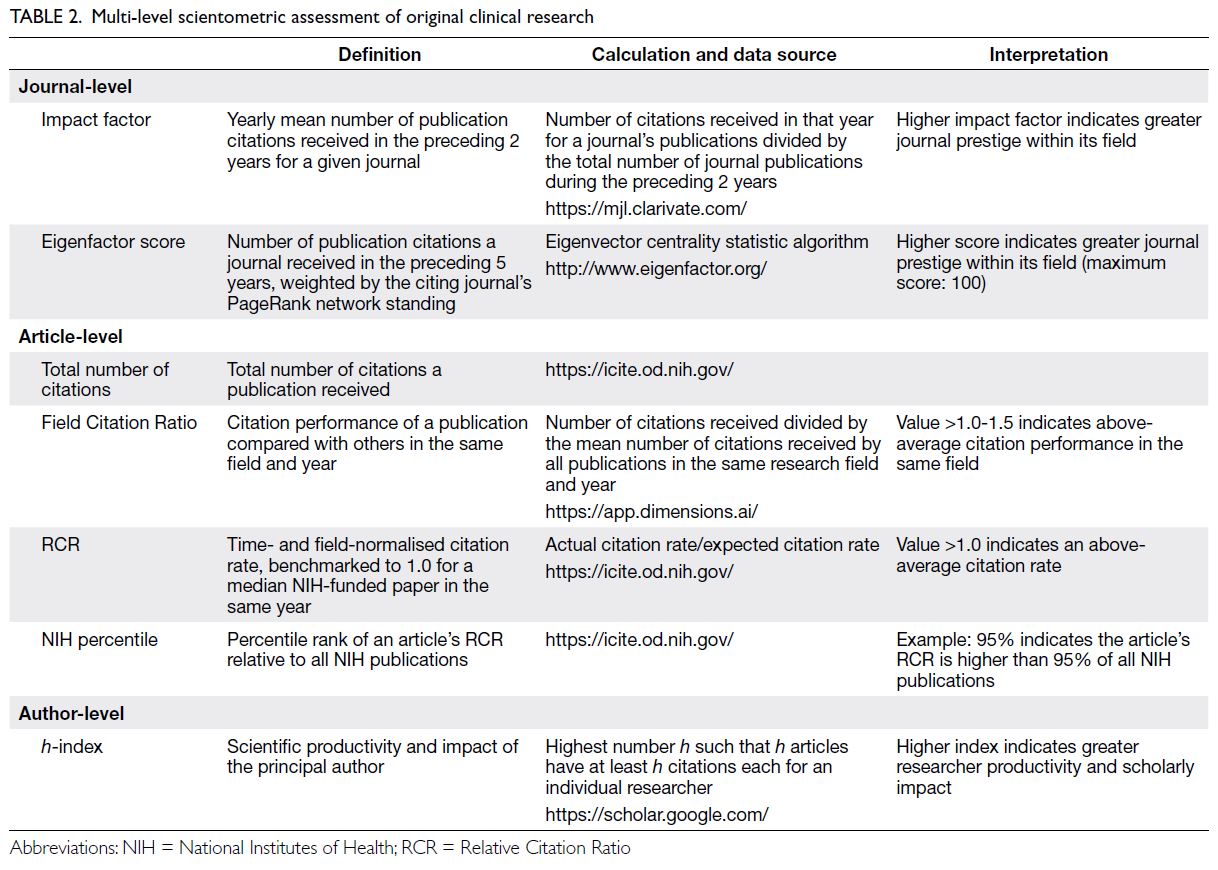
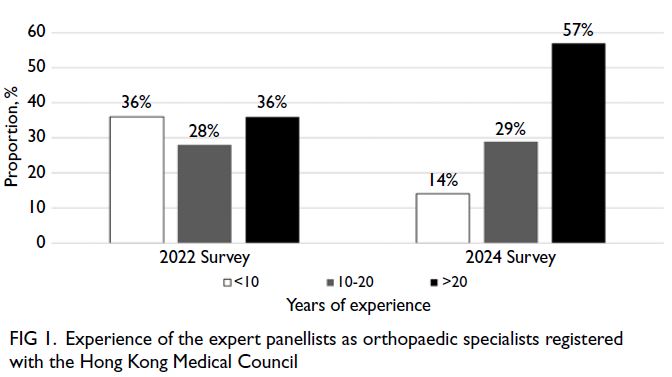
Parental depression in the relationship between parental stress and child health among lowincome families in Hong Kong
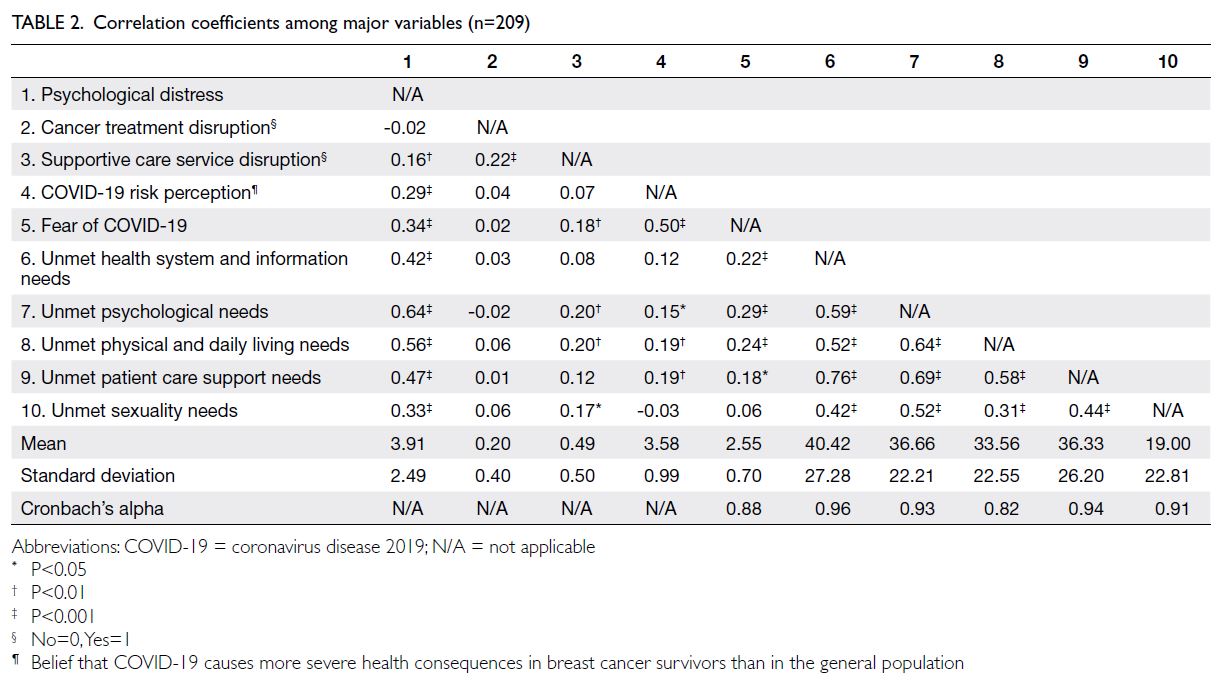
Hong Kong Med J 2025 Oct;31(5):374–83 | Epub 23 Sep 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Parental depression in the relationship between parental stress and child health among low-income families in Hong Kong
Esther YT Yu, FRACGP, FHKAM (Family Medicine)1; Eric YF Wan, PhD, CStat1,2; Rosa SM Wong, PhD3; Ivy L Mak, PhD1; Kiki SN Liu, PhD1; Caitlin HN Yeung, MB, BS, MPH1; Patrick Ip, FRCPCH, FHKAM (Paediatrics)4,5; Agnes FY Tiwari, PhD, FAAN6; Weng Y Chin, FRACGP1; Emily TY Tse, FRACGP, FHKAM (Family Medicine)1; Carlos KH Wong, PhD1,2,7; Vivian Y Guo, PhD8; Cindy LK Lam, MD, FHKAM (Family Medicine)1
1 Department of Family Medicine and Primary Care, The University of Hong Kong, Hong Kong SAR, China
2 Department of Pharmacology and Pharmacy, The University of Hong Kong, Hong Kong SAR, China
3 Department of Special Education and Counselling, The Education University of Hong Kong, Hong Kong SAR, China
4 Department of Paediatrics and Adolescent Medicine, The University of Hong Kong, Hong Kong SAR, China
5 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
6 School of Nursing, Hong Kong Sanatorium & Hospital, Hong Kong SAR, China
7 Laboratory of Data Discovery for Health Limited, Hong Kong Science Park, Hong Kong SAR, China
8 Department of Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China
Corresponding author: Dr Eric YF Wan (yfwan@hku.hk)
Abstract
Introduction: Low-income families face increased
exposure to stressors, including material hardship
and limited social support, which contribute to poor
health outcomes. The poor health and behavioural
problems in children from these families may
exacerbate parental stress. This study explored the
bidirectional relationship between parental stress
and child health, along with its mediators and
moderators, among low-income families in Hong
Kong.
Methods: In total, 217 families were recruited from
two less affluent communities between 2016 and
2017; they were followed up at 12 and 24 months.
Each parent-child pair was assessed using parent-completed
questionnaires on socio-demographics,
medical history, parental stress, health-related
quality of life, child health and behaviour, family
harmony, parenting style, and neighbourhood
cohesion.
Results: Thirty-eight parents (17.5%) reported
significantly higher levels of stress than the control
group. These individuals were more likely to be
single parents (41.2% vs 18.5%), victims of intimate
partner abuse (23.7% vs 10.9%), have a household
income below 50% of the Hong Kong population
median (50.0% vs 29.9%), and be diagnosed with
mental illnesses (23.7% vs 5.1%). A bidirectional
inverse relationship was observed between parental
stress and child health at respective time points,
with cross-effects from baseline child health to later
parental stress, and from baseline parental stress to
later child health. The relationship was mediated by
the level of parental depression.
Conclusion: Parental stress both precedes and
results from child health and behavioural problems, with reciprocal short-term and long-term effects.
Screening and intervention for parental depression
are needed to mitigate the impacts of stress on health
among parents and children.
New knowledge added by this study
- Single parents, victims of intimate partner abuse, individuals with mental illnesses, and/or those living in poverty reported significantly higher levels of stress compared to other low-income parents in Hong Kong.
- A bidirectional inverse relationship was observed between general parental stress and child health over a 24-month period among low-income families in Hong Kong.
- Parental depression mediated the relationship between parental stress and child health.
- Active screening for parental depression among at-risk parents in low-income communities is urgently needed to enable early intervention and reduce long-term negative impacts on child health.
Introduction
Low-income families face increased exposure to
stressors,1 2 such as material hardship, dispossession,
limited social support,3 4 trauma, and violence,1 5
which subsequently affect family relationships
and the physical and mental health of parents,6 7 8
contributing to household-wide feelings of
stigma, isolation, and exclusion. These stressors
are particularly relevant to Hong Kong, where
approximately one-fifth of the population lives
below the poverty line.9 Adults from low-income
families in Hong Kong have reported significantly
lower health-related quality of life (HRQOL) than
age- and sex-matched individuals from the general
population; low income is significantly associated
with poorer mental health.10
Stressors may persist across the life course
and affect the next generation, resulting in
intergenerational socio-economic inequality and
health disparities. Early caregiving experiences
have been linked to later-life child health outcomes
through physiological stress responses.11 Moreover,
poor mental health in parents may lead to family
disharmony and maladaptive parenting practices,
which can increase a child’s risk of adverse health
outcomes.7 8 Specifically, children of parents with depression tend to exhibit more difficult
temperaments and diminished psychosocial
functioning.12 13 Children from low-income families
in Hong Kong have reported poorer health and more
behavioural problems relative to population norms
for similar age-groups.14 15 Without adequate parental
care and guidance, such children may be more
vulnerable to academic difficulties and behavioural
problems, thereby exacerbating parental stress. A
bidirectional relationship between parental stress
and child health has been documented in Western
studies6 8 but not within the Chinese context.
Stress coping can be mediated or moderated
by various social factors.16 17 For instance, stressed
parents may contribute to family disharmony, which
mediates diminished child health. Neighbourhood
cohesion may moderate this relationship by
alleviating parental stress and enhancing children’s
well-being. The identification of mediators and
moderators that may influence the relationship
between parental stress and child health enables
development of targeted interventions and policy
recommendations. Despite strong associations of
parental depression with stress18 and child health,12 13
its mediating role in this relationship remains unclear.
A recent study demonstrated mediation between
parental stress and parent-infant bonding,19 but
evidence concerning overall child health is lacking.
This study aimed to explore whether a bidirectional
relationship exists between parental stress and child
health and to identify its mediators and moderators,
with the goal of promoting health among parents
and children from low-income families in Hong
Kong. We hypothesised that parental stress precedes
and results from child health, with mediating and
moderating effects exerted by factors illustrated in
Figure 1.
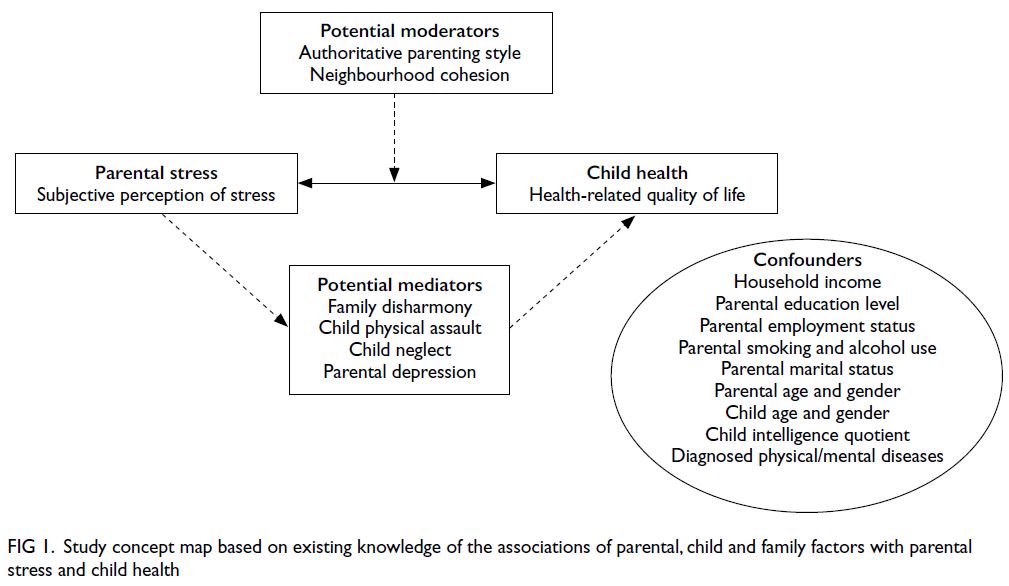
Figure 1. Study concept map based on existing knowledge of the associations of parental, child and family factors with parental stress and child health
Methods
Study design
This prospective cohort study involved 217 parent-child
pairs in which at least one parent was the
primary caregiver and at least one parent was
employed, with a monthly household income lower
than 75% of the Hong Kong median at baseline. This
income criterion included working poor families who
lived above the poverty line (50% of the population
median) and received limited government support.
Families were recruited by research staff when
attending health assessments during our previous
cohort study20 performed in two less affluent Hong
Kong communities between May 2016 and October
2017. Parents unable to communicate in Chinese,
as well as children born prematurely and/or with
congenital deformities, were excluded. All parents
provided written informed consent for themselves
and their child to participate in the study. Sample size was determined based on the need to detect
a difference in Child Health Questionnaire (CHQ)
scores between children of parents with high and low
stress levels, classified according to the Depression
Anxiety Stress Scales (DASS) stress subscale scores.
Our previous cohort study showed that average
CHQ general health perceptions subscale scores in
children of parents with high and low DASS stress
subscale scores were 59 (standard deviation [SD]=17)
and 65 (SD=16), respectively20 (effect size=0.4). A
sample size of 200 (100 per group) parent-child
pairs was required to detect a difference of 6 points
in CHQ general health perceptions subscale score
between groups using an independent t test with
80% power and a 5% level of significance.
Data collection
Each parent-child pair was invited to complete a
comprehensive questionnaire survey at three time
points (ie, baseline, 12 months, and 24 months)
covering parental stress, HRQOL, and mental health;
child’s general health, HRQOL, and behaviour;
family harmony; parenting style; and neighbourhood
cohesion, as reported by the parent. Potential
confounders were recorded at baseline, including
parental age, gender, education level, marital status,
employment status, household income, smoking
habits, and alcohol consumption, as well as the
child’s age, gender, estimated intelligence quotient,
and special education needs. Physical and mental co-morbidities
in parents and children were recorded at
all three time points.
Study instruments
Exposure
Parental stress was measured using the stress
subscale of the DASS–21 items questionnaire.21
A cut-off score of ≥15 indicated the presence of
significant parental stress.21 The scale has been validated in a Chinese population.22
Primary outcome
Child health was measured using the general health
perceptions subscale score from the CHQ–Parent
Form 50.23 A higher score indicates better perceived
physical and psychological HRQOL in the child
based on parental proxy report. The Chinese version
has demonstrated good psychometric properties in
local Chinese children.20
Potential mediators/moderators
The Patient Health Questionnaire–9 (PHQ-9)24 was
used to screen for parental depression. A cut-off
score of ≥10 was regarded as clinically significant
depression. The Chinese version of the PHQ-9 was
validated and used in our previous study.20 Family
harmony was measured using the Family Harmony
Scale–Short Form (FHS-5).25 Higher single-factor
harmony scores reflect greater harmony. The Chinese
version has demonstrated good psychometric
properties in local Chinese households.25 Parent-child
interaction was assessed using the Child
Physical Assault and Neglect subscales of the Parent–Child Conflict Tactics Scale (CTSPC).26 Higher scores indicate higher frequencies of respective
issues in the past 12 months. The translated
traditional Chinese version has demonstrated good
psychometric properties.27 Parenting style was assessed using the Authoritative Parenting Style
subscale of the short version of the Parenting Style
and Dimensions Questionnaire.28 A higher score
indicates a stronger tendency towards authoritative
parenting. The questionnaire has been validated
in the Chinese cultural setting.29 Neighbourhood
support was measured using the Neighbourhood
Collective Efficacy Scale.30 Higher scores indicate
greater neighbourhood cohesion. The scale has been
tested in Chinese in a local study.31
Data analysis
Baseline characteristics of parent-child pairs and
their households were summarised using descriptive
statistics. Differences between groups according to
parental stress level were assessed using independent
t tests for continuous variables and the Chi squared
test for categorical variables.
The longitudinal bidirectional relationship
between parental stress and child health was
assessed using a cross-lagged panel model. Multiple
indicators were utilised to evaluate model goodness-of-fit. A statistically non-significant Chi squared
P value, Comparative Fit Index and Tucker-Lewis
Index >0.95, root mean square error of approximation
≤0.05, and standardised root mean residual >0.08
were considered indicative of desirable goodness-of-fit. The final model was selected using root mean
square error of approximation–based forward
stepwise selection.
A mediation model was used to evaluate
candidate mediators. Model estimates were obtained
using 5000 bootstrapping samples. A statistically
significant indirect effect, along with a reduced direct
effect magnitude relative to the total effect, indicated
that a given mediator explained the relationship
between parental stress and child health.32 A multi-mediator
model was constructed; differences in
indirect effects between mediators were estimated
via pairwise comparison.
Potential moderating effects of neighbourhood
cohesion and parenting style on the relationship
between parental stress and child health were
examined by multivariable linear regression. A
statistically significant interaction term coefficient
indicated a moderation effect. All variables were
centred to a mean of zero to reduce multicollinearity
related to interaction terms. Confounders were
included to improve model goodness-of-fit; R2 and
adjusted R2 values were used to evaluate model
performance.
All descriptive analyses were performed using
Stata 16 (StataCorp LLC, College Station [TX],
US); all model analyses were carried out using the lavaan package33 version 0.6-6, in R version 4.0.1
(R Foundation for Statistical Computing, Vienna,
Austria). Data completion rates are presented
in online supplementary Table 1. Complete case
analyses were conducted. All tests were two-tailed; P
values <0.05 were considered statistically significant.
Results
Among the 217 parent-child pairs recruited at
baseline, 175 (80.6%) and 184 (87.6%) pairs attended
the 12-month and 24-month follow-ups, respectively
(online supplementary Fig 1). Their characteristics at
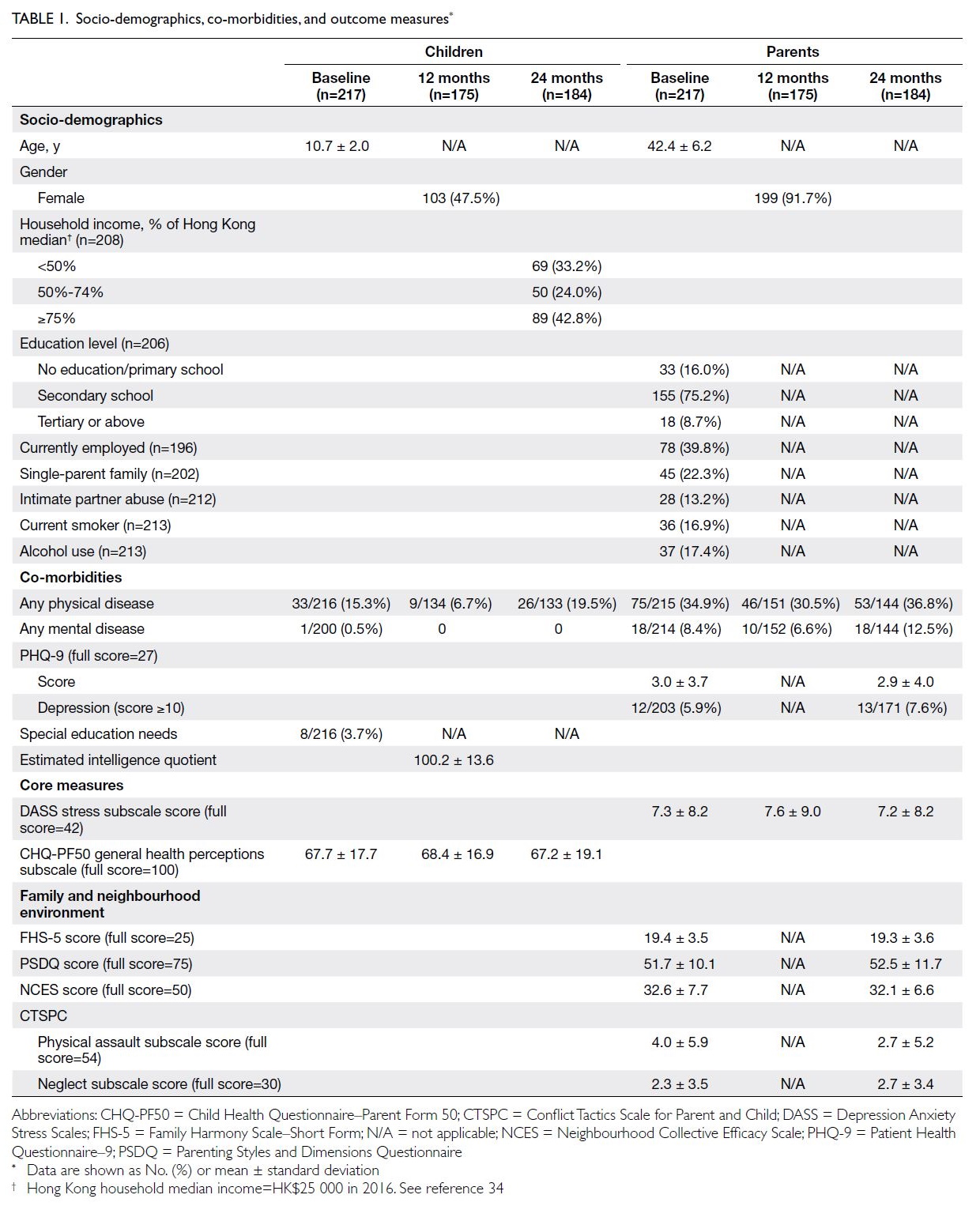
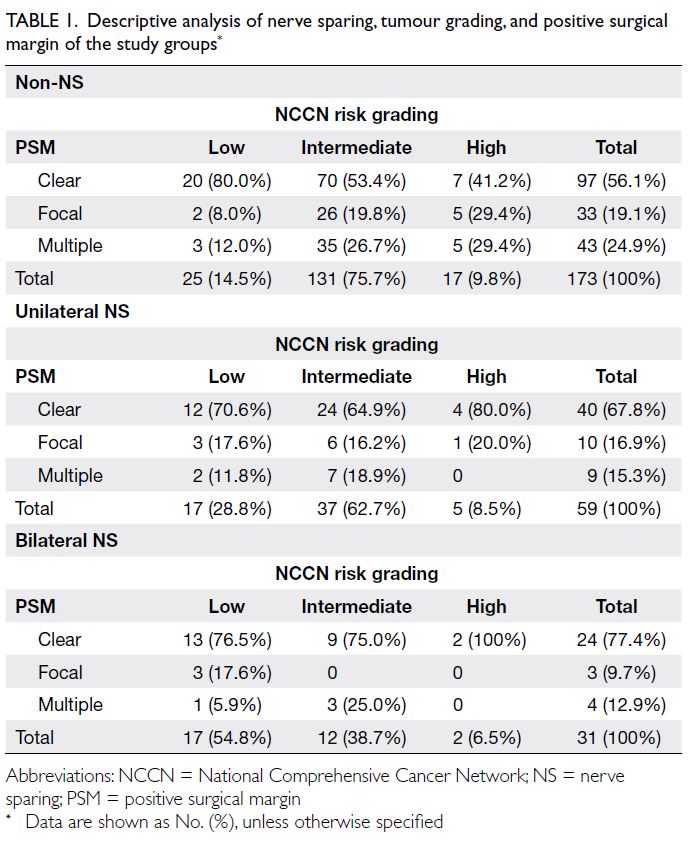
each of the three time points are detailed in Table 1.
Baseline characteristics of parent-child pairs
At baseline, the ages of parents and children (mean ± SD) were 42.4 ± 6.2 years and 10.7 ± 2.0 years,
respectively. Approximately half of the children
were girls (47.5%), whereas the parents involved
were predominantly mothers (91.7%). The majority
(75.2%) of parents had completed secondary
education. Approximately 39.8% of primary parents
were employed, and 57.2% of families had a monthly
household income below 75% of the 2016 Hong
Kong median (ie, HK$25 000).34
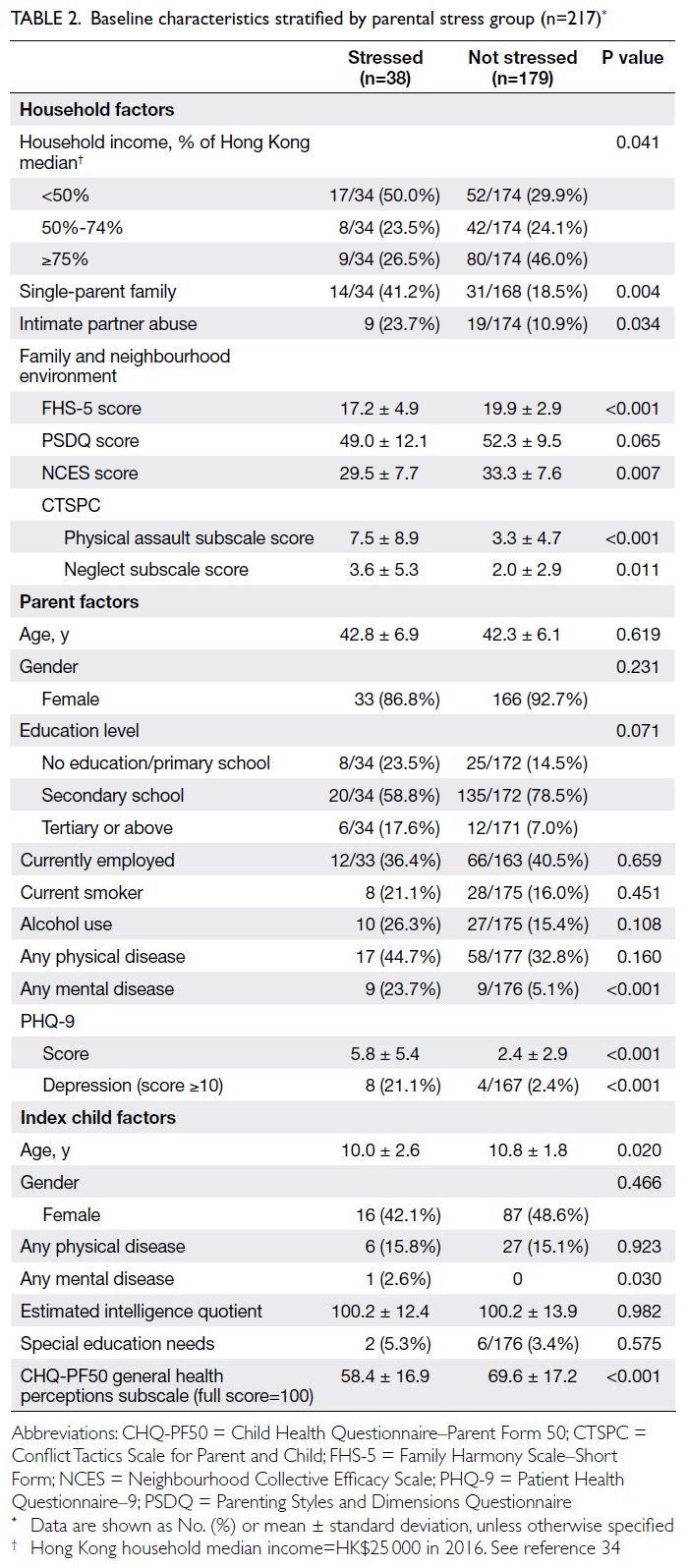
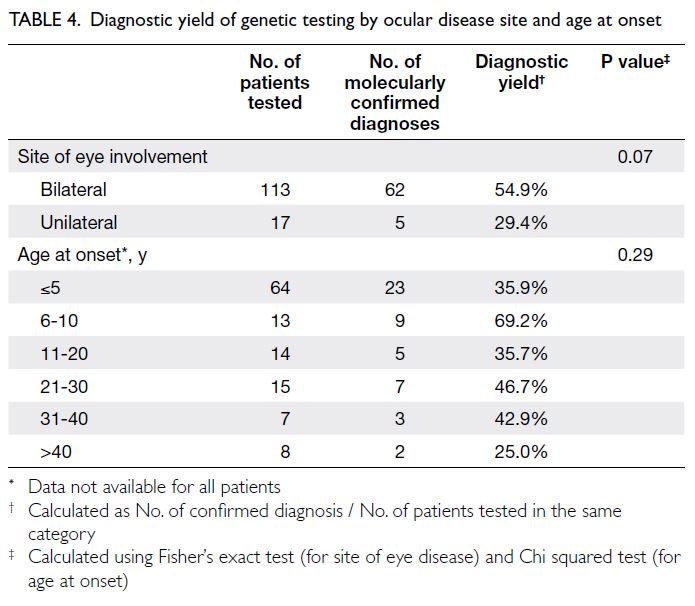
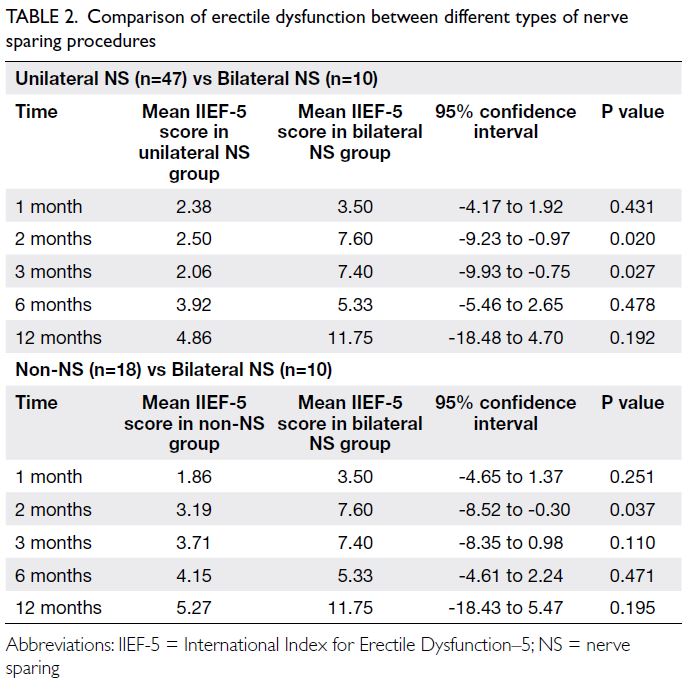
Thirty-eight parents (17.5%) experienced
significant stress, indicated by a DASS stress
subscale score of 15 or above at baseline.
Considerable differences were evident in their
baseline characteristics compared with parents who
were not stressed. Stressed parents were more likely
to be single parents (41.2% vs 18.5%) and to have
a household income below 50% of the Hong Kong
median (50.0% vs 29.9%). A greater proportion of
stressed parents reported being victims of intimate
partner abuse (23.7% vs 10.9%). Diagnosed mental
illnesses (23.7% vs 5.1%) and depression, indicated
by a PHQ-9 score ≥10 (21.1% vs 2.4%), were more
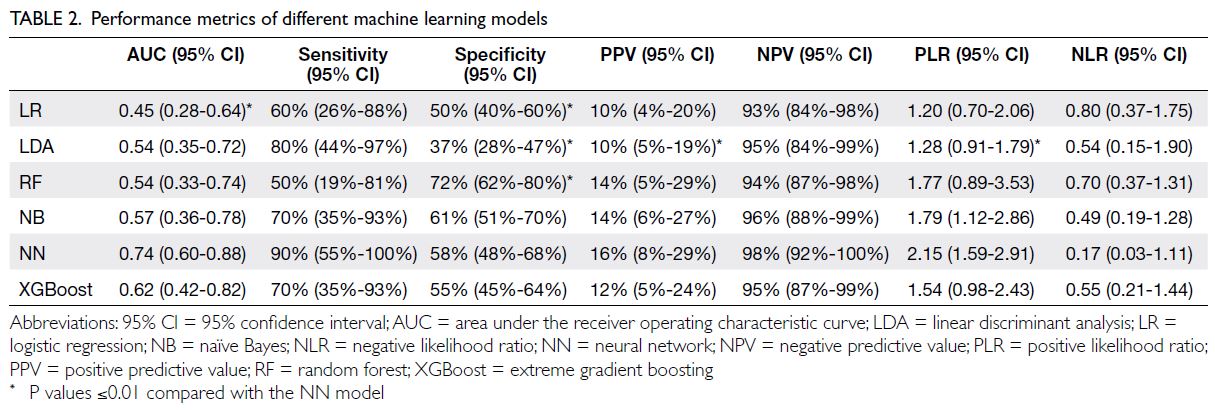
prevalent among these parents (Table 2). Both their
physical and mental HRQOL were significantly
worse (physical component score=42.5 ± 9.9 vs
49.1 ± 8.2; mental component score=38.1 ± 10.0 vs
55.5 ± 8.7; P<0.001).
Compared with children of parents who were
not stressed, children of stressed parents were
younger (age=10.0 ± 2.6 years vs 10.8 ± 1.8 years;
P=0.020) and had worse general health and HRQOL,
as reflected by lower scores in every subscale of the
CHQ–Parent Form 50 except bodily pain and self-esteem.
In particular, large differences were observed
in four subscales: parental impact—emotional,
parental impact—time, family activities, and family
cohesion.
Moreover, stressed parents reported lower
scores in family harmony (FHS-5) and neighbourhood
cohesion (Neighbourhood Collective Efficacy Scale).
Although parenting style did not differ significantly, stressed parents showed a greater tendency for
physical punishment, as reflected by higher scores
on the CTSPC–physical assault subscale, and for
neglect, as indicated by higher CTSPC–neglect
subscale scores, compared with parents who were
not stressed (Table 2).
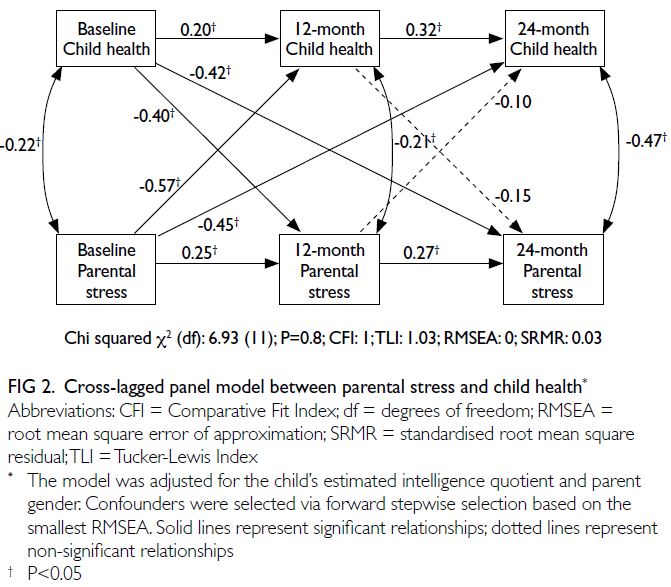
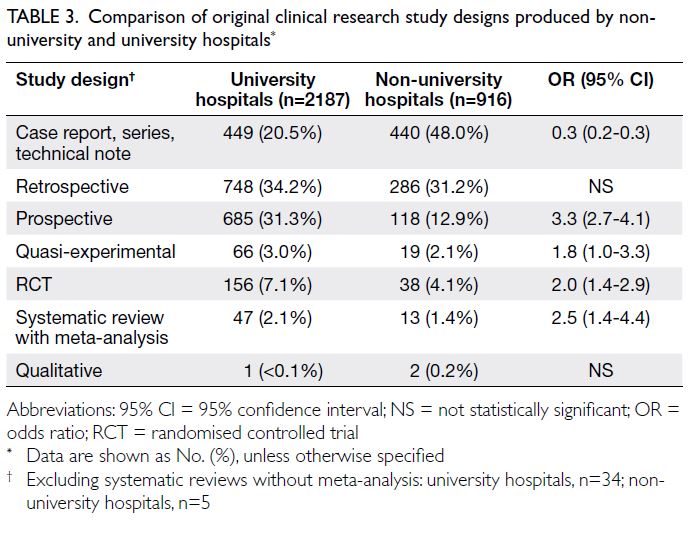
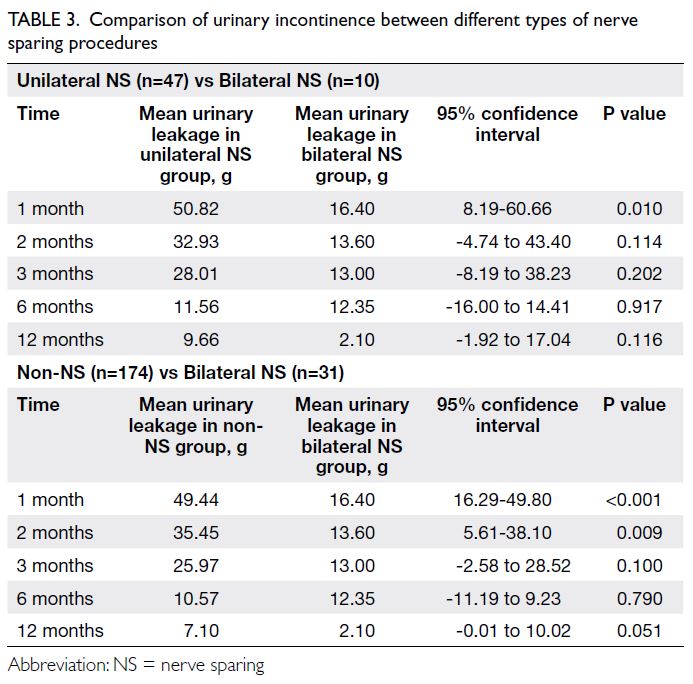
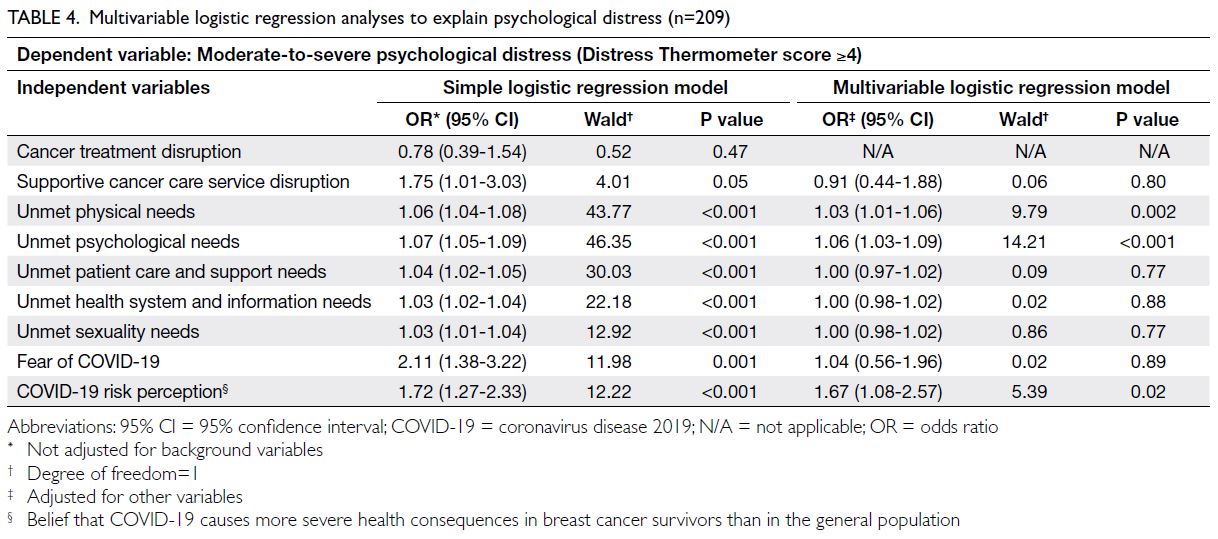
Relationship between parental stress and
child health over time
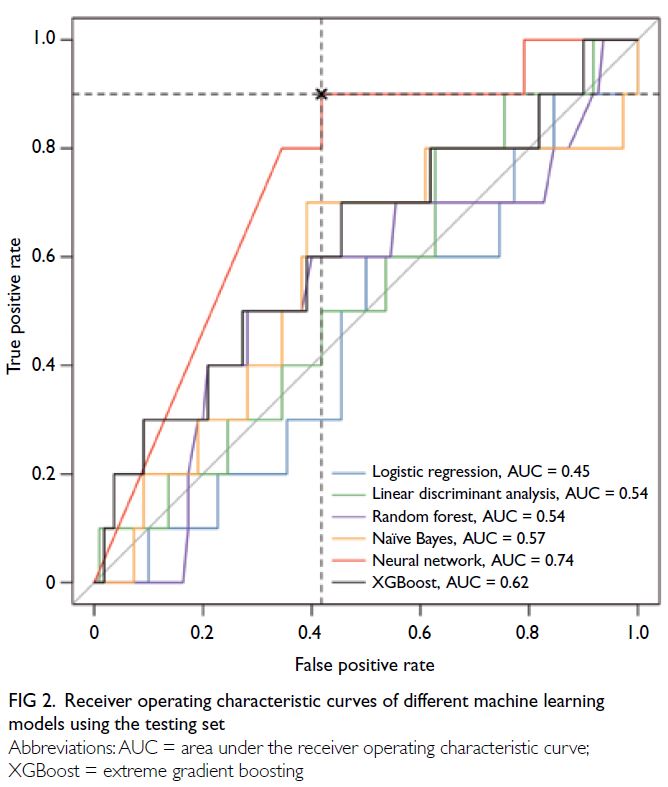
Figure 2 shows the cross-lagged panel model
examining the bidirectional relationship between
parental stress and child health. A bidirectional
relationship between child health and parental
stress was confirmed. Significant associations
were observed between parental stress and
child health at each time point (estimates:
baseline=-0.22, 12 months=-0.21, 24 months=-0.47); between baseline child health and parental
stress at 12 months (estimate=-0.40) and 24 months
(estimate=-0.42); and between baseline parental
stress and child health at 12 months (estimate=-0.57)
and 24 months (estimate=-0.10).
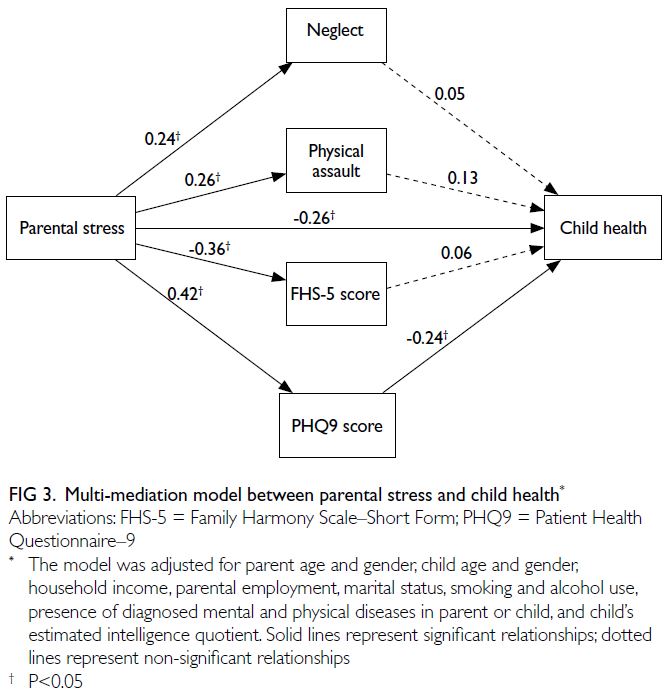
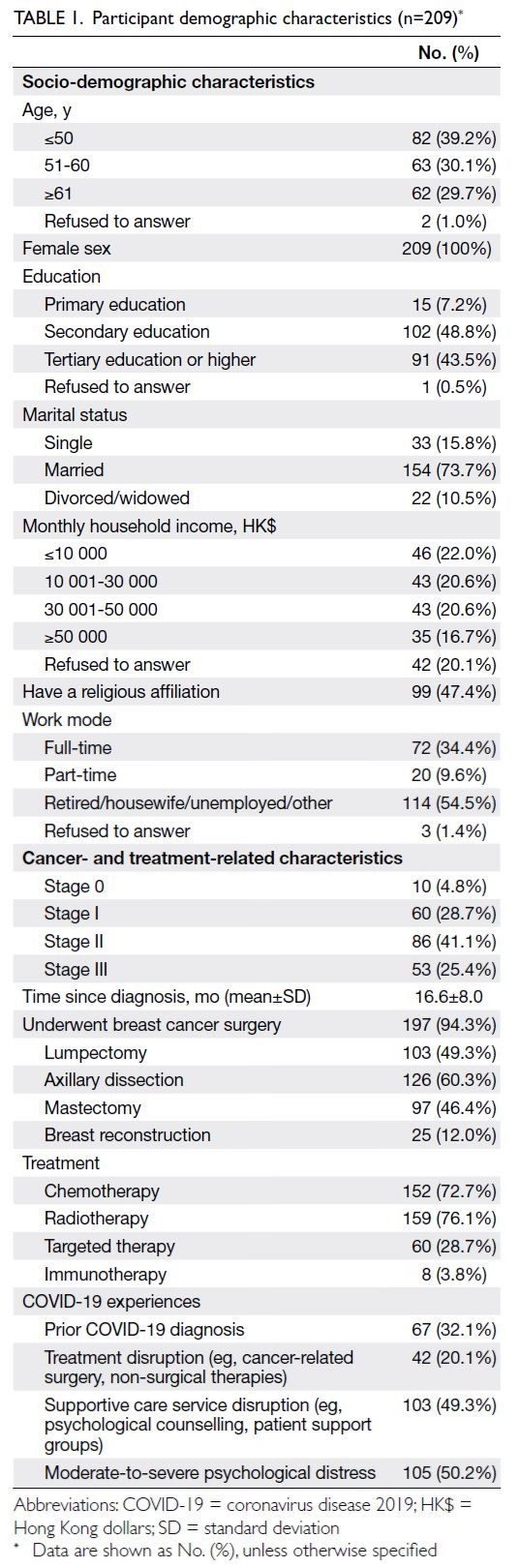
Mediators and moderators of the parent-child
health relationship over time
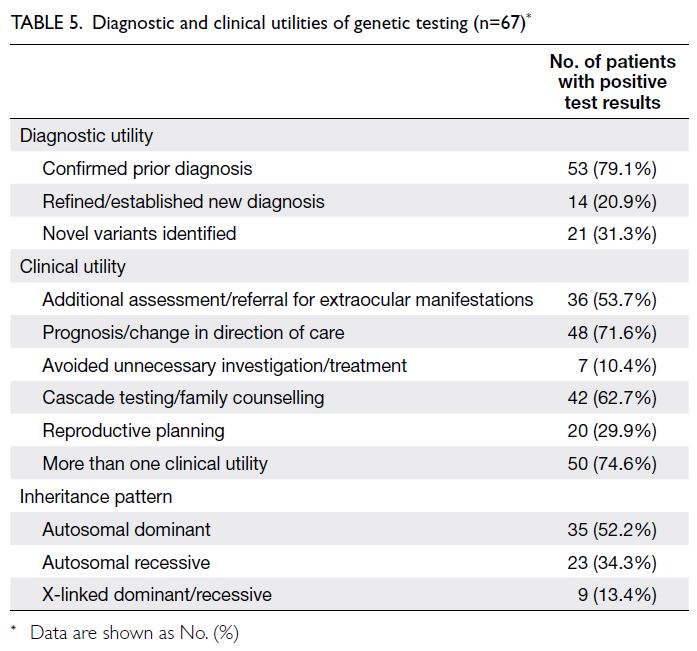
The multi-mediation model results generated by
bootstrapping are illustrated in Figure 3; the model
estimates and goodness-of-fit statistics are presented
in online supplementary Table 2. The total effect of
the relationship between parental stress and child
health was reduced when mediators were included
in the model. Significant positive associations of
parental stress were observed with the PHQ-9 score,
as well as the physical assault and neglect subscales
of the CTSPC. A significant negative association
was noted between parental stress and the FHS-5
score. Among mediators, only the PHQ-9 exerted a
significant negative effect on child health.
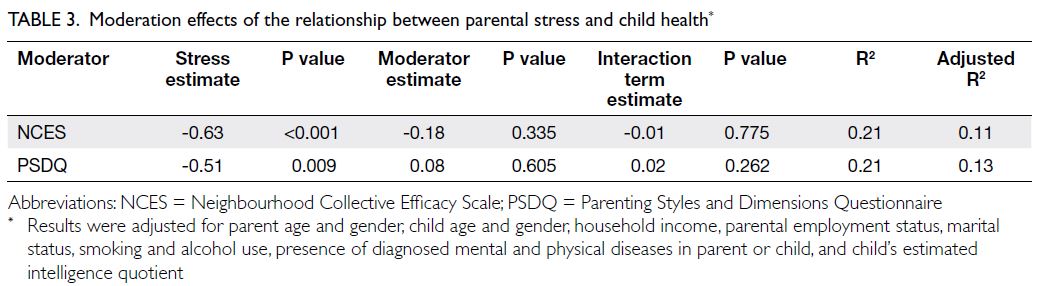
Table 3 presents the moderation model.
Neither neighbourhood cohesion nor parenting style
demonstrated a moderating effect on the relationship
between parental stress and child health. Estimates
for the interaction terms were negligible. The R2
values were around 0.21, and the adjusted R2 values
were slightly lower (0.11-0.13), indicating modest
explanatory power of the model after adjusting for
confounders.
Discussion
Our study demonstrated that a substantial
proportion of low-income parents experienced
stress (17.5%), which was associated with multiple
stressors including poverty, marital problems,
intimate partner abuse, family disharmony, and
reduced neighbourhood support. Children of
stressed parents reported worse general health and
HRQOL, as well as more behavioural problems. A short-term and long-term bidirectional inverse
relationship between parental stress and child
health was confirmed; this relationship was partially
mediated by the level of parental depression.
Compared with the general Hong Kong
population, the parent-child pairs in this study
were more exposed to various known stressors in
addition to low income. The prevalences of single-parent
families (22.3% vs 9.8%35) and intimate
partner abuse (13.2% vs 7.2%36) were higher, and
more parents reported regular alcohol consumption
(17.4% vs 8.7%37). Therefore, it is not surprising that
a considerably greater proportion of parents in this
study experienced elevated levels of stress (17.5%
vs 5.2%38) and depression (5.9% vs 1.2%37). The
persistently high level of parental stress observed
during the study period may be attributed partly
to ongoing exposure to various stressors over time
and partly to constant exposure to chronic stressors.
Both scenarios highlight the urgent need to ensure
assessment and intervention for these disadvantaged
parents.
Previous studies have demonstrated
bidirectional interactions between parental stress
and child health in relation to both internalising and
externalising behaviours.6 8 Increases in behavioural
problems have been shown to raise parental stress
over time, which in turn exacerbates behavioural
issues in children.39 Our study adds to this body of
evidence by confirming significant bidirectional
effects between general parental stress and child
health at each time point. Cross-effects were
observed from baseline child health to later parental
stress, and from baseline parental stress to later child
health at both 12 and 24 months. These findings
suggest that parental stress both precedes and
results from child health, with reciprocal short-term
and long-term influences.40
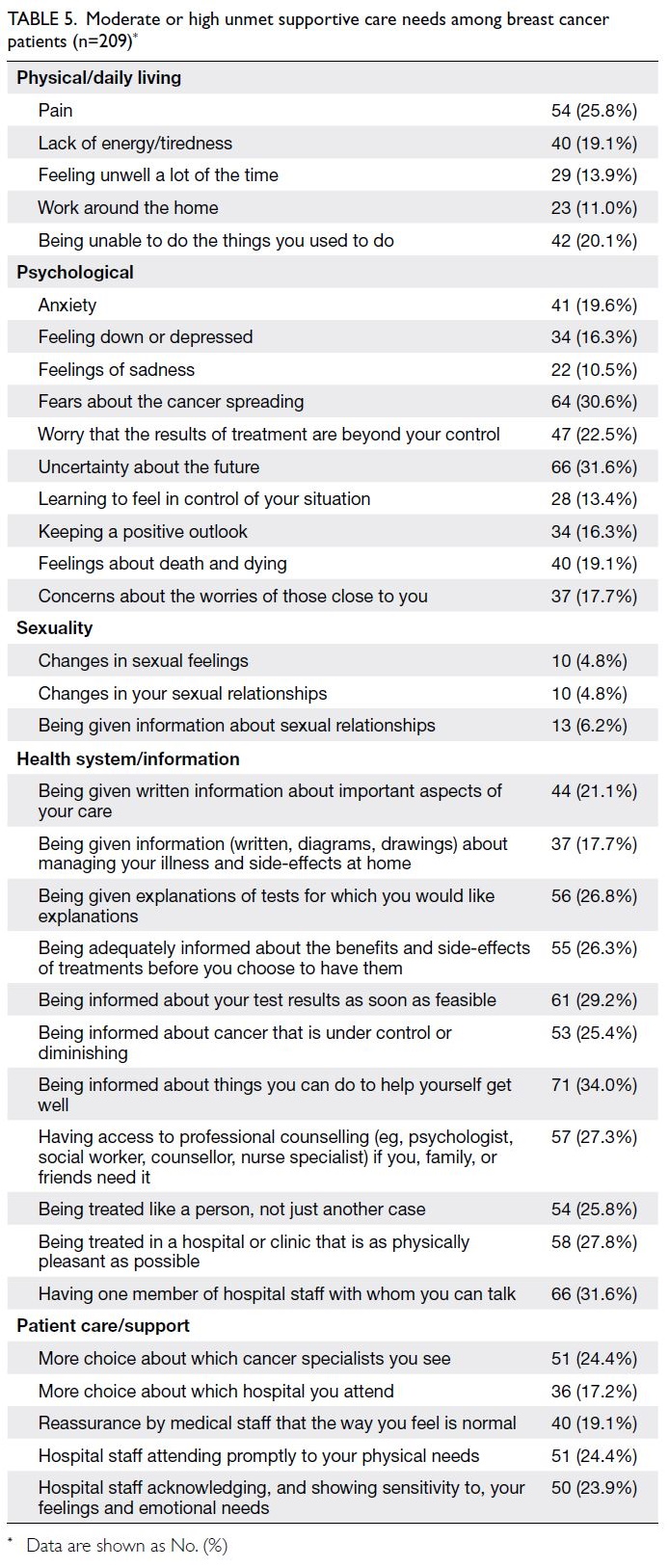
In our attempt to identify pathways through
which parental stress affects child health, we
observed that only parental depression significantly
mediated the relationship. This result is consistent
with previous findings that maternal depression and
perceived stress directly and negatively influence
child development.41 One possible explanation is that
depressed mothers may lack the energy or capacity
to provide adequate care and support for their child’s
health. Research into this mediation effect remains
limited; however, one recent study reported similar
outcomes regarding the indirect impact of workrelated
stress on child health, mediated by maternal
depression.42 The implementation of screening
and intervention for parental depression is both
imperative and urgent to counteract the adverse
effects of stress on parental and child health. Medical
and social service providers should collaborate to
actively screen at-risk parents from low-income
families in the community. Early intervention through lifestyle-based care—such as physical
activity, relaxation techniques, and mindfulness-based
therapies—can help to prevent43 44 and
manage45 46 depression, thus mitigating long-term
negative impacts on child health.
However, it must be noted that parents with
depression may be biased towards over-reporting
their child’s problems,47 compared with other
informants such as teachers and the children
themselves.48 Further research is warranted to
identify individual and family characteristics that
may influence discrepancies between informants.
Other potential factors examined in previous
studies—such as household structure (dual- vs
multi-generational), parental rearing behaviours,
and confident and affective social support—might
also contribute to the relationship between parental
stress and child health; they should be explored in
future studies with larger sample sizes.
Strengths and limitations
This is one of the first studies to examine the longitudinal relationship between general parental
stress and child health, enabling assessment of
possible causal relationships between the two
outcomes. Specifically, we recruited vulnerable
families with substantial socio-economic
disadvantages who experience high levels of stress
and would benefit most from future interventions.
Furthermore, a high response rate was maintained
throughout the study, ensuring adequate power for
the analyses.
However, the findings of our study must be
interpreted in light of the following limitations. First,
although we conducted a comprehensive analysis of
factors related to parental stress and child health, the
outcomes were based on self-reported assessments,
which are susceptible to respondent bias. Only three
measurements, taken 1 year apart, were performed
in this study due to concerns regarding practicality
and the burden on participating families. Therefore,
caution should be exercised in generalising the
results with respect to longitudinal trends, given
that substantial intra-individual fluctuations may
have occurred but were not captured in this study.
Second, both parental stress and child health
were assessed using parent-report questionnaires,
which may contribute to increased shared method
variance. Additionally, aspects of the child’s health
or behaviour considered problematic by the parent may not align with assessments made by other
individuals (eg, teachers). As mentioned earlier,
parents with depression may be biased towards
over-reporting problems and are more likely to
report behavioural issues in their child compared
with other informants.47 48 The validity of parent-perceived
measures of child health—particularly
in relation to parental depression—and their
agreement with other caregivers should be examined
in future trials specifically designed for this purpose.
Third, there were unmeasured confounders in this
observational study, such as exercise and social
functioning. Moreover, certain socio-demographic
factors, including marital and employment statuses,
were assumed to be static throughout the study. It
remains uncertain whether changes in these factors,
if any, may have influenced the observed results.
Additional information regarding participant
characteristics, observational measures of child
behaviour, or objective indicators of child health (eg,
cortisol levels) could improve the reliability of the
findings.
Conclusion
This study showed that a substantial proportion of
parents from low-income families in Hong Kong
experienced general stress due to multiple stressors,
which was negatively associated with their child’s
health. A bidirectional relationship was observed
between parental stress and child health over time,
which may be partly mediated by parental depression.
Prompt screening and appropriate intervention are
necessary to prevent adverse health outcomes for
parents and children in low-income families.
Author contributions
Concept or design: EYT Yu, RSM Wong, AFY Tiwari, CKH Wong, VY Guo, CLK Lam.
Acquisition of data: RSM Wong, KSN Liu.
Analysis or interpretation of data: EYT Yu, EYF Wan, RSM Wong, IL Mak, AFY Tiwari, CKH Wong, VY Guo, CLK Lam.
Drafting of the manuscript: EYT Yu, RSM Wong, IL Mak, CHN Yeung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: RSM Wong, KSN Liu.
Analysis or interpretation of data: EYT Yu, EYF Wan, RSM Wong, IL Mak, AFY Tiwari, CKH Wong, VY Guo, CLK Lam.
Drafting of the manuscript: EYT Yu, RSM Wong, IL Mak, CHN Yeung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As advisors of the journal, EYT Yu and CKH Wong were
not involved in the peer review process. Other authors have
disclosed no conflicts of interest.
Acknowledgement
The authors are grateful for the support from Kerry Group
Kuok Foundation (Hong Kong) Limited in conducting this
study on participants of the Trekkers Family Enhancement
Scheme. The authors’ sincere gratitude goes to the
Neighbourhood Advice-Action Council, Hong Kong Sheng
Kung Hui Lady MacLehose Centre, and Shek Lei Community
Hall for their assistance in participant recruitment and
provision of venues for data collection, respectively. The
authors thank the Social Science Research Centre of The
University of Hong Kong (HKU) for their timely completion
of the telephone surveys, and Department of Paediatrics
and Adolescent Medicine of HKU for performing the assays
for DNA extraction and telomere length measurement. The
authors also thank the hard work of their research staff in data
collection and analysis.
Declaration
The study results were disseminated through a poster
presentation at the Health Research Symposium 2021 (23
November 2021, hybrid conference), entitled “In-depth
exploration of a bidirectional parent-child health relationship
and its mediating and moderating factors among low-income
families in Hong Kong”.
Funding/support
This research was supported by the Health and Medical Research Fund of the Health Bureau, Hong Kong SAR
Government (Ref No.: HMRF 14151571). The funder
had no role in the study design, data collection/analysis/interpretation, or manuscript preparation.
Ethics approval
This research was approved by the Institutional Review
Board of The University of Hong Kong/Hospital Authority
Hong Kong West Cluster, Hong Kong (Ref No.: UW 16-415).
Informed consent was obtained from patients when baseline
data were collected.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Santiago CD, Kaltman S, Miranda J. Poverty and mental
health: how do low-income adults and children fare in
psychotherapy? J Clin Psychol 2013;69:115-26. Crossref
2. Smith MV, Mazure CM. Mental health and wealth:
depression, gender, poverty, and parenting. Annu Rev Clin
Psychol 2021;17:181-205. Crossref
3. Evans GW, Kim P. Childhood poverty, chronic stress, self-regulation,
and coping. Child Dev Perspect 2013;7:43-8. Crossref
4. Adjei NK, Jonsson KR, Straatmann VS, et al. Impact of
poverty and adversity on perceived family support in
adolescence: findings from the UK Millennium Cohort
Study. Eur Child Adolesc Psychiatry 2024;33:3123-32. Crossref
5. Alto ME, Warmingham JM, Handley ED, Manly JT,
Cicchetti D, Toth SL. The association between patterns of
trauma exposure, family dysfunction, and psychopathology
among adolescent females with depressive symptoms from
low-income contexts. Child Maltreat 2023;28:130-40. Crossref
6. van Dijk W, de Moor MH, Oosterman M, Huizink AC,
Matvienko-Sikar K. Longitudinal relations between
parenting stress and child internalizing and externalizing
behaviors: testing within-person changes, bidirectionality
and mediating mechanisms. Front Behav Neurosci
2022;16:942363. Crossref
7. Neece CL, Green SA, Baker BL. Parenting stress and child
behavior problems: a transactional relationship across
time. Am J Intellect Dev Disabil 2012;117:48-66. Crossref
8. Stone LL, Mares SH, Otten R, Engels RC, Janssens JM.
The co-development of parenting stress and childhood
internalizing and externalizing problems. J Psychopathol
Behav Assess 2016;38:76-86. Crossref
9. Economic Analysis Division Economic Analysis
and Business Facilitation Unit Financial Secretary’s
Office; Census and Statistics Department, Hong Kong
SAR Government. Hong Kong Poverty Situation
Report 2013. Oct 2014. Available from: https://www.commissiononpoverty.gov.hk/eng/pdf/poverty_report13_rev2.pdf. Accessed 31 Jul 2023.
10. Lam CL, Guo VY, Wong CK, Yu EY, Fung CS. Poverty and
health-related quality of life of people living in Hong Kong:
comparison of individuals from low-income families and
the general population. J Public Health (Oxf) 2017;39:258-65.Crossref
11. Luecken LJ, Lemery KS. Early caregiving and physiological
stress responses. Clin Psychol Rev 2004;24:171-91. Crossref
12. Hanington L, Ramchandani P, Stein A. Parental depression
and child temperament: assessing child to parent effects
in a longitudinal population study. Infant Behav Dev
2010;33:88-95. Crossref
13. Associations between depression in parents and parenting,
child health, and child psychological functioning. In:
England MJ, Sim LJ, editors. Depression in Parents,
Parenting, and Children: Opportunities to Improve
Identification, Treatment, and Prevention. Washington
(DC): National Academies Press (US); 2009: 119-82.
14. Lee SL, Cheung YF, Wong HS, Leung TH, Lam T, Lau YL.
Chronic health problems and health-related quality of life
in Chinese children and adolescents: a population-based
study in Hong Kong. BMJ Open 2013;3:e001183. Crossref
15. Chan KL, Lo CK, Ho FK, Chen Q, Chen M, Ip P. Modifiable
factors for the trajectory of health-related quality of life
among youth growing up in poverty: a prospective cohort
study. Int J Environ Res Public Health 2021;18:9221. Crossref
16. Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol
2013;25:577-85. Crossref
17. Evans GW, Kim P, Ting AH, Tesher HB, Shannis D.
Cumulative risk, maternal responsiveness, and allostatic
load among young adolescents. Dev Psychol 2007;43:341-51. Crossref
18. Hammen C. Stress and depression. Annu Rev Clin Psychol
2005;1:293-319. Crossref
19. Power C, Weise V, Mack JT, Karl M, Garthus-Niegel S.
Does parental mental health mediate the association
between parents’ perceived stress and parent-infant
bonding during the early COVID-19 pandemic? Early
Hum Dev 2024;189:105931. Crossref
20. Fung CS, Yu EY, Guo VY, et al. Development of a Health
Empowerment Programme to improve the health of
working poor families: protocol for a prospective cohort
study in Hong Kong. BMJ Open 2016;6:e010015. Crossref
21. Lovibond SH, Lovibond PF; Psychology Foundation of
Australia. Manual for the Depression Anxiety Stress Scales.
Sydney: Sydney Psychology Foundation; 1995. Crossref
22. Wang K, Shi HS, Geng FL, et al. Cross-cultural validation of
the Depression Anxiety Stress Scale–21 in China. Psychol
Assess 2016;28:e88-100. Crossref
23. Landgraf JM. Child Health Questionnaire (CHQ). In:
Maggino F, editor. Encyclopedia of Quality of Life and
Well-being Research. Cham: Springer; 2020: 1-6. Crossref
24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity
of a brief depression severity measure. J Gen Intern Med
2001;16:606-13. Crossref
25. Kavikondala S, Stewart SM, Ni MY, et al. Structure and
validity of Family Harmony Scale: an instrument for
measuring harmony. Psychol Assess 2016;28:307-18. Crossref
26. Straus MA. Measuring intrafamily conflict and violence:
the Conflict Tactics (CT) Scales. J Marriage Fam
1979;41:75-88. Crossref
27. Chan KL, Brownridge DA, Fong DY, Tiwari A, Leung WC,
Ho PC. Violence against pregnant women can increase the
risk of child abuse: a longitudinal study. Child Abuse Negl
2012;36:275-84. Crossref
28. Robinson CC, Mandleco B, Olsen SF, Hart CH. The
Parenting Styles and Dimensions Questionnaire (PSDQ).
In: Perlmutter BF, Touliatos J, Holden GW, editors.
Handbook of Family Measurement Techniques: Vol 3.
Instruments & Index. Thousand Oaks: Sage; 2001: 319-21.
29. Wu P, Robinson CC, Yang C, et al. Similarities and
differences in mothers’ parenting of preschoolers in China
and the United States. Int J Behav Dev 2002;26:481-91. Crossref
30. Sampson RJ, Raudenbush SW, Earls F. Neighborhoods
and violent crime: a multilevel study of collective efficacy.
Science 1997;277:918-24. Crossref
31. Chou KL. Perceived discrimination and depression among
new migrants to Hong Kong: the moderating role of social
support and neighborhood collective efficacy. J Affect
Disord 2012;138:63-70. Crossref
32. Baron RM, Kenny DA. The moderator–mediator variable
distinction in social psychological research: conceptual,
strategic, and statistical considerations. J Pers Soc Psychol
1986;51:1173-82. Crossref
33. Rosseel Y. lavaan: an R package for structural equation
modeling. J Stat Softw 2012;48:1-36. Crossref
34. Census and Statistics Department, Hong Kong SAR
Government. Hong Kong 2016 Population By-census–Thematic Report: Household Income Distribution in Hong Kong. Jun 2017. Available from: https://www.censtatd.gov.hk/en/data/stat_report/product/B1120096/att/B11200962016XXXXB0100.pdf . Accessed 25 Aug 2025.
35. Census and Statistics Department, Hong Kong SAR
Government. 2021 Population Census—Thematic
Report: Children. Feb 2023. Available from: https://www.census2021.gov.hk/doc/pub/21c-Children.pdf. Accessed 25 Aug 2025.
36. Chan KL. Intimate partner violence in Hong Kong. In:
Chan KL, editor. Preventing Family Violence: A
Multidisciplinary Approach. Hong Kong: Hong Kong
University Press; 2012: 19-58. Crossref
37. Non-Communicable Disease Branch, Centre for Health
Protection, Hong Kong SAR Government. Report of
Population Health Survey 2020-22 (Part I); 2022. Available
from: https://www.chp.gov.hk/files/pdf/dh_phs_2020-22_part_1_report_eng_rectified.pdf. Accessed 31 Jul 2023.
38. Chan SM, Wong H, Chung RY, Au-Yeung TC. Association
of living density with anxiety and stress: a cross-sectional
population study in Hong Kong. Health Soc Care
Community 2021;29:1019-29. Crossref
39. Baker BL, McIntyre LL, Blacher J, Crnic K, Edelbrock C,
Low C. Pre-school children with and without developmental
delay: behaviour problems and parenting stress over time. J
Intellect Disabil Res 2003;47:217-30. Crossref
40. Motrico E, Bina R, Kassianos AP, et al. Effectiveness of
interventions to prevent perinatal depression: an umbrella
review of systematic reviews and meta-analysis. Gen Hosp
Psychiatry 2023;82:47-61. Crossref
41. Vameghi R, Amir Ali Akbari S, Sajedi F, Sajjadi H, Alavi
Majd H. Path analysis association between domestic
violence, anxiety, depression and perceived stress in
mothers and children’s development. Iran J Child Neurol
2016;10:36-48. Crossref
42. Xu L, Xu J. The impact of maternal occupation on
children’s health: a mediation analysis using the parametric
G-formula. Soc Sci Med 2024;343:116602. Crossref
43. Bellón JÁ, Conejo-Cerón S, Sánchez-Calderón A, et al.
Effectiveness of exercise-based interventions in reducing
depressive symptoms in people without clinical depression:
systematic review and meta-analysis of randomised
controlled trials. Br J Psychiatry 2021;219:578-87. Crossref
44. Newland P, Bettencourt BA. Effectiveness of mindfulness-based
art therapy for symptoms of anxiety, depression,
and fatigue: a systematic review and meta-analysis.
Complement Ther Clin Pract 2020;41:101246. Crossref
45. Marx W, Manger SH, Blencowe M, et al. Clinical guidelines
for the use of lifestyle-based mental health care in major
depressive disorder: World Federation of Societies for
Biological Psychiatry (WFSBP) and Australasian Society
of Lifestyle Medicine (ASLM) taskforce. World J Biol
Psychiatry 2023;24:333-86. Crossref
46. Recchia F, Leung CK, Chin EC, et al. Comparative
effectiveness of exercise, antidepressants and their
combination in treating non-severe depression: a systematic
review and network meta-analysis of randomised
controlled trials. Br J Sports Med 2022;56:1375-80. Crossref
47. Chi TC, Hinshaw SP. Mother–child relationships of
children with ADHD: the role of maternal depressive
symptoms and depression-related distortions. J Abnor
Child Psychol 2002;30:387-400. Crossref
48. Richters JE. Depressed mothers as informants about their
children: a critical review of the evidence for distortion.
Psychol Bull 1992;112:485-99. Crossref